Abstract
Background
Ischemic injury by hepatic artery ligation (HAL) during obstructive cholestasis induced by bile duct ligation (BDL) results in bile duct damage, which can be prevented by administration of VEGF-A. The potential regulation of VEGF and VEGF receptor expression and secretion by bile acids in BDL with HAL is unknown.
Aims
We evaluated whether taurocholic acid (TC) can prevent HAL-induced cholangiocyte damage via the alteration of VEGFR-2 and/or VEGF-A expression.
Methods
Utilizing BDL, BDL+TC, BDL+HAL, BDL+HAL+TC, and BDL+HAL+wortmannin+TC treated rats, we evaluated cholangiocyte apoptosis, proliferation, and secretion as well VEGF-A and VEGFR-2 expression by immunohistochemistry. In vitro, we evaluated the effects of TC on cholangiocyte secretion of VEGF-A and the dependence of TC-induced proliferation on the activity of VEGFR-2.
Results
In BDL rats with HAL, chronic feeding of TC prevented HAL-induced loss of bile ducts and HAL-induced decreased cholangiocyte secretion. TC also prevented HAL-inhibited VEGF-A and VEGFR-2 expression in liver sections and HAL-induced circulating VEGF-A levels, which were blocked by wortmannin administration. In vitro, TC stimulated increased VEGF-A secretion by cholangiocytes, which was blocked by wortmannin and stimulated cholangiocyte proliferation that was blocked by VEGFR-2 kinase inhibitor.
Conclusion
TC prevented HAL-induced biliary damage by upregulation of VEGF-A expression.
Keywords: Apoptosis, cAMP, intrahepatic biliary epithelium, mitosis, VEGF
Introduction
Cholangiocytes are the epithelial cells that line the intrahepatic biliary tree [1, 2] and modify canalicular bile [3] by a series of basal and hormone-regulated absorptive and secretory events before reaching the small intestine [2, 4-8]. The gastrointestinal hormone secretin stimulates bile secretion by interaction with secretin receptors (expressed only by cholangiocytes in rat liver) [2, 9] by enhanced adenosine 3′,5′-monophosphate (cAMP) levels [2, 5, 7, 10].
Cholangiocytes have a low basal mitotic rate in normal rodent liver [1, 11, 12], but proliferate in a number of experimental models of cholestasis including bile duct ligation (BDL) [4, 11, 13]. In rodent models in which cholangiocytes proliferate (e.g., BDL or partial hepatectomy), there is elevated secretin receptor gene expression and enhanced secretin-stimulated cAMP levels, and secretin-induced bile and bicarbonate secretion [4, 12, 14, 15]. However, reduced cholangiocyte proliferation and increased cholangiocyte apoptosis was observed following vagotomy, hepatic artery ligation (HAL), endogenous bile acid depletion, or acute carbon tetrachloride (CCl4) administration [16, 17]. In these models, decreased cholangiocyte proliferation was associated with impairment of basal and secretin-stimulated cAMP levels and bile and bicarbonate secretion [14-17].
Proliferating cholangiocytes act as a neuroendocrine compartment in the diseased liver allowing for the regulation of cholangiocyte proliferation to occur in autocrine and paracrine mechanisms [18]. We have shown that cholangiocyte proliferation is regulated by neuropeptides, bile acids, gastrointestinal hormones and growth factors such as vascular endothelial growth factor (VEGF) [13, 14, 16-25]. Indeed, rat cholangiocytes express the message and protein for VEGF-A, secrete VEGF-A, and express the VEGF receptor subtypes, VEGFR-2 and VEGFR-3 [22]. In fact, VEGF secretion is increased during cholestasis induced by BDL and stimulates cholangiocyte proliferation via an autocrine mechanism [17, 22].
The intrahepatic biliary system is nourished by the peribiliary vascular plexus (PBP), which originates from the hepatic artery [26]. During cholestasis induced by BDL, ligation of the hepatic artery: (i) induced the disappearance of the PBP, (ii) increased apoptosis and impaired cholangiocyte proliferation and secretin-stimulated ductal secretion, and (iii) decreased cholangiocyte VEGF secretion [17]. Interestingly, administration of recombinant-VEGF-A to BDL rats prevented HAL-induced damage, by maintaining the integrity of the PBP and cholangiocyte proliferation and VEGF expression/secretion [17].
Bile acids, which accumulate during cholestatic liver diseases [27], play critical roles in the regulation of cholangiocyte function [16, 20, 28]. In vivo, chronic feeding of taurocholic acid (TC) to normal rats increased cholangiocyte proliferation and secretin-stimulated ductal secretion [20]. TC prevents caffeic acid-induced bile duct damage by increasing cholangiocyte VEGF expression/secretion [29]. However, there is no information regarding: (i) the potential regulation of cholangiocyte VEGF expression/secretion by bile acids with regard to changes in biliary functions following ligation of the hepatic artery, the main blood supply of the biliary epithelium [30]; and (ii) the role of PI3-K in the modulation of TC induced changes in cholangiocyte VEGF-A/VEGFR-2 expression. Thus, our study sought to address whether TC has a protective effect during HAL-induced bile duct damage [26] and if this event involved alterations of cholangiocyte VEGFR-2 and/or VEGF-A expression/secretion.
Material and Methods
Materials
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. The monoclonal mouse antibody against proliferating cellular nuclear antigen (PCNA) was purchased from DAKO (Kyoto, Japan). The antibodies against VEGF-A and the VEGF-A receptor subtype, VEGFR-2, were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). (Z)-3-[(2,4-Dimethyl-3-(ethoxycarbonyl)pyrrol-5-yl)methylidenyl]indolin-2-one (VEGFR-2 Kinase Inhibitor I) and wortmannin were purchased from EMD Chemicals, Inc (Gibbstown, NJ). The apoptosis detection kit (TACS™ TdT kit) for evaluating apoptosis by terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) analysis [13, 31] was purchased from R&D systems, Minneapolis, MN. The RIA kit for cAMP measurements was purchased from Perkin Elmer, Shelton, CT.
Animal Models
Male Fischer 344 rats (150 to 175 gm) were purchased from Charles River (Wilmington, MA). The animals were kept in a temperature-controlled environment (22°C) with a 12-hour light-dark cycle and fed ad libitum rat chow. The studies were performed in: (i) BDL (for isolation of cells) [4, 11] or bile duct incannulated (BDI, for bile collection) [4] rats that (immediately after BDL or BDI) were fed bile acid control diet or 1% taurocholic acid diet (which represents an approximate dose of 275 μmol/day) [20, 29] for 1 week; (ii) rats that (immediately after BDL or BDI + HAL) were fed bile acid control diet or 1% taurocholic acid diet; and (iii) rats that (immediately after BDL or BDI + HAL) were fed 1% TC for 1 week in the presence of daily injections of 0.9% NaCl or wortmannin (0.7 mg/kg body weight) [32]. The groups of animals used in the study are summarized in Table 1. Since we have previously shown that daily injections of wortmannin or DMSO (in which wortmannin is dissolved) to BDL or BDI rats do not affect cholangiocyte apoptosis, proliferation and functional activity [23, 33], these groups of animals were not included in the study. BDL, BDI and HAL were performed as described [4, 34]. Before each procedure, animals were anesthetized with sodium pentobarbital (50 mg/kg body weight, IP). Study protocols were performed in compliance with IACUC guidelines of Scott and White/Texas A&M Health Science Center.
Table 1.
Animal Models.
| Treatment | Treatment |
|---|---|
| BDL or BDI rats + BA control feeding for 1 week | Immediately after surgery, rats were fed BA control diet for 1 week |
| BDL or BDI rats + 1% TC feeding for 1 week | Immediately after surgery, rats were fed 1% TC for 1 week |
| BDL or BDI rats + HAL + bile acid control diet feeding for 1 week in the presence of daily injections of NaCl | Immediately after surgeries, rats were fed bile acid control diet for 1 week in the presence of daily injections of NaCl |
| BDL or BDI rats + HAL + 1% TC feeding for 1 week in the presence of daily injections of NaCl | Immediately after surgeries, rats were fed 1% TC for 1 week in the presence of daily injections of NaCl |
| BDL or BDI rats + HAL + 1% TC feeding for 1 week in the presence of daily injections of wortmannin | Immediately after surgeries, rats were fed 1% TC for 1 week in the presence of daily injections of wortmannin |
BA = bile acid; BDI = bile duct incannulated; BDL = bile duct ligated; HAL = hepatic artery ligation; TC = taurocholic acid.
Isolated Cholangiocytes and Cholangiocyte Cultures
Cholangiocytes were purified by immunoaffinity separation as described by us [11, 35]. For the in vitro studies, we utilized our normal rat intrahepatic cholangiocyte (NRIC) cell line, which retains morphological, phenotypic and functional characteristics similar to that of freshly isolated cholangiocytes [36, 37].
Measurement of Cholangiocyte VEGF-A and VEGFR-2 Protein Expression in Liver Sections
We evaluated the protein expression for VEGF-A and VEGFR-2 by immunohistochemistry [22, 26] in paraffin-embedded liver sections (4-5 μm). For each primary antibody, negative controls were performed by replacing the primary antibody with similarly diluted normal serum from the same species, keeping all the other steps the same. Following staining, sections were analyzed in a coded fashion with an Olympus BX-51 microscope (Tokyo, Japan) equipped with a Videocam (Spot Insight, Diagnostic Instrument, Inc. Sterling Heights, MI) and processed with an Image Analysis System (IAS - Delta Sistemi, Roma- Italy). The semi-quantitative evaluation of VEGFA and VEGFR-2 immunostaining were assessed in a coded fashion (in 10 different non-overlapping fields of 3 different slides taken from each of six blocks randomly obtained from the medial lobe of each experimental group.
Evaluation of Cholangiocyte Apoptosis and Proliferation
We evaluated cholangiocyte apoptosis by TUNEL analysis [13, 31] in liver sections (4 μm, 3 slides evaluated for each group) from the selected groups of animals. Following counterstaining with hematoxylin solution, liver sections were evaluated in a coded fashion with the microscope ECLIPSE E600 (Nikon Eclipse, Tokyo, Japan). Approximately 100 cells per slide were counted in a coded fashion in ten non-overlapping fields.
Cholangiocyte proliferation was evaluated by expression of PCNA and intrahepatic bile duct mass (IBDM, by CK-19 immunohistochemistry) in paraffin-embedded liver sections from the selected group of rodents [22]. Following the selected staining and counterstaining with hematoxylin and eosin, the semi-quantitative immunohistochemical evaluation was assessed (using an Olympus BX 51 light microscope, Tokyo, Japan) in a coded fashion on 10 different non-overlapping fields of 3 different slides taken from each of six blocks randomly obtained from medial lobe of each experimental group. For PCNA, the data were expressed as percentage of positive cells. For bile duct mass, the data were expressed as percentage of cholangiocyte area/total surface. Proliferation was also evaluated by immunoblots for PCNA protein expression [14] in purified cholangiocytes from the in vivo treatment groups.
Measurement of Basal and Secretin-Stimulated cAMP Levels and Bile Secretion
We evaluated the effect of secretin on cAMP levels (in cholangiocytes) and bile and bicarbonate secretion (in bile fistula rats), two functional parameters of cholangiocyte proliferation [4, 9, 11, 12, 14, 20, 33, 38, 39]. For the measurement of cAMP levels, cholangiocytes were incubated for 1 hour at 37°C and incubated for 5 minutes at room temperature with 0.2% bovine serum albumin (BSA, basal) or 100 nM secretin with 0.2% BSA [2, 5, 7, 11, 12]. Measurement of cAMP levels was performed by RIA. Following anesthesia, the selected rats were surgically prepared for bile collection as described [4]. When steady-state bile flow was reached (60-70 minutes from the infusion of Krebs Ringer Henseleit, KRH), the selected animal was infused at a jugular vein with secretin (100 nM) for 30 minutes and by a final infusion of KRH for 30 minutes. Bile was collected every 10 minutes in pre-weighed tubes and immediately stored at -70°C before determination of bicarbonate concentration. Biliary bicarbonate concentration (measured as total CO2) was determined by an ABL™ 520 Blood Gas System (Radiometer Medical A/S, Copenhagen, Denmark).
Determination of VEGF-A Serum Levels and VEGF-A Secretion by Cholangiocytes
We evaluated VEGF-A concentration in: (i) serum and in cholangiocyte supernatants (after 6 hour of incubation at 37°C) [31] obtained from the selected groups of animals of Table 1; and (ii) supernatants from NRIC treated in vitro with taurocholic acid (20 μM) [40] for 24 hours in the absence or presence of PI3-K inhibitor wortmannin (100 nM) [40]. VEGF levels were measured by ELISA using commercially available kits (RayBiotech, Norcross, GA).
Evaluation of the Role of VEGF-A Secretion in Taurocholate-Mediated NRIC Proliferation
After trypsinization, NRIC were seeded into 96-well plates (10,000 cells/well) in a final volume of 200 μl of medium. NRIC were stimulated in vitro for 48 hours with taurocholic acid (20 μM) [19] in the absence or presence of 1-hour preincubation with wortmannin (100 nM) or VEGFR-2 Kinase Inhibitor I (100 nM). The proliferation of NRIC was evaluated by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega Corp., Madison, WI) [21]. Absorbance was measured at 490 nm on a microplate spectrophotometer (Versamax, Molecular Devices, Sunnyvale, CA). Data were expressed as the fold change of treated cells as compared to vehicle-treated controls. To support the concept that the supernatant of NRIC stimulates cholangiocyte proliferation to different extents (depending on the amount of VEGF present in the supernatant of these cells), we treated NRIC for 24 hours at 37°C with the supernatant of NRIC obtained after 24 hours of incubation with BSA or 20 μM TC (containing higher levels of VEGF-A compared to BSA-treated supernatant) in the absence or presence of 1-hour pre-incubation with wortmannin (100 nM) before measuring cell growth by immunoblots for PCNA [13].
Statistical Analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by the Student's unpaired t-test when two groups were analyzed and analysis of variance (ANOVA) when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Effect of Taurocholic Acid Feeding on Cholangiocyte VEGF-A and VEGFR-2 Protein Expression in Liver Sections
Immunohistochemistry in BDL liver sections shows that intrahepatic bile ducts express VEGF-A and VEGFR-2 (Figures 1 A and B, see arrows, and Table 2). In agreement with our previous studies [17], HAL decreased the percentage of cholangiocytes positive for VEGF-A and VEGFR-2 (Figures 1 A and B, and Table 2). TC feeding to BDL + HAL rats prevented HAL-induced decrease in VEGF-A and VEGFR-2 protein expression (Figures 1 A and B, and Table 2). The effect of taurocholic acid feeding was blocked by the simultaneous administration of wortmannin (Figures 1 A and B, and Table 2).
Figure 1.
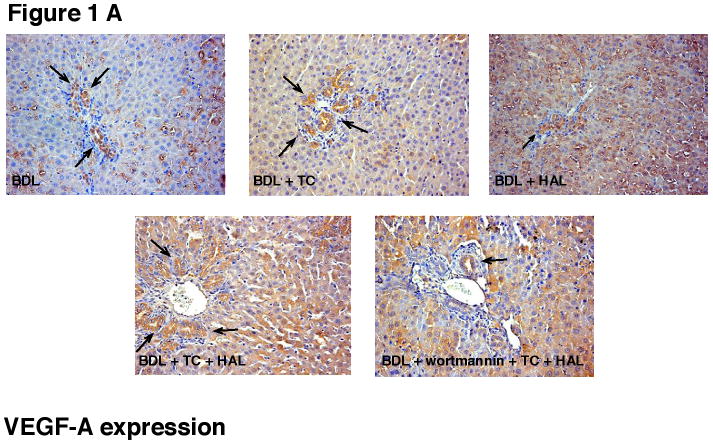
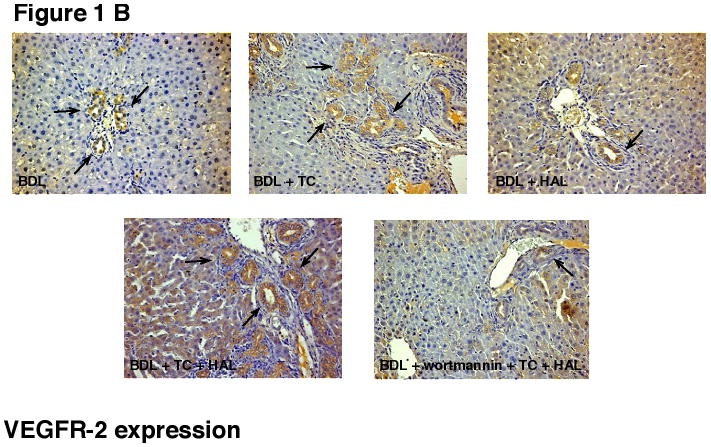
Immunohistochemistry for [A] VEGF-A and [B] VEGFR-2 in liver sections from the selected groups of animals of Table 1. [A-B] Immunohistochemistry shows that bile ducts express VEGF-A and VEGFR-2 (arrows). HAL decreased the % of cholangiocytes positive for VEGF-A and VEGFR-2 (see Table 2 for semiquantitative analysis). Taurocholic acid feeding to BDL + HAL rats prevented HAL-induced decrease in VEGF-A and VEGFR-2 protein expression (see Table 2 for semiquantitative analysis), decrease that was blocked by the simultaneous administration of wortmannin (see Table 2). Original magnification 20×. VEGF-A = vascular endothelial growth factor-A; VEGF-R2 = vascular endothelial growth factor-receptor 2.
Table 2.
Immunohistochemical evaluation of the percentage of cholangiocytes positive for VEGF-A, and VEGFR-2 in liver sections.
| Parameters | BDL + BA control feeding 1 week | BDL + TC feeding 1 week | BDL + HAL + BA control feeding + NaCl daily injections 1 week | BDL + HAL + TC feeding + NaCl daily injections 1 week | BDL + HAL + TC feeding + wortmannin daily injections 1 week |
|---|---|---|---|---|---|
| VEGF-A | 70.4 ± 0.2 | 73.7 ± 3.0 | 38.0 ± 1.4* | 80.2 ± 2.1 | 50.3 ± 2.0* |
| VEGFR-2 | 45.2 ± 3.0 | 48.5 ± 2.5 | 34.3 ± 1.8* | 46.8 ± 1.8 | 28.3 ± 1.6* |
HAL decreased the percentage of cholangiocytes positive for VEGF-A, and VEGFR-2. Taurocholic acid feeding to BDL + HAL rats prevented HAL-induced decrease in VEGF-A and VEGFR-2 protein expression, decrease that was blocked by the simultaneous administration of wortmannin.
p<0.05 vs. the corresponding value of BDL rats fed bile acid control diet.
Data are mean ± SEM of cumulative values obtained from the evaluation of 10 different fields from three different sections (6 sections analyzed per group) for each group. BA = bile acid; BDL = bile duct ligation; HAL = hepatic artery ligation; TC = taurocholic acid.
Measurement of Cholangiocyte Apoptosis and Proliferation
The percentage of apoptotic cholangiocytes in the liver sections of BDL rats was low (Table 3). Taurocholic acid feeding did not change cholangiocyte apoptosis in BDL rats, but prevented HAL-induced increases in cholangiocyte apoptosis (Table 3). TC prevention of HAL-induced apoptosis was blocked by wortmannin treatment (Table 3). In agreement with previous findings [20, 41], TC increased IBDM of BDL rats (Figure 2 and Table 3). HAL-induced a decrease in the percentage of PCNA-positive cholangiocytes (Table 3) and intrahepatic bile duct mass (IBDM) (Figure 2 and Table 3), decreases that were prevented by taurocholic acid feeding (Figure 2 and Table 3). Taurocholic acid prevention of HAL-induced decrease in cholangiocyte proliferation (by PCNA and CK-19 immunohistochemistry) was blocked by wortmannin treatment (Figure 2 and Table 3).
Table 3.
Determination of the percentage of cholangiocytes positive for TUNEL and PCNA and intrahepatic ductal mass.
| Parameters | BDL + BA control feeding for 1 week | BDL + TC feeding for 1 week | BDL + HAL + BA control feeding + NaCl daily injections for 1 week | BDL + HAL + TC feeding + NaCl daily injections for 1 week | BDL + HAL + TC feeding + wortmannin daily injections for 1 week |
|---|---|---|---|---|---|
| % TUNEL-positive cholangiocytes | 3.12 ± 0.45 | 2.21 ± 0.38 | 4.56 ± 0.57a | 3.70 ± 0.50 | 4.12 ± 0.48 a |
| % PCNA-positive cholangiocytes | 25.40 ± 1.08 | 29.16 ± 1.47 | 14.30 ± 0.74b | 21.57 ± 0.84 | 15.36 ± 1.35b |
| Intrahepatic bile duct mass (% surface) | 3.99±0.24 | 4.57±0.32b | 2.41±0.29c | 3.75±0.34 | 2.64±0.21b |
BDL = bile duct ligation; CK-19 = cytokeratin-19; TC = taurocholic acid. Data are mean ± SEM of cumulative values obtained from the evaluation of 10 different fields from three different sections (5 μm, 3 sections analyzed per group) for each group.
HAL-induced increases in cholangiocyte apoptosis in liver sections from BDL rats was prevented by TC feeding. TC prevention of HAL-induced cholangiocyte apoptosis was blocked by wortmannin treatment.
p<0.05 vs. cholangiocyte apoptosis of BDL rats fed bile acid control diet.
HAL-induced decrease in cholangiocyte proliferation in liver sections from BDL rats was prevented by TC feeding. TC prevention of HAL-induced decrease in cholangiocyte proliferation was blocked by wortmannin treatment.
p<0.05 vs. the number of CK-19-positive cholangiocytes of BDL rats fed bile acid control diet.
Figure 2.
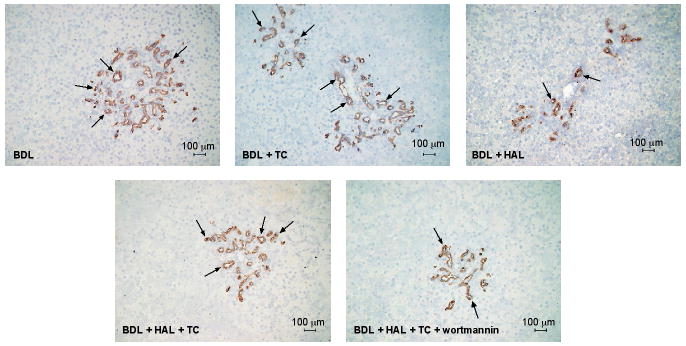
Measurement of intrahepatic bile duct mass (IBDM) in the selected groups of Table 1. TC feeding increased IBDM of BDL rats compared to BDL rats fed bile acid control diet. HAL-induced a decrease in IBDM, a decrease that was prevented by taurocholic acid feeding. Taurocholic acid prevention of HAL-induced decrease in IBDM was blocked by wortmannin treatment. *p<0.05 vs. the corresponding value of BDL rats fed bile acid control diet for 1 week. HAL = hepatic artery ligation IBDM = intrahepatic bile duct mass.
Protein expression for PCNA increased in cholangiocytes from BDL rats fed taurocholic acid compared to purified cholangiocytes from BDL rats (Figure 3). HAL-induced decrease of PCNA protein expression in purified cholangiocytes was prevented by taurocholic feeding (Figure 3). Taurocholic prevention of HAL-induced decrease in PCNA protein expression was blocked by the simultaneous administration of wortmannin (Figure 3).
Figure 3.
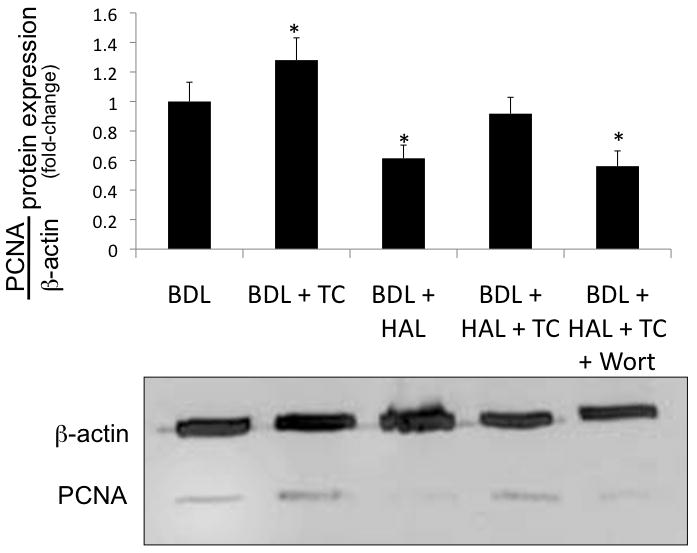
Immunoblots for PCNA in cholangiocytes purified from the experimental groups of Table 1. Taurocholic acid feeding to BDL rats increased PCNA protein expression in purified cholangiocytes compared to cholangiocytes from BDL rats. HAL-induced decrease of PCNA protein expression in purified cholangiocytes was prevented by taurocholic feeding to BDL + HAL rats. Taurocholic prevention of HAL-induced decrease in PCNA protein expression was blocked by the simultaneous administration of wortmannin. Data are mean ± SE of 4 experiments. *p < 0.05 vs. corresponding value of cholangiocytes from BDL rats. BDL = bile duct ligated; HAL = hepatic artery ligation; TC = taurocholic acid; Wort = Wortmannin.
Evaluation of Basal and Secretin-stimulated Bile and Bicarbonate Secretion
We evaluated the changes in basal and secretin-stimulated cAMP levels (in purified cholangiocytes) and bile and bicarbonate secretion (in bile fistula rats), two functional markers of cholangiocyte proliferation [5, 17]. Parallel to previous studies [20], secretin increased intracellular cAMP levels of cholangiocytes from BDL (but not BDL + HAL) rats (Figure 4). Similar to previous findings in normal rats [4, 17], taurocholic acid feeding to BDL rats increased basal and secretin-stimulated cAMP levels compared to purified cholangiocytes from BDL rats (Figure 4). In BDL + HAL rats fed taurocholic acid, secretin-stimulated cAMP levels was blocked by the simultaneous administration of wortmannin (Figure 4).
Figure 4.
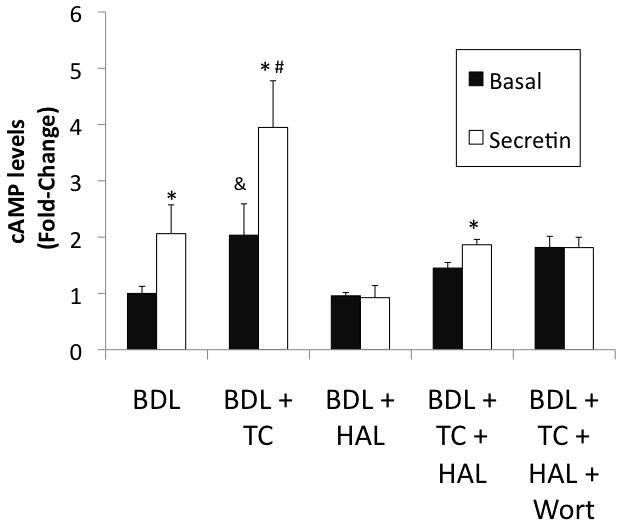
Secretin increased intracellular cAMP levels of cholangiocytes from BDL (but not BDL + HAL) rats. Taurocholic acid feeding to BDL rats increases basal and secretin-stimulated cAMP levels compared to purified cholangiocytes from BDL rats. In BDL + HAL rats fed taurocholic acid, secretin increased cAMP levels of purified cholangiocytes, increase that was blocked by the simultaneous administration of wortmannin. *p < 0.05 vs. its corresponding basal value. &p < 0.05 vs. the basal value of cholangiocytes from BDL. #p< 0.05 vs. secretin-stimulated cAMP levels of cholangiocytes from BDL rats. BDL = bile duct ligated; HAL = hepatic artery ligation; TC = taurocholic acid; Wort = Wortmannin.
Intravenous infusion of secretin-stimulated bile flow, bicarbonate concentration and secretion of BDI (but not BDI + HAL) rats (Table 4). Similar to what was shown in normal rats [20], taurocholic acid feeding increased basal and secretin-stimulated bile and bicarbonate secretion of BDI rats (Table 4). Taurocholic acid feeding prevented HAL-induced inhibition of secretin-stimulated bile and bicarbonate concentration and secretion (Table 4). The simultaneous administration of wortmannin to rats blocked the effect of taurocholic acid on HAL-induced inhibition of secretin-stimulated bile and bicarbonate secretion (Table 4).
Table 4.
Measurement of basal and secretin-stimulated bile flow, bicarbonate concentration and secretion in 1-week BDI rats and rats that [immediately after BDI + hepatic artery ligation (HAL)] were treated by daily IP injections of NaCl or wortmannin in the absence or presence of bile acid control or 1% taurocholic acid (TC) feeding for 1 week.
| Bile Flow | Bicarbonate Concentration | Bicarbonate Secretion | ||||
|---|---|---|---|---|---|---|
| Treatment | Basal (μl / min / Kg BW) | Secretin (μl / min / Kg BW) | Basal (mEq / Liter) | Secretin (mEq / Liter) | Basal (μEq / min / Kg BW) | Secretin (μEq / min / Kg BW) |
| BDI + bile acid control diet (n = 8) | 108.5 ± 4.2 | 162.9 ± 10.4 * | 36.4 ± 2.1 | 53.3 ± 5.5 * | 4.0 ± 0.3 | 8.6 ± 1.0 * |
| BDI + 1% TC (n = 8) | 164.3 ± 16.3 # | 240.3 ± 25.7 * | 44.9 ± 2.2 # | 56.9 ± 2.1 * | 7.4 ± 0.9 # | 13.8 ± 1.6 * |
| BDI + HAL + bile acid control diet (n = 6) | 91.2 ± 9.7 | 104.2 ± 9.1 ns | 40.2 ± 2.1 | 40.6 ± 1.6 ns | 3.6 ± 0.4 | 4.2 ± 0.3 ns |
| BDI + HAL + NaCl + 1% TC feeding 1 week (n = 6) | 100.4± 8.5 | 140.9 ± 13.4 * | 35.8 ± 4.9 | 60.6 ± 9.3 * | 3.4 ± 0.6 | 8.2 ± 0.7 * |
| BDI + HAL + wortmannin+ 1% TC feeding 1 week (n = 7) | 88.5 ± 13.4 | 88.5 ± 15.0 ns | 57.6 ± 2.7 | 62.4 ± 3.0 ns | 5.3 ± 0.8 | 5.8 ± 1.1 ns |
When steady spontaneous bile flow was reached (60-70 minutes from the infusion of Krebs Ringer Henseleit (KRH), rats were infused for 30 minutes with secretin followed by a final infusion of KRH for 30 minutes. After the rats were surgically prepared for bile flow experiments, bile was collected every 10 minutes in pre-weighed tubes and used for determining bicarbonate concentration. Data are mean ± SEM.
p < 0.05 vs. corresponding basal value of bile flow, bicarbonate concentration or bicarbonate secretion of BDI rats.
p < 0.05 vs. corresponding basal value of bile flow, bicarbonate concentration or bicarbonate secretion of BDI rats.
vs. corresponding basal value of bile flow, bicarbonate concentration or bicarbonate secretion of BDI rats. Differences between groups were analyzed by the Student unpaired t test when two groups were analyzed and analysis of variance (ANOVA) when more than two groups were analyzed. TC = taurocholic acid.
Measurement of VEGF-A Levels in Serum and Cholangiocyte Supernatant
Following taurocholic acid feeding to BDL rats, VEGF-A levels increased in serum and cholangiocyte supernatants compared to BDL rats fed bile acid control diet (Figure 5 A-B). In agreement with previous studies [17], VEGF-A levels decreased in serum and cholangiocyte supernatants from BDL + HAL rats; this decrease was prevented by taurocholic acid feeding (Figure 5 A-B). Taurocholic acid prevention of HAL-induced decrease of VEGF-A levels (in serum and supernatant) was partly blocked by the simultaneous administration of wortmannin (Figure 5 A-B). Following stimulation with taurocholic acid for 24 hours, we observed a significant increase in the levels of VEGF-A detected in the supernatant of NRIC treated in vitro with taurocholic acid for 24 hours (Figure 5 C). The taurocholic acid-induced increase in NRIC VEGF-A secretion was blocked by the PI3-K inhibitor, wortmannin (Figure 5 C).
Figure 5.
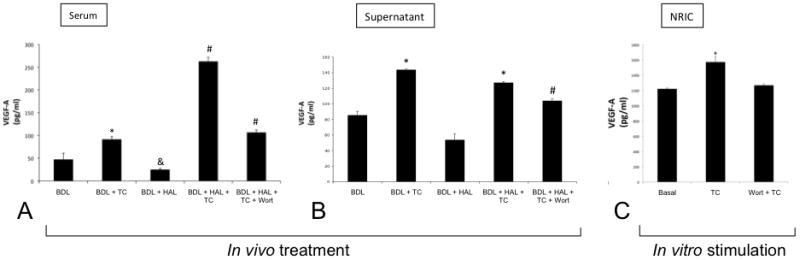
[A-B] Taurocholic acid feeding increased the levels of VEGF-A in the serum and cholangiocyte supernatant of BDL rats. VEGF-A levels were reduced in the serum and cholangiocyte supernatant of BDL rats by HAL, decreases that were prevented by TC feeding. TC prevention of HAL-induced decreases of VEGF levels in serum and cholangiocyte supernatant were blocked by wortmannin. Data are mean ± SE of 4 experiments *p < 0.05 vs. BDL. &p< 0.05 vs. BDL. #p < 0.05 vs. all the other groups. [C] In vitro treatment of NRIC with taurocholic acid (20 μM) for 24 hours induced an increase of VEGF-A levels in the supernatants collected from NRIC compared to basal NRIC: TC-induced increase in NRIC VEGF-A levels was blocked by preincubation with wortmannin. Data are mean ± SE of 4 experiments. *p < 0.05 vs. basal. BDL = bile duct ligated; HAL = hepatic artery ligation; TC = taurocholic acid; Wort = Wortmannin.
Role of VEGF-A and its Receptor, VEGFR-2, in Taurocholic Acid-Induced Cholangiocyte Proliferation
As expected [42], taurocholic acid induced a significant 2.7-fold increase in NRIC proliferation (Figure 6 A). The taurocholic acid-stimulated proliferation of NRIC was blocked by both wortmannin and the VEGFR-2 kinase inhibitor indicating the involvement of the PI3 kinase/Akt pathway and taurocholic acid induced VEGF-A expression in TC stimulation of cholangiocyte proliferation (Figure 6 A).
Figure 6.
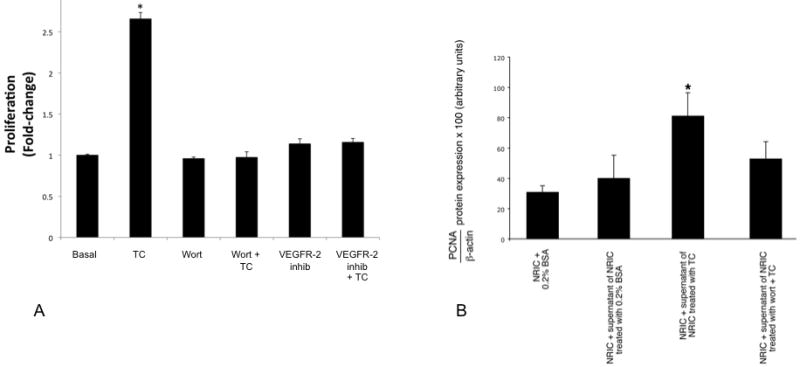
[A] NRIC were stimulated in vitro for 48 hours with taurocholic acid (20 μM) in the absence or presence of 1-hour preincubation with wortmannin (100 nM) or a VEGFR-2 Kinase Inhibitor I (100 nM). TC induced an increase in NRIC proliferation that was blocked by wortmannin and VEGFR-2 kinase inhibitor. Data are mean ± SEM of 3 experiments. *p < 0.05 vs. its corresponding basal value. [B] NRIC were stimulated for 24 hours with the supernatant of NRIC (obtained after 6 hours of incubation with 0.2% BSA or TC, 20 μM) in the absence or presence of 1-hour pre-incubation with wortmannin before measuring cell growth by MTS assays. There was a significant increase in NRIC proliferation when these cells were incubated with the supernatant of NRIC treated with TC (containing more VEGF-A) compared to NRIC treated with the supernatant of NRIC treated with BSA (containing less VEGF-A). TC-induced increase in NRIC proliferation was partly blocked by wortmannin. Data are mean ± SEM of 3 experiments. *p < 0.05 vs. its corresponding basal value.
We found a significant increase in NRIC proliferation when these cells were incubated with the supernatant of NRIC treated in vitro with TC (containing more VEGF-A) compared to NRIC treated with the supernatant of NRIC treated in vitro with BSA (containing less VEGF-A) (Figure 6 B). The increase in NRIC proliferation (induced by the VEGF-A contained in the supernatant) was blocked by wortmannin (Figure 6 B).
Discussion
Our study demonstrates that in BDL rats with HAL, chronic feeding of taurocholic acid prevents: (i) HAL-induced apoptosis of intrahepatic bile ducts; (ii) HAL-induced a decrease in VEGF-A and VEGFR-2 expression in liver sections, VEGF secretion in cholangiocyte supernatant and HAL-induced circulating VEGF-A levels; and (iii) HAL-induced decrease in secretin-stimulated cAMP levels in cholangiocytes, and secretin-stimulated bile flow and bicarbonate secretion in bile fistula rats. All the in vivo preventive effects of TC on HAL-induced changes in cholangiocyte functions were blocked by the simultaneous administration of the PI3-K inhibition, wortmannin [40]. In vitro, we demonstrated that taurocholic acid increases VEGF-A secretion by cholangiocytes (NRIC), an increase that was blocked by wortmannin. Moreover, we demonstrated that: (i) taurocholic acid-induced cholangiocyte proliferation is blocked by both wortmannin and a VEGFR-2 kinase inhibitor; and (ii) there was a significant increase in NRIC proliferation when these cells were incubated with the supernatant of NRIC treated in vitro with TC (containing more VEGF-A) compared to NRIC treated with the supernatant of NRIC treated with BSA (containing less VEGF-A); the increase in NRIC proliferation (induced by the VEGF-A contained in the supernatant) was blocked by wortmannin.
In BDL rats, the vascular system undergoes expansion through adaptive proliferation in order to support the nourishment required by a larger intrahepatic biliary tree [22, 26]. In our previous work, we demonstrated that cholangiocytes express the message and secrete VEGF-A and that VEGF-A plays a key role in the autocrine regulation of cholangiocyte proliferation, and the development of the PBP in order to sustain the enhanced functional and nutritional demands of the proliferating biliary tree [26]. Also, administration of r-VEGF-A to BDL+HAL rats sustains cholangiocyte VEGF-A expression/secretion and prevents HAL-induced disappearance of the PBP, bile duct damage and the loss of secretin-stimulated choleresis [17]. In contrast to our chronic studies over 7 days, in a model of arterial liver ischemia subsequent to complete arterial deprivation of the rat liver for 24 hours, the mRNA levels of VEGF increased in cholangiocytes [43]. The increase in VEGF levels at early time points (24 hours) is presumably due to a compensatory mechanism, whereas cholangiocyte VEGF levels decrease after chronic HAL due to the interruption of the blood flow of the hepatic artery, the main blood supply of the biliary epithelium [17, 30]. A recent study has also shown [44] that the expression of VEGF, VEGFRs, angiopoietin-1 (Ang-1) and Tie-2 is upregulated in cholangiocytes from polycystic liver diseases, and that VEGF and Ang-1 have autocrine proliferative effects on cholangiocyte growth and a paracrine effect on portal vasculature, promoting the growth of the cysts and their vascular supply.
We have previously shown that taurocholic acid both in vitro and in vivo increases the proliferation and secretin-stimulated cAMP levels and ductal secretion [19, 20]. We have also demonstrated that chronic feeding of TC ameliorates cholangiocyte damage in several animal models [32, 40]. TC feeding prevented the loss of cholangiocytes in rats with BDL following cholinergic and adrenergic denervation, which was dependent upon the activation of PI3-K/AKT dependent mechanisms [32, 40]. We demonstrated that r-VEGF-A prevents HAL-induced loss of biliary mass and secretory function [17]. The effect of TC was blocked by wortmannin, which is consistent with findings from our other studies [32, 40] and suggest that the effects of taurocholic acid involve a PI3-K/AKT-dependent signaling mechanism. Since we have previously demonstrated that VEGF-A reverses the effects of HAL-induced loss of biliary mass and function, we sought to evaluate whether TC feeding had an effect on the expression of VEGF-A and VEGFR-2 in bile ducts. In liver sections, we found consistent with our previous study [26] that HAL induces a decrease in both VEGF-A and VEGFR-2 expression. The observed HAL-induced reductions in biliary VEGF-A and VEGFR-2 expression were prevented by TC feeding, which is a protective effect that can be blocked by wortmannin. This protective effect of taurocholic acid was blocked by wortmannin. Taken together, our findings demonstrate that bile acids regulate cholangiocyte growth/loss by directly modulating cholangiocyte VEGF expression/secretion by a PI3-K-dependent mechanism. Ischemic lesions of intra- or extra-hepatic bile ducts have been described after surgical or chemical (i.e. intra-arterial chemotherapies) lesions of the hepatic artery and may also occur in the transplanted liver with negative prognostic impact on liver function [45-48]. In addition, in the course of primary sclerosing cholangitis (PSC), lesions of the hepatic artery and its branches have been considered to play a causal role in bile duct damage and in the associated ductal cholestasis [47]. In all these pathological conditions, no treatment is currently available to delay or halt the evolution of ischemic bile duct damage. We have recently demonstrated how reduction of the blood supply through the hepatic artery impairs the proliferative and the repairing capacities of damaged ducts and how exogenous VEGF may play beneficial effects (5). Findings from the present study demonstrated how bile salts, via VEGF, exert beneficial effects on ischemic bile duct damage thus providing the experimental background for testing bile salts as pharmacological treatment of the pathologies of the intra-or extra-hepatic bile ducts associated with hepatic artery lesions. Since PSC has been shown to be secondary to hepatic artery ligation after abdominal trauma [49], and since ursodeoxycholic acid can be important in preventing experimental-induced bile duct damage [32] and as therapy in the management of PSC, we also propose that UDCA should be tested in clinical trials concerning ischemic damage of bile ducts.
Acknowledgments
We thank the Texas A&M Health Science Center Microscopy Imaging Center for assistance with confocal microscopy. We would like to thank Prof. Alvaro (University of the Studies of Rome “La Sapienza” for the constructive suggestions during the resubmission of the manuscript.
Sources of Support: This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology to Dr. Alpini from Scott & White Hospital, by a grant award from Scott & White and a NIH Grant DK081442 to Dr. Glaser, by a VA Research Scholar Award, the VA Merit Award and the NIH grant DK58411, DK062975 and DK76898 to Dr. Alpini, PRIN funds 2007 from Italian MiUR (prot. 2007HPT7BA_001), and Federate Atheneum funds from “Sapienza” Rome University to Dr. Gaudio, Faculty funds from University of L'Aquila to Dr. Onori and by a grant from Health and Labor Sciences Research Grants for the Research on Measures for Intractable Diseases (from the Ministry of Health, Labor and Welfare of Japan) and from Grant-in Aid for Scientific Research C (21590822) from JSPS to Dr. Ueno.
Abbreviations used
- ASBT
Na+-dependent bile acid transporter
- BDL
bile duct ligation
- BDI
bile duct incannulation
- BSA
bovine serum albumin
- cAMP
adenosine 3′, 5′-monophosphate
- CK-19
cytokeratin-19
- CCl4
carbon tetrachloride
- HAL
hepatic artery ligation
- KRH
Krebs Ringer Henseleit
- NRIC
normal rat intrahepatic cholangiocyte cell line
- PI3-K
phosphatidylinositol-3-kinase
- PBP
peribiliary plexus
- PCNA
proliferating cellular nuclear antigen
- TC
taurocholic acid
- TUNEL
terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–69. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 3.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 4.Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaser S, Rodgers RE, Phinizy JL, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G1061–G1070. doi: 10.1152/ajpgi.1997.273.5.G1061. [DOI] [PubMed] [Google Scholar]
- 6.Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 7.Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a Cyclic AMP-mediated mechanism. J Biol Chem. 1992;267:15523–15529. [PubMed] [Google Scholar]
- 8.Cho WK, Mennone A, Rydberg SA, et al. Bombesin stimulates bicarbonate secretion from rat cholangiocytes: Implications for neural regulation of bile secretion. Gastroenterology. 1997;113:311–321. doi: 10.1016/s0016-5085(97)70109-4. [DOI] [PubMed] [Google Scholar]
- 9.Alpini G, Ulrich CD, 2nd, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 10.Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 11.Alpini G, Glaser S, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 12.LeSage G, Glaser S, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 13.Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/Elk-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-b/gastrin receptors via D-myoinositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 15.LeSage G, Glaser S, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 16.Alpini G, Glaser S, Alvaro D, et al. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226–1237. doi: 10.1053/gast.2002.36055. [DOI] [PubMed] [Google Scholar]
- 17.Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–317. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 18.Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: A neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Alpini G, Glaser S, Robertson W, et al. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G518–529. doi: 10.1152/ajpgi.1997.273.2.G518. [DOI] [PubMed] [Google Scholar]
- 20.Alpini G, Glaser SS, Ueno Y, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: Evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 21.Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to h1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Glaser S, Alvaro D, Francis H, et al. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of AKT. Am J Physiol Gastrointest Liver Physiol. 2006;290:G813–826. doi: 10.1152/ajpgi.00306.2005. [DOI] [PubMed] [Google Scholar]
- 24.Glaser SS, Ueno Y, DeMorrow S, et al. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest. 2007;87:914–926. doi: 10.1038/labinvest.3700602. [DOI] [PubMed] [Google Scholar]
- 25.LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte proliferation. Liver. 2001;21:73–80. doi: 10.1034/j.1600-0676.2001.021002073.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: A morphological study. Gastroenterology. 1996;111:1118–1124. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 27.Bomzon A, Holt S, Moore K. Bile acids, oxidative stress, and renal function in biliary obstruction. Semin Nephrol. 1997;17:549–562. [PubMed] [Google Scholar]
- 28.Barone M, Maiorano E, Ladisa R, et al. Ursodeoxycholate further increases bile-duct cell proliferative response induced by partial bile-duct ligation in rats. Virchows Arch. 2004;444:554–560. doi: 10.1007/s00428-004-0998-0. [DOI] [PubMed] [Google Scholar]
- 29.Mancinelli R, Onori P, Gaudio E, et al. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med (Maywood) 2009;234:462–474. doi: 10.3181/0808-RM-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavoloni N, Schaffner F. The intrahepatic biliary epithelium in the guinea pig: Is hepatic artery blood flow essential in maintaining its function and structure? Hepatology. 1985;5:666–672. doi: 10.1002/hep.1840050424. [DOI] [PubMed] [Google Scholar]
- 31.Taffetani S, Glaser S, Francis H, et al. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol. 2007;7:6. doi: 10.1186/1472-6793-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzioni M, Francis H, Benedetti A, et al. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398–409. doi: 10.2353/ajpath.2006.050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeSage G, Alvaro D, Benedetti A, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa M, Fjii M, Iuchi M, et al. Effect of intrahepatic omental implantation on angiogenesis in rat liver with hepatic artery ligation. Clin Exp Med. 2001;1:27–33. doi: 10.1007/s10238-001-8006-3. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M, Vroman B, LaRusso NF. Isolation and morphological characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 36.Alpini G, Phinizy JL, Glaser S, et al. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1066–1073. doi: 10.1152/ajpgi.00260.2002. [DOI] [PubMed] [Google Scholar]
- 37.Strazzabosco M, Fiorotto R, Melero S, et al. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA, editors. The Liver; Biology & Pathobiology. 4th. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 421–435. [Google Scholar]
- 39.Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Marucci L, Alpini G, Glaser S, et al. Taurocholate feeding prevents CCl4-induced damage of large cholangiocytes through pi3-kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;284:G290–301. doi: 10.1152/ajpgi.00245.2002. [DOI] [PubMed] [Google Scholar]
- 41.Marzioni M, LeSage G, Glaser S, et al. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2003;284:G837–G852. doi: 10.1152/ajpgi.00398.2002. [DOI] [PubMed] [Google Scholar]
- 42.Alpini G, Glaser SS, Rodgers R, et al. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734–1740. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- 43.Fouassier L, Beaussier M, Schiffer E, et al. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G25–35. doi: 10.1152/ajpgi.00175.2006. [DOI] [PubMed] [Google Scholar]
- 44.Fabris L, Cadamuro M, Fiorotto R, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 45.Siegel EG, Schmidt WE, Folsch UR. Severe ischemic-type biliary strictures due to hepatic artery occlusion seven years after liver transplantation: A rare cause of late cholestatic graft failure. Z Gastroenterol. 1998;36:509–513. [PubMed] [Google Scholar]
- 46.Ben-Ari Z, Pappo O, Mor E. Intrahepatic cholestasis after liver transplantation. Liver Transplantation. 2003:1005–1018. doi: 10.1053/jlts.2003.50212. [DOI] [PubMed] [Google Scholar]
- 47.Batts KP. Ischemic cholangitis. Mayo Clin Proc. 1998;73:380–385. doi: 10.1016/S0025-6196(11)63706-3. [DOI] [PubMed] [Google Scholar]
- 48.Beaussier M, Wendum D, Fouassier L, et al. J Hepatol. 2005;42:257–265. doi: 10.1016/j.jhep.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Martín de Carpi J, Tarrado X, Varea V. Sclerosing cholangitis secondary to hepatic artery ligation after abdominal trauma. Eur J Gastroenterol & Hepatol. 2005;17:987–990. doi: 10.1097/00042737-200509000-00016. [DOI] [PubMed] [Google Scholar]


