Abstract
Glutamate plays a pivotal role in regulating drug self-administration and drug-seeking behavior, and the past decade has witnessed a substantial surge of interest in the role of Group I metabotropic glutamate receptors (mGlu1 and mGlu5 receptors) in mediating these behaviors. As will be reviewed here, Group I mGlu receptors are involved in normal and drug-induced synaptic plasticity, drug reward, reinforcement and relapse-like behaviors, and addiction-related cognitive processes such as maladaptive learning and memory, behavioral inflexibility, and extinction learning. Animal models of addiction have revealed that antagonists of Group I mGlu receptors, particularly the mGlu5 receptor, reduce self-administration of virtually all drugs of abuse. Since inhibitors of mGlu5 receptor function have now entered clinical trials for other medical conditions and appear to be well-tolerated, a key question that remains unanswered is - what changes in cognition are produced by these compounds that result in reduced drug intake and drug-seeking behavior? Finally, in contrast to mGlu5 receptor antagonists, recent studies have indicated that positive allosteric modulation of mGlu5 receptors actually enhances synaptic plasticity and improves various aspects of cognition, including spatial learning, behavioral flexibility, and extinction of drug-seeking behavior. Thus, while inhibition of Group I mGlu receptor function may reduce drug reward, reinforcement, and relapse-related behaviors, positive allosteric modulation of the mGlu5 receptor subtype may actually enhance cognition and potentially reverse some of the cognitive deficits associated with chronic drug use.
Keywords: drug addiction, rodent models, glutamate, metabotropic glutamate receptor, allosteric modulators, learning, memory, cognition, extinction
1. Introduction
Drug addiction is a multifaceted disorder that places an enormous socioeconomic, legal, and medical burden on society, in addition to the destructive influences it has on the addict and his or her family and peers. Current evidence suggests that drug addiction is a result of complex interactions between environmental, developmental, and genetic factors (Koob and Le Moal, 2007; Le Moal, 2009; Spanagel, 2009). At the behavioral level, drug addiction is typically characterized by a transition from casual, intermittent drug use to compulsive, uncontrolled drug intake coupled with repeated failed attempts at cessation of or curtailing drug use. At the cellular and molecular levels, repeated intake of drugs of abuse produce lasting neuroadaptations in gene expression, cytoarchitecture, and synaptic plasticity in various circuitries of the brain, including the limbic, prefrontal executive control, and reward systems (Christie, 2008; Crews and Boettiger, 2009; Feltenstein and See, 2008; Kalivas, 2008; Koob and Volkow, 2010; Robbins and Arnsten, 2009; Shaham and Hope, 2005; Thomas et al., 2008).
Although there is a substantial body of evidence supporting a role for the excitatory amino acid neurotransmitter glutamate and its ligand-gated ionotropic receptors (i.e., N-methyl-D-aspartate (NMDA), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), and kainic acid (KA) subtypes) in mediating addictive behaviors that dates back more than two decades (Gass and Olive, 2008; Kalivas, 2004; Tzschentke and Schmidt, 2003; Wolf, 1998), it is only within the last decade or so that it has become apparent that metabotropic glutamate (mGlu) receptors are also involved in the neural mechanisms underlying drug addiction. Studies using pharmacological and genetic approaches have revealed clear evidence for a role of Group I (mGlu1 and mGlu5) receptors in regulating drug intake, reward, reinforcement, and reinstatement of drug-seeking behavior (Olive, 2009a). However, Group I mGlu receptors also mediate cognitive processes such as learning and memory, behavioral flexibility, and extinction (Darrah et al., 2008; Gass and Olive, 2009a; Moghaddam, 2004; Shipe et al., 2005; Simonyi et al., 2005), and deficits in these forms of cognition are frequently observed in drug addicts.
The purpose of the present review will be to summarize evidence that Group I mGlu receptors are involved in normal and drug-induced synaptic plasticity new evidence that Group I mGlu receptors are involved in various cognitive aspects of addiction, particularly learning and memory processes and associated synaptic plasticity. In addition, the effects of Group I mGlu receptor antagonists on drug self-administration, reward, and reinstatement are be reviewed along with possible neuroanatomical sites of action, underlying neural mechanisms, and effects of mGlu5 receptor inhibition on cognition in recent clinical trials. Finally, recent findings on the ability of newly developed mGlu5 receptor positive allosteric modulators (PAMs) to enhance cognition, synaptic plasticity, and extinction learning will also be reviewed, which provides a highly novel therapeutic avenue for potentially reversing some of the cognitive deficits that result from chronic heavy drug use.
1.1. Role of learning and memory processes in drug addiction
Drug addiction, referred to as “substance dependence” in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 2002) is characterized by compulsive drug use despite negative consequences, numerous failed attempts at abstinence, and a narrowing of the behavioral repertoire towards drug-seeking and consumption and away from normal social, occupational, or academic functioning. Evidence for deficits in numerous cognitive functions in drug addicts is manifested by behavioral perseverance and continued drug intake despite negative consequences, lack of impulse control, poor decision-making, working memory impairment, and attentional deficits (Goldstein and Volkow, 2002; London et al., 2005; Volkow and Fowler, 2000; Volkow et al., 1992).
It has become increasingly apparent that the brain circuits, neurotransmitter systems, and cellular and molecular substrates that mediate drug addiction have considerable overlap with those that underlie normal learning and memory processes (Berke and Hyman, 2000; Dalley and Everitt, 2009; Hyman et al., 2006; Kelley, 2004; Koob, 2009; Nestler, 2002; Robbins et al., 2008; White, 1996). For example, acute and/or subchronic passive (i.e., experimenter-administered) delivery of various drugs of abuse such as cocaine, amphetamines, nicotine, opiates, or alcohol and can induce or modulate long-term potentiation (LTP) or long-term depression (LTD) of synaptic strength, two widely established cellular hallmarks of neural plasticity, in brain structures such as the hippocampus, mesoaccumbens dopamine pathway, and various regions of the limbic system (see Section 1.2. below). These same regions also mediate contextual, episodic, and emotional memory as well as spatial, habit, and incentive learning. The enduring neuroadaptations produced by chronic drug exposure can lead to the perseveration of drug-seeking behavior (Davis and Gould, 2008; Kalivas and O’Brien, 2008; Kauer, 2004; Kauer and Malenka, 2007), hypersalience of drug-associated stimuli, and a learned “imprint” of drug use in the brain (Boning, 2009).
One of the predominant forms of maladaptive learning that occurs as a result of chronic drug use is classical (Pavlovian) conditioning of the associations between the drug’s effects, drug-specific withdrawal symptoms, and the environmental cues and contexts that are present at the time these drug-related effects are experienced (Robbins and Everitt, 2002). To give an example, suppose it takes 10 puffs to smoke a cigarette, and an active smoker smokes one pack of 20 cigarettes per day. In the course of one year, this would allow for approximately 7,300 pairings between the psychoactive ingredients of cigarette smoke (i.e., nicotine) and the cues and contexts that are present when cigarettes are smoked. As a result of these numerous drug-cue or context pairings, these external stimuli become overconditioned (a form of associative overlearning), and when experienced in the absence of the drug they can elicit expectation of drug availability and/or activate memories of previous euphoric experiences under the influence of a particular drug, which (Grant et al., 1996; Volkow et al., 2002a). Activation of these drug-related memories in turn can result in drug craving, drug-seeking behavior, and ultimately relapse (the so-called “addiction cycle”; see Carter and Tiffany, 1999; Dackis and O’Brien, 2005; Everitt et al., 2008; O’Brien et al., 1998; Weiss, 2005). Two key brain regions that mediate cue- and context-drug associations are the amygdala and hippocampus, respectively (Gould, 2006; Olive, 2009b), the activity of which is governed by the prefrontal cortex (PFC). Another form of maladaptive learning that occurs as a result of chronic drug use is drug-taking behaviors that become compulsive and automatic (i.e., instrumental overlearning), which is likely a result of perturbations in corticostriatal habit circuitry (Everitt et al., 2008; Kalivas, 2008; Kalivas et al., 2005; Koob and Volkow, 2010; Sesack and Grace, 2010).
As can be seen, a common neuroanatomical element involved in both of these maladaptive learning phenomena is the PFC, a multicomponent brain region that exerts executive control over numerous brain habit and motivational circuitries and is also involved in impulse control, working memory, attention, cue salience and extinction learning (Crews and Boettiger, 2009; Robbins and Arnsten, 2009). As will be discussed in Section 3.1., hypofunctioning of the PFC cortex is widely believed to mediate many of the cognitive deficits, including impaired decision making and impulse control, that are observed in chronic drug users.
1.2. Role of Group I mGlu receptors in addiction-related synaptic plasticity and learning and memory
Drugs of abuse including cocaine, amphetamines, nicotine, opiates, can induce and/or modulate forms of synaptic plasticity, such as LTP and LTD, in brain regions known to be involved in addiction, learning, and memory, including the hippocampus, ventral tegmental area (VTA), nucleus accumbens, and amygdala (Bao et al., 2007; Bellone and Luscher, 2006; Borgland et al., 2004; Delanoy et al., 1983; Goussakov et al., 2006; Grueter et al., 2008; Jones et al., 2000; Liu et al., 2005; Nugent et al., 2007; Saal et al., 2003; Thomas et al., 2001; Thompson et al., 2005; Ungless et al., 2001; Wanat et al., 2009; Yin et al., 2007). While the aforementioned studies examined changes in synaptic transmission after passive exposure to drugs of abuse, both LTP and/or LTD of glutamatergic synapses in the nucleus accumbens from the ventral prefrontal afferents can be reduced or abolished or following active self-administration of the drug (Martin et al., 2006; Moussawi et al., 2009; Schramm-Sapyta et al., 2006), but remain unaffected in other regions such as the hippocampus (Del Olmo et al., 2006; Thompson et al., 2004). This “metaplasticity” of glutamatergic cortico-accumbens transmission may play a role in the development of maladaptive motivated behaviors in addiction such as the perseverance of drug-seeking, while the persistence of synaptic plasticity in the hippocampus may underlie the long-lasting nature of drug-associated memories.
mGlu1 and mGlu5 receptors have an almost complementary pattern of anatomical distribution in the brain (Shigemoto and Mizuno, 2000). High levels of mGlu5 receptors found in forebrain regions such as the cerebral cortex, dorsal and ventral striatum, olfactory bulb and tubercle, lateral septum, and hippocampus (Romano et al., 1995; Shigemoto et al., 1993). In contrast, expression levels of mGlu1 receptors in these regions are weak, except for the hippocampus, where it is highly expressed primarily in the CA3 region and dentate gyrus. Regions containing high levels of expression of mGlu1 receptors include the cerebellum, thalamus, hypothalamus, and pallidal regions (Shigemoto et al., 1992), where mGlu5 expression levels are often low or absent. Thus, the hippocampus is one of the few brain structures where high levels of both mGlu1 and mGlu5 receptors are found, and as such many investigations into the ability of these receptors to regulate synaptic plasticity have focused on this region.
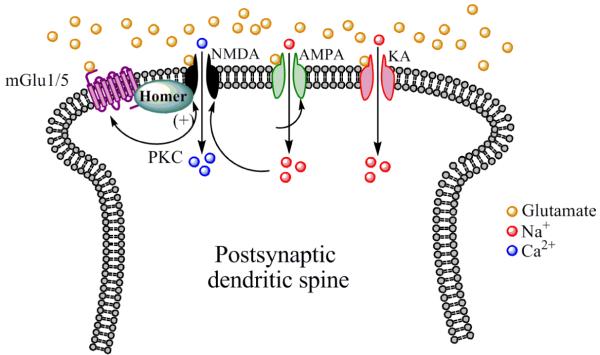
It is well established that Group I mGlu receptors play a pivotal role in the induction and maintenance of synaptic plasticity (Anwyl, 2009; Bellone et al., 2008; Gladding et al., 2009). Electron microscopy studies have shown that Group I mGlu receptors are predominantly localized postsynaptically on perisynaptic annulus of dendritic spines (Romano et al., 1995; Shigemoto et al., 1997; Shigemoto et al., 1993), where they are structurally linked to NMDA receptors by various scaffolding proteins such as the Homer family of proteins (see Fig. 1) as well as other proteins including Shank and guanylate kinase-associated protein (GKAP) (Niswender and Conn, 2010). A small percentage of Group I mGlu receptors have been found on presynaptic terminals (Shigemoto et al., 1997), where they are thought to modulate neurotransmitter release (Anwyl, 1999).
Fig. 1.
Diagram of a dendritic spine and postsynaptic localization of ionotropic and Group I mGlu receptors. Synaptically released glutamate binds to ionotropic glutamate receptors (NMDA, AMPA, and KA receptors), which allows the influx of Na+ and/or Ca2+ ions to depolarize the postsynaptic membrane. Group I mGlu receptors are localized to the perisynaptic annulus of the dendritic spine where they are stimulated by high levels of synaptic glutamate release or glutamate released from glia. Group I mGlu are structurally coupled to NMDA receptor via Homer proteins (as well as other scaffolding proteins not shown), and are biochemically coupled to NMDA receptor function via PKC. Activation of Group I mGlu receptors increases the activity of NMDA receptors (denoted by a +), and NMDA receptor activation can positively influence Group I mGlu receptor function as well.
Due to the predominant postsynaptic localization of Group I mGlu receptors, these receptors primarily regulate induction or maintenance of LTP or LTD by postsynaptic mechanisms such as potentiation of NMDA receptor function (discussed in further detail below), de novo synthesis of GluR2 subunits the AMPA receptor and other proteins, release of calcium ions from intracellular stores which activate a host of calcium-dependent effector proteins such as mitogen-activated and extracellular signal-regulated protein kinases (MAP and ERK kinases, respectively) which in turn modulate gene expression, as well as changes in phospholipid signaling (Anwyl, 2009; Bellone et al., 2008; Gladding et al., 2009). Group I mGlu receptors also regulate the synthesis of endocannabinoids, which act as retrograde signaling molecules to stimulate presynaptically localized cannabinoid CB1 receptors to induce plasticity in axon terminals. One of the most widely studied mechanisms by which Group I mGlu receptors regulate LTP via postsynaptic mechanisms is through their aforementioned positive coupling to NMDA receptor function, whereby activation of these receptors increases NMDA receptor function (Alagarsamy et al., 1999; Attucci et al., 2001; Awad et al., 2000; Benquet et al., 2002; Doherty et al., 1997; Kotecha and MacDonald, 2003; Pisani et al., 2001; Ugolini et al., 1999). This positive coupling is primarily mediated by Group I mGlu receptor-induced activation of protein kinase C (PKC), which can phosphorylate (among other substrates) specific subunits of the NMDA receptor, resulting in enhanced NMDA receptor function (Lu et al., 1999).
In accord with this well-established positive coupling between Group I mGlu and NMDA receptors, there is a wealth of evidence suggesting that Group I mGlu receptors play an important role in NMDA-dependent synaptic plasticity and learning and memory processes (Riedel et al., 2003; Simonyi et al., 2005). Genetic deletion or pharmacological blockade of Group I mGlu receptors inhibits the induction and/or expression of LTP in various brain regions including the CA1 and dentate gyrus regions of the hippocampus (Balschun et al., 1999; Balschun and Wetzel, 2002; Bikbaev et al., 2008; Jia et al., 1998; Lu et al., 1997; Manahan-Vaughan and Braunewell, 2005; Naie and Manahan-Vaughan, 2004; 2005; Neyman and Manahan-Vaughan, 2008), amygdala (Fendt and Schmid, 2002; Rodrigues et al., 2002), and dorsal and ventral striatum (Gubellini et al., 2003; Schotanus and Chergui, 2008), which are addiction-related brain regions that are involved in contextual/spatial learning, reward cue/emotional learning, and habit/incentive learning, respectively. Accordingly, microinjection studies have shown that local infusions of Group I mGlu receptor antagonists into the hippocampus produce decrements in contextual learning (Maciejak et al., 2003), disruption of emotional learning when infused into the amygdala (Bonini et al., 2003; Rodrigues et al., 2002), disruption of habit learning when infused into the dorsal striatum (Packard et al., 2001), and inhibition of reinforcer seeking when infused into the ventral striatum (Backstrom and Hyytia, 2007; Cozzoli et al., 2009; Gass and Olive, 2009b; Kumaresan et al., 2009). In contrast, local stimulation of Group I mGlu receptors in the amygdala has been shown to augment emotional learning (Rudy and Matus-Amat, 2009).
Emerging evidence suggests that Group I mGlu receptors also play a role in the neuronal plasticity and neuroadaptations produced by drugs of abuse (Bird and Lawrence, 2009a; Grueter et al., 2007). For example, acute cocaine-induced synaptic plasticity in the VTA (i.e., increased AMPA-mediated postsynaptic currents and associated insertion of high conductance GluR2-lacking AMPA receptors into the postsynaptic membrane) can be rapidly reversed by activation of mGlu1 receptors (Bellone and Luscher, 2006), resulting in LTD characterized by endocytosis of GluR2-lacking AMPA receptors and their replacement by low-conductance GluR2-containing AMPA receptors replacement (Bellone et al., 2008; Grueter et al., 2007; Mameli et al., 2007). While acute cocaine-induced synaptic plasticity in the VTA is transient in nature (Ungless et al., 2001), functional disconnection of mGlu1 receptors from Homer scaffolding proteins in the VTA allows for cocaine-induced synaptic plasticity in this region to become more persistent, and also leads to increased synaptic plasticity (LTD) in the nucleus accumbens, which may ultimately lead to the persistence of drug-seeking behavior (Mameli et al., 2009). In the bed nucleus of the stria terminalis (BNST), a region of the extended amygdala that mediates the negative emotional states produced by chronic drug use, LTD produced by repeated cocaine exposure is regulated by mGlu5 receptors (Grueter et al., 2006; Grueter et al., 2008). Nicotine-induced enhancement of LTP in the hippocampus is also regulated by mGlu5 receptors (Welsby et al., 2006). Thus, Group I mGlu receptors appear to regulate drug-induced synaptic plasticity in various addiction-related regions of the brain and may therefore serve as “gatekeepers” of the neuroadaptions produced by drugs of abuse, and ultimately the persistence of drug-seeking behavior that is characteristic of addiction (Bird and Lawrence, 2009a).
While the primary focus of this review is on Group I mGlu receptors, it should be noted that many neurotransmitter systems other than glutamate also modulate synaptic plasticity and learning and memory processes relevant to addiction, including acetylcholine, monoamines, endocannabinoids, GABA, and various neuropeptide systems (Koob and Volkow, 2010). Thus, the aforementioned contributions of Group I mGlu receptors to addiction related learning and synaptic plasticity are likely a single component of numerous neurotransmitter systems acting in concert.
2. Reductions in drug intake, reward, and relapse produced by Group I mGlu receptor antagonists
Although the Group I mGlu receptors were first isolated in the early 1990’s (reviewed in Ferraguti and Shigemoto, 2006), their role in drug addiction did not become evident until almost a decade later in a highly influential study published by Chiamulera and colleagues (Chiamulera et al., 2001). These investigators demonstrated that mice carrying a targeted deletion of the mGlu5 receptor gene did not demonstrate hyperlocomotion in response to acute administration of various doses of cocaine (10, 20 or 40 mg/kg i.p.), and failed to acquire intravenous self-administration of cocaine. This latter phenomenon was not due to deficits in the ability to learn an operant task, as acquisition of lever pressing for food was unaltered in these mice. These investigators further confirmed a role for mGlu5 receptors in regulating cocaine self-administration by demonstrating that intravenous administration of the mGlu5 receptor antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) dose-dependently reduced cocaine self-administration without producing non-specific motor effects (as indicated by a lack of alteration in the rate of lever pressing). This pivotal study provided the first evidence that either genetic or pharmacological inhibition of mGlu5 receptor function reduces the reinforcing effects of cocaine, and has provoked a wealth of speculation that suggested that mGlu5 receptor antagonists may prove useful in treating drug addiction in humans (Bird and Lawrence, 2009b; Carroll, 2008; Cryan et al., 2003; Heilig and Egli, 2006; Jaeschke et al., 2008; Jupp and Lawrence, 2009; Kenny and Markou, 2004; Olive, 2005; 2009a; Spooren et al., 2001). Subsequent studies examining the effects of inhibition of Group I mGlu receptor function on the rewarding and reinforcing properties of cocaine and other drugs of abuse, and relapse-like behaviors, will now be reviewed.
2.1. Results from studies using the conditioned place preference paradigm
The conditioned place preference (CPP) paradigm is frequently used to assess the conditioned rewarding effects of a drug of abuse. In a typical CPP paradigm, an animal is passively administered a drug of abuse (such as cocaine) and then placed in a conditioning compartment with unique tactile, visual, and/or olfactory environmental cues. Drug conditioning sessions are alternated with saline conditioning session, where saline is administered and the animal is placed in an adjacent compartment with tactile, visual, and/or olfactory cues that are distinct from the drug conditioning compartment. During repetition of these conditioning procedures, contextual associative learning occurs between euphorigenic and physiological effects of the drug with the physical environment in which it is received. When given the opportunity to explore both compartments (i.e., the test phase), the animal will demonstrate an increased amount of time spent in the drug-paired compartment as compared to time spent in the saline-paired compartment or compared to the amount of time spent in the drug-paired compartment prior to drug conditioning (i.e., a conditioned place preference). Experimental manipulations (i.e., Group I mGlu receptor antagonist treatment) that are performed prior to drug conditions sessions are intended to determine effects on the acquisition of CPP, whereas such manipulations immediately prior to the test session are intended to determine effects on the expression of CPP. The CPP paradigm is also amenable to the study of extinction, which is accomplished either by repeatedly pairing the previously drug-paired compartment with saline, or by allowing the CPP to dissipate over a period of several weeks with repeated testing of place preference in the absence of further conditioning (see Section 3.3. for results of the effects of mGlu5 receptor positive allosteric modulation on extinction of a cocaine CPP).
Following the landmark study by Chiamulera and colleagues, work from the author’s laboratory demonstrated that pharmacological blockade of mGlu5 receptors with MPEP (up to 20 mg/kg) dose-dependently attenuates the acquisition of a cocaine CPP in mice but did not alter the acquisition of a CPP for morphine, ethanol, nicotine, or amphetamine (Mcgeehan and Olive, 2003). Consistent with these findings, mGlu5 receptor-deficient mice are capable of acquiring a CPP for ethanol (Bird et al., 2008). It is somewhat intriguing that the effects of MPEP on the acquisition of the CPP were selective for cocaine. However, other CPP studies have shown inhibitory effects of MPEP on CPP for other drugs of abuse. For example, in rats, MPEP at doses of 9 and 12 mg/kg has been shown to reduce (but not completely eliminate) the acquisition of a nicotine CPP in rats (Yararbas et al., in press), and higher doses of MPEP (30 mg/kg i.p.) have been shown to reduce the acquisition of a morphine CPP (Popik and Wrobel, 2002). Other studies have shown that MPEP actually potentiates the acquisition of a heroin- and ketamine-induced CPP in rats (van der Kam et al., 2009a). Some of these discrepancies may be attributable to procedural differences such as the use of rats vs. mice, the timing of MPEP administration prior to drug conditioning, the number and duration drug conditioning sessions, etc.
With regards to the expression of drug-induced CPP, MPEP has been shown to attenuate the expression of a CPP for morphine (Herzig and Schmidt, 2004), amphetamine (Herzig et al., 2005), and ethanol (Lominac et al., 2006) but not for cocaine, methylenedioxymethamphetamine (MDMA), or food (Herzig et al., 2005; Herzig and Schmidt, 2004). While the lack of consistency of effects of MTEP across different drugs of abuse may be attributable to procedural differences mentioned above, another possible explanation is a differential involvement of mGlu5 receptors in the actual contextual learning process (CPP acquisition) versus the behavioral manifestation of that learning (CPP expression). Nonetheless, these studies show that mGlu5 receptors are involved in the acquisition and expression of drug reward and drug-context associative learning for most drugs of abuse. Surprisingly, no studies on the effects of mGlu1 receptor antagonists on drug CPP have been published. Since contextual learning that occurs during place conditioning is highly dependent on the hippocampus, where both mGlu1 and mGlu5 receptors are abundantly expressed and involved in synaptic plasticity, it is also surprising that inhibitory effects of mGlu5 receptor antagonism on the acquisition of CPP were confined to only cocaine, nicotine and morphine. It is likely that specific combination of the neurochemical mechanisms of action of certain drugs of abuse and the resulting changes in neurotransmitter release that are induced by these drugs (i.e., increased dopamine and glutamate release), as well as procedural variables, are important determinants of any observed effects of Group I mGlu receptor antagonists on drug reward as measured by the CPP paradigm.
It is worth mentioning at this point that MPEP has been found to have numerous off-target effects at high (micromolar) concentrations, including direct inhibition of NMDA receptor function, inhibition of norepinephrine transporter activity, and activity at mGlu4 receptors (see Lea and Faden, 2006 for review). As a result, the findings of some of the aforementioned studies that used high doses of MPEP (i.e., 30-50 mg/kg; Herzig and Schmidt, 2004; Popik and Wrobel, 2002) should be interpreted with caution, as these effects might not be mediated solely by mGlu5 receptor antagonism. Along these lines, a dose of 20 mg/kg i.p. of MPEP has been shown to produce neither conditioned rewarding or aversive effects when used alone as the conditioning drug in mice (Mcgeehan and Olive, 2003). However, MPEP has been shown to induce a CPP in rats when administered at doses of 3 and 10 mg/kg intravenously but not intraperitoneally (van der Kam et al., 2009b), which is likely attributable to the resulting high concentrations of MPEP in the brain following intravenous administration, which could directly inhibit NMDA receptor function and therefore to produce a CPP (Layer et al., 1993).
2.2. Results from studies using oral or intravenous self-administration paradigms
Although some of the results of CPP studies have been mixed, more consistent effects of mGlu5 receptor antagonism have been observed in paradigms where animals have been trained to either consume ethanol orally or self-administer other drugs of abuse intravenously. Systemic administration of mGlu5 receptor antagonists such as MPEP or the more selective compound 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) reduces operant self-administration of cocaine (Chiamulera et al., 2001; Iso et al., 2006; Kenny et al., 2005; Kenny et al., 2003; Lee et al., 2005; Martin-Fardon et al., 2009; Platt et al., 2008; Tessari et al., 2004), nicotine (Paterson et al., 2003; Tessari et al., 2004), heroin (van der Kam et al., 2007), ketamine (van der Kam et al., 2007), methamphetamine (Gass et al., 2009), and ethanol (Backstrom et al., 2004; Gupta et al., 2008; Hodge et al., 2006; Lominac et al., 2006; McMillen et al., 2005; Olive et al., 2005; Schroeder et al., 2005). The ability of MPEP and MTEP to suppress drug self-administration is due, at least in part, by a reduction in the reinforcing efficacy of the self-administered drug, as it has been shown that MPEP and MTEP can reduce breakpoints for drug self-administration on a progressive ratio schedule of reinforcement (Besheer et al., 2008b; Gass et al., 2009; Paterson and Markou, 2005). However, mGlu5 receptor antagonism can also interfere with the interoceptive effects of some drugs of abuse such as ethanol (Besheer et al., 2006) and cocaine (Lee et al., 2005), which may underlie the ability of this compound to reduce self-administration of these drugs.
With regards to mGlu1 receptor-mediated regulation of drug self-administration, most studies have focused on effects of mGlu1 receptor antagonists on voluntary ethanol consumption. Several studies have found a consistent lack of effect of mGlu1 receptor antagonism by 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) on operant ethanol self-administration in rodents (Hodge et al., 2006; Schroeder et al., 2005); however Lominac and colleagues found an inhibitory effect of CPCCOEt on ethanol self-administration (Lominac et al., 2006). The reasons for these discrepant findings remain unclear. A more recent study examining the effects of the highly potent mGlu1 receptor antagonist JNJ16259685 also showed inhibitory effects on ethanol self-administration as well as reducing breakpoints in a progressive ratio paradigm, but nonspecific impairments in motor activity were also induced by this compound (Besheer et al., 2008a; b). Thus, to date the role of mGlu1 receptors in regulating ethanol consumption are equivocal, and further studies are needed examining effects of mGlu1 receptor antagonists on self-administration of other drugs of abuse are needed. Given the apparent locomotor inhibitory effects of such ligands noted by Besheer and colleagues, such studies should closely examine whether any observed reductions in drug self-administration are not the result of nonspecific motor inhibitory effects.
2.3. Results from studies using the reinstatement paradigm
Group I mGlu receptors also appear to regulate relapse-like behaviors, as measured by the reinstatement paradigm (increases in the number of operant responses that previously led to drug delivery prior to extinction procedures, a phenomenon that can be induced by presentation of drug-associated cues, contexts, drug priming, or stressors). In the case of alcohol, the alcohol deprivation effect (a transient increase in ethanol consumption following a short period of forced abstinence) is sometimes used. Reinstatement of drug-seeking behavior evoked by drug-associated cues has shown to be attenuated by mGlu5 receptor antagonists in rats with a history of self-administration of cocaine (Kumaresan et al., 2009; Lee et al., 2005; Martin-Fardon et al., 2009; Moussawi et al., 2009), alcohol (Backstrom et al., 2004; Schroeder et al., 2008), nicotine (Bespalov et al., 2005) or methamphetamine (Gass et al., 2009). mGlu5 receptor antagonism also attenuates the alcohol deprivation effect (Backstrom et al., 2004; Schroeder et al., 2005). Reinstatement of drug-seeking behavior evoked by acute drug exposure (drug priming) is also attenuated by mGlu5 receptor antagonists in rats with a history of self-administration of cocaine (Backstrom and Hyytia, 2006; Kumaresan et al., 2009) or nicotine (Tessari et al., 2004).
Although most reinstatement studies have focused on effects of mGlu5 receptor antagonists, a recent study demonstrated that the selective mGlu1 receptor antagonist (3-ethyl-2-methyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)-methanone (EMQMCM) attenuated the reinstatement of nicotine-seeking behavior elicited by nicotine priming and nicotine-associated cues (Dravolina et al., 2007). These recent findings suggest that studies need to be conducted that examine the role of mGlu1 receptors in mediating relapse-like behaviors in animals with a history of self-administration of other drugs of abuse such as cocaine, methamphetamine, heroin, and alcohol.
2.4. Possible underlying neural mechanisms of action
Most studies examining the effects of Group I mGlu receptor antagonists on drug reinforcement, reward and relapse-like behavior have used peripheral routes of administration. However, a handful of recent studies have employed intracranial microinjection procedures to determine potential neuroanatomical sites of action of these compounds. Thus far, mGlu5 receptors in the nucleus accumbens have been shown to regulate ethanol consumption and reinstatement of cocaine-seeking behavior, and mGlu1 receptors in the hippocampus have been demonstrated to regulate contextual reinstatement of cocaine-seeking behavior. More specifically, blockade of mGlu5 receptors in the nucleus accumbens shell but not core subregion with MTEP attenuated intravenous alcohol reinforcement in rats (Gass and Olive, 2009b), an effect that was dependent on the activity of epsilon isoform of PKC (PKCε), a downstream signaling target of mGlu5 receptors (Olive et al., 2005). Similar findings were reported by Cozzoli and colleagues, who demonstrated that intra-accumbens infusion of MPEP reduced binge-like alcohol consumption in mice (Cozzoli et al., 2009). Interestingly, Cozzoli and colleagues also demonstrated that disconnection of mGlu5 receptors from Homer scaffolding proteins by incorporating a singe amino acid substitution in the Homer-binding domain of the mGlu5 receptor eliminated the ability of intra-accumbens MPEP to reduce alcohol consumption. These data support the notion that the Homer family of scaffolding proteins that structurally link mGlu5 to NMDA receptors are important mediators of drug self-administration as well as drug sensitivity (Szumlinski et al., 2004, 2005, 2006). Blockade of mGlu5 receptors in the nucleus accumbens also attenuates cocaine- or cue-primed reinstatement of cocaine-seeking behavior (Backstrom and Hyytia, 2007; Kumaresan et al., 2009).
An important role for mGlu1 receptors in the dorsal hippocampus in regulating cocaine-seeking behavior evoked by cocaine-associated contextual cues was recently demonstrated (Xie et al., 2010), consistent with the established role for the hippocampus in mediating drug-context associations and their ability to evoke drug-seeking behavior (Fuchs et al., 2005). Thus, it seems that Group I mGlu receptors in multiple brain regions mediate different aspects of drug addiction, such as drug reinforcement, self-administration, and cue- and drug-primed reinstatement (which are mediated, at least in part, by mGlu5 receptors in the nucleus accumbens) and the ability of drug-associated contextual cues to elicit drug-seeking behavior (which are mediated, at least in part, by mGlu1 receptors in the hippocampus). Studies examining the effects of pharmacological manipulation of Group I mGlu receptors in other brain regions known to be involved in addiction (i.e., the amygdala, VTA, PFC, etc.) on drug self-administration and relapse are needed to provide a more comprehensive understanding of the neural circuitry in which these receptors regulate various aspects of drug addiction.
On a cellular level, it is of interest to know precisely how inhibition of Group I mGlu receptor function attenuates drug reward, reinforcement, or relapse-like behaviors. A likely mechanism can be postulated as follows: inhibition of Group I mGlu receptors, primarily the mGlu5 receptor subtype, dampens of the activity of medium spiny neurons in the nucleus accumbens, which comprise more than 90% of the neurons within this region and exhibit high levels of mGlu5 expression (Romano et al., 1995; Shigemoto et al., 1993). Although this has not been demonstrated directly, it seems to be a plausible hypothesis as it has recently been shown that mGlu5 receptor activation increases the spike firing and excitability of medium spiny neurons in the nucleus accumbens (D’Ascenzo et al., 2009). Therefore, mGlu5 receptor antagonism in this region could possibly reduce the excitability of these neurons, and as a consequence, dampen the outflow of neural information from the nucleus accumbens to its target regions including the ventral pallidum and VTA. These regions are well known to be a part of the circuitry that mediates adaptive motivated behaviors (Kalivas and Nakamura, 1999; Kalivas et al., 2006; Koob and Volkow, 2010). However, given that effects of Group I mGlu receptor antagonists administered into regions other than nucleus accumbens or hippocampus on addiction-related behaviors have not yet been investigated, one cannot rule the potential role of other structures, circuitries, or physiological mechanisms at this point.
Another mechanism by which Group I mGlu receptor antagonists may reduce drug reward and reinforcement and relapse is by altering the functionality of the brain’s reward circuitry. For example, Kenny and colleagues showed that a 9 mg/kg dose of MPEP elevated thresholds for intracranial self-stimulation of the medial forebrain bundle (Kenny et al., 2005), a well-established assay of brain reward function. Typically, elevations in thresholds for intracranial self-stimulation indicate a decrease in functioning of the brain’s reward system are have been interpreted as representing an anhedonic or dysphoric state such as that produced by drug withdrawal (Markou and Koob, 1992). Thus, Kenny and colleagues have proposed that MPEP-induced deficits in brain reward function mediated the ability of this compound to reduce self-administration of cocaine (Kenny et al., 2005). While reductions in brain reward circuitry function could indeed contribute to the ability of mGlu5 receptor antagonists to attenuate drug self-administration, findings that MPEP at a dose of 20 mg/kg does not produced conditioned aversive effects (Mcgeehan and Olive, 2003), and that MTEP does not alter self-administration of food (a non-drug reward) on fixed or progressive ratios schedule of reinforcement (Gass et al., 2009), suggest that additional mechanisms may be involved.
2.5. Cognitive effects of Group I mGlu antagonists in humans
Given the wealth of preclinical evidence suggesting that Group I mGlu receptor antagonists may be of use in the treatment of drug addiction, one critical unanswered question is - what cognitive effects will such ligands have in human beings with respect to drug craving and subjective drug effects? Several mGlu5 (but not mGlu1) receptor antagonists (or negative allosteric modulators, NAMs) have now entered Phase I and II clinical trials for the treatment of other medical conditions, such as Fragile X syndrome, gastroesophageal reflux disease (GERD), anxiety, depression, and L-dopa induced dyskinesias in Parkinson’s disease (Jaeschke et al., 2008).. While the few reports detailed below on the clinical efficacy of these compounds as well as their cognitive (side) effects provide are relatively limited in detail, thus far they indicate that such ligands are fairly well tolerated and result in few serious adverse cognitive effects.
Several early clinical studies were conducted with [N-(3-chlorophenyl-N’-(4,5)-dihydro-1-methyl-4-oxo-1H-imidazol-2-yl)urea (fenobam) for the treatment of anxiety (Friedmann et al., 1980; Itil et al., 1978; Pecknold et al., 1980; Pecknold et al., 1982). While at the time of these studies fenobam had an unknown mechanism of action, it was later found to be an mGlu5 receptor antagonist with greater selectivity for the receptor than MPEP (Montana et al., 2009; Porter et al., 2005). The results of these early clinical studies demonstrated clear anxiolytic effects of fenobam, equivalent to those produced by diazepam, but adverse side effects such as nausea, dizziness, insomnia, blurred vision, depersonalization, and perceptual alterations were reported in approximately 25-40% of the patients taking fenobam, and many patients who experienced these side effects discontinued the study. Such adverse side effects occurred primarily in patients taking higher doses of fenobam (200-600 mg/day). Despite its apparent efficacy as an anxiolytic, the relatively high prevalence rates of adverse side effects associated with fenobam resulted in a discontinuation of clinical trials of this compound.
Recently, however, a re-examination of the therapeutic potential of fenobam for treating Fragile X syndrome, a common form of mental retardation, has been initiated by several pharmaceutical companies (Jaeschke et al., 2008). In a recent preliminary report on the safety and tolerability of fenobam in subjects with Fragile X syndrome, it was reported that doses of fenobam in the 50-150 mg/day range produced clinical improvements in cognitive function in 9 out of 12 patients, including increased eye contact and social interaction, and reduced anxiety and hyperactivity (Berry-Kravis et al., 2009). The only side effect observed in 3 of the 12 patients was mild sedation, and no subjects discontinued the trial.
The results of a clinical trial of the mGlu5 receptor negative allosteric modulator (NAM) ADX10059 for the treatment of GERD have also recently been made public (Keywood et al., 2009). Although little has been revealed about the precise pharmacological properties of ADX10059, it is designated as an mGlu5 receptor NAM rather than an antagonist, most likely because it dampens but does not completely antagonize the functioning of this receptor, as is the case with MPEP, MTEP, and fenobam. The mechanistic rationale for the treatment of GERD with compounds that reduce mGlu5 receptor function has been based on the localization of mGlu5 receptors on vagal afferent nerve endings that innervate and control the contractility of the lower esophageal sphincter, thereby regulating reflux of stomach acid into the esophagus (Young et al., 2007). In the study by Keywood and colleagues, 24 subjects with GERD received placebo prior to a high fat meal on the first day of reflux assessment, followed by ADX10059 at a dose of either 50 or 250 mg/day (n=12 per group) prior to a high fat meal on the second day of reflux assessment. ADX10059 was shown to be effective in reducing acid reflux, and no subjects taking either dose of ADX10059 discontinued the trial. In subjects receiving the 50 mg dose, 1 out of 12 subjects reported mild sedation, cough, and rhinorrhea. In subjects receiving the 250 mg dose, side effects such as dizziness and nausea occurred in 9 out of 12 participants. However, these side effects resolved following the first or second dose of ADX10059, and none were considered severe. Thus, like fenobam, ADX10059 appears to be fairly well tolerated with few adverse side effects that precipitate discontinuation of the medication. Another mGlu5 receptor NAM, ADX48621, has recently entered clinical trials for the treatment of depression and anxiety (Jaeschke et al., 2008). Thus, mGlu5 receptor antagonists or NAMs such as fenobam and ADX10059 appear to be well-tolerated in humans with few side effects, and future clinical trials on their effects on the craving, use, and subjective effects of drugs of abuse in human addicts are eagerly awaited.
3. Plasticity- and cognition-enhancing of mGlu5 positive allosteric modulators (PAMs)
In Section 1, the positive coupling of Group I mGlu receptors to NMDA receptor function was described along with the ability of antagonists of these receptors to negatively influence synaptic plasticity. In Section 2, studies providing a clear rationale for the use of ligands that reduce or inhibit Group I mGlu receptor function in reducing drug reward, reinforcement, and relapse-like behaviors were summarized, along with recent exciting preliminary data on the favorable safety and tolerability of mGlu5 receptor antagonist fenobam and the mGlu5 receptor NAM ADX10059 in humans. Recently, however, efforts have also been made to develop compounds that indirectly enhance NMDA receptor function via positive allosteric modulation of Group I mGlu receptors (see Fig. 1) for the purposes of reversing the hypothesized NMDA receptor “hypofunction” that is believed to contribute to cognitive deficits in schizophrenia (Chen and Conn, 2008; Conn et al., 2009a; Conn et al., 2009b; Lindsley et al., 2006; Niswender and Conn, 2010; Rodriguez and Williams, 2007). Thus far, significant progress has been made in the development of mGlu5 receptor positive allosteric modulators (PAMs). These compounds bind to an allosteric site that is distinct from the orthosteric glutamate binding site of the receptor, and while devoid of any agonistic activity on their own, they potentiate the receptor response to endogenously released glutamate. Due to the more widespread distribution of mGlu5 vs. mGlu1 receptors in the brain, particularly in forebrain regions, greater effort has been devoted to the development of mGlu5 over mGlu1 receptor PAMs.
The first mGlu5 receptor PAM to be characterized was 3,3′-difluorobenzaldazine (DFB) (O’Brien et al., 2003), which is structurally related to MPEP and MTEP. However, DFB exhibits low potency, poor solubility in aqueous solutions, and does not cross the blood-brain barrier. These same investigators subsequently identified a more potent mGlu5 receptor PAM, N-[4-chloro-2-[1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl]-2-hydroxybenzamide (CPPHA) (O’Brien et al., 2004). In the initial characterization of this compound, it was demonstrated that CPPHA was 7 to 8 times more potent than DFB in potentiating mGlu5 receptor function in vitro. In addition, CPPHA potentiated NMDA receptor-mediated currents in rat hippocampal slices in the presence of threshold concentrations of the Group I mGlu receptor agonist dihydroxyphenylglycine (DHPG), and potentiated DHPG-induced depolarizations of rat subthalamic nucleus neurons. Unfortunately, like DFB, CPPHA exhibited poor solubility in aqueous solutions and did not show significant brain penetrance following systemic administration.
The first mGlu5 receptor PAM that showed significant CNS activity following peripheral administration was 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB), which was shown to reverse amphetamine-induced disruption of prepulse inhibition, an animal model of sensorimotor gating deficits in schizophrenia (Kinney et al., 2005; Lindsley et al., 2004). Subsequently, the characterization and antipsychotic profile of another mGlu5 receptor PAM, [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone] (ADX47273) has also been described (Liu et al., 2008; Schlumberger et al., 2009). As will be discussed below, mGlu5 receptor PAMs may be novel therapeutic approaches to enhancing synaptic plasticity and improving cognitive function in drug addicts.
3.1. Cognitive deficits produced by drug addiction
Chronic drug use leads to numerous deficits in cognitive function, including loss of executive control, impulsivity, poor decision making, behavioral inflexibility, memory impairments, amongst many others. One area of the brain that is particularly vulnerable to drug-induced neurological impairments which result in cognitive deficits is the PFC (Goldstein and Volkow, 2002; London et al., 2005; Volkow and Fowler, 2000; Volkow et al., 1992). The PFC is comprised of various subregions of the dorsal forebrain, including the medial prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex. As a whole, the PFC mediates a host of functions including working memory, impulsivity and response inhibition, selective attention, salience attribution, reward expectancy, decision-making, goal-directed behavior, drive and motivation, and emotional learning (Fuster, 1997; Shidara and Richmond, 2002; Tzschentke, 2000; Vertes, 2006). The PFC has reciprocal connections with numerous other regions of the brain including the dorsal and ventral striatum, ventral midbrain, brainstem, thalamus, hippocampus, amygdala, and other cortical regions (Likhtik et al., 2005; Morgane et al., 2005; Sesack et al., 1989; Vertes, 2004). As a result of chronic drug use, a reduction in function and activity of PFC is often observed in drug addicts, a phenomenon commonly referred to as “hypofrontalism”. Loss of PFC functionality is believed to contribute to the loss of executive function and inhibitory control over compulsive behaviors, improper decision making, and the maladaptive motivational salience of drug-associated cues that are characteristic of addiction (Crews and Boettiger, 2009; Everitt et al., 2008; Isoda and Hikosaka, 2007; Jentsch and Taylor, 1999; Kalivas, 2008; Schoenbaum et al., 2006; Volkow et al., 2002b). Thus, “hypofrontal” drug addicts exhibit behavioral perseverance and inflexibility, and thus have difficulty changing their drug-taking behavior from compulsive and uncontrolled to self-regulated. Impaired functioning of the PFC may be due, in part, to drug-induced alterations of mGlu5 receptor function or expression, as infusions of MPEP into the PFC has been shown to impair object recognition and spatial short-term memory (Christoffersen et al., 2008), while repeated drug exposure or drug self-administration alters expression levels of mGlu5 receptors in this region (Ben-Shahar et al., 2009; Winstanley et al., 2007). As will be discussed below, mGlu5 receptor PAMs appear to possess pro-cognitive properties and reverse experimentally-induced deficits in PFC function, and may therefore improve functionality of the PFC and reverse some of the cognitive deficits commonly observed in drug addiction.
3.2. Effects of mGlu5 receptor PAMs on synaptic plasticity and cognition
Recently it was reported that the CDPPB analog VU-29 facilitated the induction of LTP in slice preparations of the CA1 region of the hippocampus, and these effects were blocked by AP-5, confirming a role for increased NMDA receptor function in this phenomenon (Ayala et al., 2009). VU-29 also potentiated DHPG-induced LTD in this region. In this same report, it was shown that systemic administration of CDPPB or ADX47273 improved performance in the Morris water maze, a hippocampus-dependent spatial memory task. Consistent with these findings, intracerebroventricular infusion of DFB has been shown to improve spatial memory in a Y-maze task (Balschun et al., 2006), whereas genetic deletion or pharmacological blockade of mGlu5 receptors impairs performance in the Morris water maze and other spatial navigation tasks (Car et al., 2007; Christoffersen et al., 2008; Jacob et al., 2009; Manahan-Vaughan and Braunewell, 2005). CDPPB and ADX47273 have also been reported to improve cognition as measured by novel object recognition and five-choice serial reaction time tests, which involve proper functioning of the hippocampus and frontal cortex. In accordance with the observed ability of mGlu5 receptor PAMs to facilitate the induction of synaptic plasticity, CDPPB and ADX47273 increase the expression of markers of synaptic plasticity in the PFC and hippocampus, such as increased phosphorylation of the NR1 and NR2B subunits of the NMDA receptor and the GluR1 subunit of the AMPA receptor, and activate α-calmodulin-dependent kinase II, cAMP-responsive element binding protein, and extracellular signal-regulated kinase (Liu et al., 2008; Uslaner et al., 2009). Further support for mGlu5 receptor PAM-induced increases in PFC functioning have been recently demonstrated using in vivo electrophysiological techniques, where CDPPB was shown to reverse changes in frontal cortex neuronal firing patterns induced by the psychotomimetic NMDA receptor antagonist MK-801 (Homayoun and Moghaddam, 2006; 2008). Finally, and perhaps most importantly, CDPPB has been shown to reverse MK-801 induced impairments in performance in behavioral flexibility tasks (Darrah et al., 2008), which highly involve proper functioning of the orbitofrontal cortex. Thus, it is possible that enhanced functionality of the PFC produced by mGlu5 receptor PAMs might reduce the behavioral inflexibility and perseverative nature of compulsive and uncontrolled drug-seeking and drug self-administration behavior that is a defining characteristic of drug addiction. Other cognition-enhancing agents such as inhibitors of acetylcholinesterase and norepinephrine transporters, nicotinic acetylcholine receptor partial agonists, and α2-adrenergic receptor agonists, have also been proposed to be of potential benefit in reversing some of the cognitive impairments associated with chronic drug use (Sofuoglu, 2010).
3.3. Effects of mGlu5 receptor PAMs on extinction of drug-seeking behavior
Drug-associated memories evoked by exposure to contexts or distinct environmental stimuli that have been paired with drug intake can result in drug craving and ultimately relapse. It is therefore important that during the course of treatment of addiction, a weakening of drug-related memories and the motivational salience of drug-associated cues and contexts is needed, as is the formation of newer associations between environmental stimuli and drug effects or availability (Centonze et al., 2005; Diergaarde et al., 2008; Taylor et al., 2009). While weakening drug memories by interfering with the mechanisms of memory reconsolidation is one potential approach (Diergaarde et al., 2008; Lee, 2009; Taylor et al., 2009), these approaches would likely involve manipulation of long-term aspects of synaptic plasticity such as dendritic remodeling, de novo protein synthesis, and changes in gene expression related to synaptic plasticity, which may be difficult to achieve without affecting normal brain function.
An alternative approach to overcoming drug-associated memories is via facilitation of extinction learning. It is well accepted that extinction of conditioned associations between external stimuli and maladaptive behaviors or responses (such as drug craving and drug-seeking behavior) is a form of new and active learning. However, treatment approaches such as cue exposure therapy that are used to desensitize the addict to the psychological and physiological responses evoked by drug-associated stimuli have shown only modest success rates (Conklin and Tiffany, 2002; Havermans and Jansen, 2003). Therefore, pharmacological approaches to facilitating extinction learning could provide a novel avenue for treatment of drug addiction.
Indeed, there is recent data demonstrating that increasing glutamategic transmission via activation or positive allosteric modulation of Group I mGlu receptors can enhance extinction learning. For example, intra-hippocampal infusions of an mGlu1 receptor antagonist attenuates the extinction of inhibitory avoidance learning (Simonyi et al., 2007), and infusions of mGlu1 receptor antagonists into the amygdala attenuate the extinction of conditioned fear (Kim et al., 2007). It has also been shown that mGlu5 receptor-deficient mice fail to extinguish cue- and contextually conditioned fear behavior (Xu et al., 2009). Relevant to drug addiction, studies from the author’s laboratory have shown that CDPPB facilitates the extinction of a cocaine-induced conditioned place preference (Gass and Olive, 2009a) and reduces extinction responding following intravenous cocaine self-administration (see Fig. 2). Although excessive NMDA activation can produce excitotoxicity, repeated CDPPB administration does not produce evidence of neurotoxicity (Gass and Olive, 2009a). Thus, mGlu5 receptor PAMs may be a novel class of compounds by which to facilitate the extinction of drug-stimuli or drug-context associations.
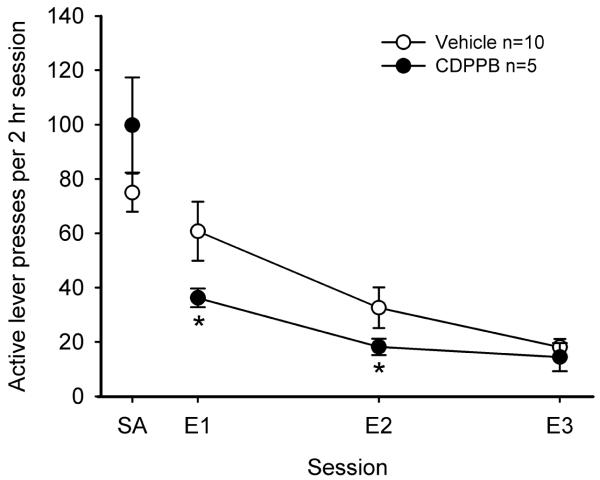
Fig. 2.
The mGlu5 receptor positive allosteric modulator CDPPB reduces extinction responding following intravenous cocaine self-administration. Male Sprague-Dawley rats were trained to self-administer cocaine at a dose of 0.33 mg/kg/infusion under an FR1 schedule of reinforcement in 2 hr daily sessions. Following 12 days of active cocaine self-administration (the average of the last two days is depicted in the graph as SA), animals underwent extinction by eliminating infusions as a consequence of active lever presses, while stimuli that were previously presented during each cocaine infusion (i.e., light/tone stimulus and syringe pump activation) were still presented. Animals were administered vehicle (20% 2-hydroxypropyl-β-cyclodextrin) or CDPPB (30 mg/kg i.p.) 30 min prior to daily 2 hr extinction sessions (E1, E2, or E3). CDPPB significantly (*P<0.05) reduced extinction responding on extinction days E1 and E2, possibly reflecting increased extinction learning, as this dose of CDPPB has no effect on motor activity (Gass and Olive, 2009a).
4. Conclusions and treatment implications
Group I mGlu receptors are intricately involved in regulating synaptic plasticity, including plasticity produced by drugs of abuse, as well as associated normal and pathogenic learning and memory processes. Group I mGlu receptor antagonists or NAMs may be of potential benefit in reducing drug reward, reinforcement and relapse-related behaviors, and a few recent clinical trials on such compounds acting on mGlu5 receptors (i.e., fenobam and ADX10059) for other medical conditions have revealed that these compounds are safe and well tolerated. Thus, clinical trials examining the effects of these or similar compounds on drug intake and relapse are now clearly warranted, as are studies of their effects on addiction-related cognitive phenomena such as drug craving and subjective drug effects. On the other hand, there now is evidence that mGlu5 receptor PAMs, which indirectly increase NMDA receptor function and enhance synaptic plasticity, improve cognitive function in certain tasks, facilitate the extxinction of drug-associated contextual memories, and reverse experimentally-induced deficits in PFC function. Therefore, the clinical utility of pharmacologically inhibiting or enhancing Group I mGlu receptor function may ultimately depend on the specific aspect of the addict’s condition that is most problematic (i.e., reducing the drug’s reinforcing effects by mGlu5 receptor antagonists or NAMs, or improving executive control, behavioral flexibility, and extinction learning by mGlu5 receptor PAMs). Furthering the advancement of both types of ligands in clinical trials, as well as improving our understanding of the neural circuitry in which they exert their effects and the behavioral paradigms in which they most effective, should be top priorities for researchers in this field of study.
5. Acknowledgements
This work was supported by grants AA013852, DA024355 and DA025606 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Alagarsamy S, Marino MJ, Rouse ST, Gereau R.W.t., Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat. Neurosci. 1999;2:234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatry Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision American Psychiatric Press; Washington DC: 2002. [Google Scholar]
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br. J. Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J. Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Balschun D, Manahan-Vaughan D, Wagner T, Behnisch T, Reymann KG, Wetzel W. A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning. Learn. Mem. 1999;6:138–152. [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol. Biochem. Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Bao G, Kang L, Li H, Li Y, Pu L, Xia P, Ma L, Pei G. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology. 2007;32:1738–1749. doi: 10.1038/sj.npp.1301308. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell. Mol. Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor reponses via two distinct metabotropic glutamate receptor subtypes. J. Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open-label single-dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol. 2008a;42:13–20. doi: 10.1016/j.alcohol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res. 2008b;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur. J. Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn J, Nicoletti F, Manahan-Vaughan D. MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS ONE. 2008;3:e2155. doi: 10.1371/journal.pone.0002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int. J. Neuropsychopharmacol. 2008;11:765–774. doi: 10.1017/S1461145708008572. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr. Mol. Pharmacol. 2009a;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. The promiscuous mGlu5 receptor - a range of partners for therapeutic possibilities? Trends Pharmacol. Sci. 2009b;30:617–623. doi: 10.1016/j.tips.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Boning J. Addiction memory as a specific, individually learned memory imprint. Pharmacopsychiatry. 2009;42(Suppl 1):S66–68. doi: 10.1055/s-0029-1216357. [DOI] [PubMed] [Google Scholar]
- Bonini JS, Rodrigues L, Kerr DS, Bevilaqua LR, Cammarota M, Izquierdo I. AMPA/kainate and group-I metabotropic receptor antagonists infused into different brain areas impair memory formation of inhibitory avoidance in rats. Behav. Pharmacol. 2003;14:161–166. doi: 10.1097/00008877-200303000-00008. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Car H, Stefaniuk R, Wisniewska RJ. Effect of MPEP in Morris water maze in adult and old rats. Pharmacol. Rep. 2007;59:88–93. [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann. N. Y. Acad. Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Centonze D, Siracusano A, Calabresi P, Bernardi G. Removing pathogenic memories: a neurobiology of psychotherapy. Mol. Neurobiol. 2005;32:123–132. doi: 10.1385/MN:32:2:123. [DOI] [PubMed] [Google Scholar]
- Chen Y, Conn PJ. mGluR5 positive allosteric modulators. Drugs Fut. 2008;33:355–360. [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br. J. Pharmacol. 2008;154:384–396. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen GR, Simonyi A, Schachtman TR, Clausen B, Clement D, Bjerre VK, Mark LT, Reinholdt M, Schmith-Rasmussen K, Zink LV. MGlu5 antagonism impairs exploration and memory of spatial and non-spatial stimuli in rats. Behav. Brain Res. 2008;191:235–245. doi: 10.1016/j.bbr.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009a;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009b;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol. Biochem. Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Gasparini F, van Heeke G, Markou A. Non-nicotinic neuropharmacological strategies for nicotine dependence: beyond bupropion. Drug Discov. Today. 2003;8:1025–1034. doi: 10.1016/s1359-6446(03)02890-3. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents. J. Physiol. 2009;587:3233–3250. doi: 10.1113/jphysiol.2009.172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat. Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav. Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Associative learning, the hippocampus, and nicotine addiction. Curr. Drug Abuse Rev. 2008;1:9–19. doi: 10.2174/1874473710801010009. [DOI] [PubMed] [Google Scholar]
- Del Olmo N, Miguens M, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Solis JM, Ambrosio E. Enhancement of hippocampal long-term potentiation induced by cocaine self-administration is maintained during the extinction of this behavior. Brain Res. 2006;1116:120–126. doi: 10.1016/j.brainres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Delanoy RL, Tucci DL, Gold PE. Amphetamine effects on long term potentiation in dentate granule cells. Pharmacol. Biochem. Behav. 1983;18:137–139. doi: 10.1016/0091-3057(83)90263-0. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Pharmacological manipulation of memory reconsolidation: towards a novel treatment of pathogenic memories. Eur. J. Pharmacol. 2008;585:453–457. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br. J. Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Schmid S. Metabotropic glutamate receptors are involved in amygdaloid plasticity. Eur. J. Neurosci. 2002;15:1535–1541. doi: 10.1046/j.1460-9568.2002.01988.x. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Friedmann CTH, Davis LJ, Ciccone PE, Rubin RT. Phase II double-blind controlled study of a new anxiolytic, fenobam (McN-3377) vs. placebo. Curr. Ther. Res. 1980;27:144–151. [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex: anatomy, physiology and neuropsychology of the frontal lobe. Lippincott Williams & Wilkins; Baltimore: 1997. [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol. Psychiatry. 2009a;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCε) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009b;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol. Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol. Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussakov I, Chartoff EH, Tsvetkov E, Gerety LP, Meloni EG, Carlezon WA, Jr., Bolshakov VY. LTP in the lateral amygdala during cocaine withdrawal. Eur. J. Neurosci. 2006;23:239–250. doi: 10.1111/j.1460-9568.2005.04538.x. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of ths stria terminalis is disrupted by cocaine administration. J. Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG. In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J. Neurosci. 2008;28:9261–9270. doi: 10.1523/JNEUROSCI.2886-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol. Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Costa C, Tropepi D, Bernardi G, Conquet F, Calabresi P. Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology. 2003;44:8–16. doi: 10.1016/s0028-3908(02)00214-9. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol drinking-in-the-dark in male C57BL/6J mice. Alcohol. Clin. Exp. Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Havermans RC, Jansen AT. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict. Behav. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol. Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict. Biol. 2005;10:243–249. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb. Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18041–18046. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM, Sanker S, Roth BL, Tueckmantel W, Kozikowski AP. Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J. Med. Chem. 2006;49:1080–1100. doi: 10.1021/jm050570f. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Itil TM, Seaman PA, Huque M, Mukhopadhyay S, Blasucci D, Tat K, Ciccone PE. The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Curr. Ther. Res. 1978;24:708–724. [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Jaeschke G, Wettstein JG, Nordquist RE, Spooren W. mGlu5 receptor antagonists and their therapeutic potential. Exp. Opin. Ther. Patents. 2008;18:123–142. [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn. Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J. Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Lawrence AJ. New horizons for therapeutics in drug and alcohol abuse. Pharmacol Ther. doi: 10.1016/j.pharmthera.2009.11.002. in press. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr. Opin. Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox. Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr. Opin. Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol. Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu. Rev. Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann. N. Y. Acad. Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Keywood C, Wakefield M, Tack J. A proof of concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastro-esophageal reflux disease. Gut. 2009;58:1192–1199. doi: 10.1136/gut.2008.162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, Shin K, Choi S. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem. Biophys. Res. Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J. Pharmacol. Exp. Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur. Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha SA, MacDonald JF. Signaling molecules and receptor transduction cascades that regulate NMDA receptor-mediated synaptic transmission. Int. Rev. Neurobiol. 2003;54:51–106. doi: 10.1016/s0074-7742(03)54003-x. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav. Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer RT, Kaddis FG, Wallace LJ. The NMDA receptor antagonist MK-801 elicits conditioned place preference in rats. Pharmacol. Biochem. Behav. 1993;44:245–247. doi: 10.1016/0091-3057(93)90306-e. [DOI] [PubMed] [Google Scholar]
- Le Moal M. Drug abuse: vulnerability and transition to addiction. Pharmacopsychiatry. 2009;42(Suppl 1):S42–55. doi: 10.1055/s-0029-1216355. [DOI] [PubMed] [Google Scholar]
- Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J. Pharmacol. Exp. Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J. Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr., Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr. Top. Med. Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O’Brien J,A, Lemaire W, Williams DL, Jr., Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J. Med. Chem. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Wantuch C, Popiolek M, Day M, Khawaja X, Smith D, Olsen M, Kouranova E, Gilbert A, Lai M, Pausch MH, Pruthi F, Pulicicchio C, Brandon NJ, Comery TA, Beyer CE, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273: a novel metabotropic glutamate receptor 5 selective positive allosteric modulator with preclinical antipsychotic-like and pro-cognitive activities. J. Pharmacol. Exp. Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between group I mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol. Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat. Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejak P, Taracha E, Lehner M, Szyndler J, Bidzinski A, Skorzewska A, Wislowska A, Zienowicz M, Plaznik A. Hippocampal mGluR1 and consolidation of contextual fear conditioning. Brain Res. Bull. 2003;62:39–45. doi: 10.1016/j.brainresbull.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb. Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J. Pharmacol. Exp. Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Mcgeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 2005;40:494–497. doi: 10.1093/alcalc/agh200. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RW. The mGlu5 antagonist fenobam is analgesic and has improved in vivo selectivity as compared to the prototypical antagonist MPEP. J. Pharmacol. Exp. Ther. 2009;330:834–843. doi: 10.1124/jpet.109.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb. Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-D-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur. J. Neurosci. 2005;21:411–421. doi: 10.1111/j.1460-9568.2005.03864.x. [DOI] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur. J. Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J. Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Lemaire W, Chen TB, Chang RS, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, Conn PJ, Williams DL., Jr. A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol. Pharmacol. 2003;64:731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL. A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 (mGluR5) in rat forebrain. J. Pharmacol. Exp. Ther. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- Olive MF. mGlu5 receptors: neuroanatomy, pharmacology, and role in drug addiction. Curr. Psychiatry Rev. 2005;1:197–214. [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for drug addiction. Curr. Drug Abuse Rev. 2009a;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Role of the amygdala in drug-related memories. CellScience. 2009b;6:87–103. [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase Cε-dependent mechanism. Mol. Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Packard MG, Vecchioli SF, Schroeder JP, Gasbarri A. Task-dependent role for dorsal striatum metabotropic glutamate receptors in memory. Learn. Mem. 2001;8:96–103. doi: 10.1101/lm.37401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L. Fenobam in anxious outpatients. Curr. Ther. Res. 1980;27:119–123. [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J. Clin. Psychopharmacol. 1982;2:129–133. [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 2008;200:167–176. doi: 10.1007/s00213-008-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard T, Buettelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muehlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated non-benzodiazepine anxiolytic is a potent, selective and non-competitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platta B, Micheaub J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J. Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Williams R. Recent progress in the development of allosteric modulators of mGluR5. Curr. Opin. Drug Discov. Devel. 2007;10:715–722. [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. DHPG activation of group 1 mGluRs in BLA enhances fear conditioning. Learn. Mem. 2009;16:421–425. doi: 10.1101/lm.1444909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Klein KU, Greco S, More L, Danysz W. Comparison of the mGlu5 receptor positive allosteric modulator ADX47273 and the mGlu2/3 receptor agonist LY354740 in tests for antipsychotic-like activity. Eur. J. Pharmacol. 2009;623:73–83. doi: 10.1016/j.ejphar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacology. 2008;54:837–844. doi: 10.1016/j.neuropharm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Olsen CM, Winder DG. Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmacology. 2006;31:1444–1451. doi: 10.1038/sj.npp.1300918. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat. Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Mizuno N. Metabotropic glutamate receptors - immunocytochemical and in situ hybridization analysis. In: Ottersen OP, Storm-Mathisen J, editors. Handbook of Chemical Neuroanatomy: Metabotropic Glutamate Receptors: Immunocytochemical and In Situ Hybridization Analyses. Vol. 18. Elsevier; London: 2000. pp. 63–98. [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J. Comp. Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Shipe WD, Wolkenberg SE, Williams DL, Jr., Lindsley CW. Recent advances in positive allosteric modulators of metabotropic glutamate receptors. Curr. Opin. Drug Discov. Devel. 2005;8:449–457. [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect. 2005;18:353–361. doi: 10.1358/dnp.2005.18.6.927927. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Shelat PB, Dopheide MM, Coulibaly AP, Schachtman TR. Differential roles of hippocampal metabotropic glutamate receptors 1 and 5 in inhibitory avoidance learning. Neurobiol. Learn. Mem. 2007;88:305–311. doi: 10.1016/j.nlm.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu5 receptors and CNS disorders. Trends Pharmacol. Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3d, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr. Opin. Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugman M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J. Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur. J. Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127:177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Wagner JJ. Cocaine-induced modulation of long-term potentiation in the CA1 region of rat hippocampus. Neuropharmacology. 2005;49:185–194. doi: 10.1016/j.neuropharm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as part of the brain reward system. Amino Acids. 2000;19:211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol. Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by the specific mGluR5 agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav. Pharmacol. 2007;18:717–724. doi: 10.1097/FBP.0b013e3282f18d58. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. 2-Methyl-6(phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat. Eur. J. Pharmacol. 2009a;606:94–101. doi: 10.1016/j.ejphar.2008.12.042. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. The mGlu5 receptor antagonist 2-methyl-6(phenylethynyl)-pyridine (MPEP) supports intravenous self-administration and induces conditioned place preference in the rat. Eur. J. Pharmacol. 2009b;607:114–120. doi: 10.1016/j.ejphar.2009.01.049. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav. Pharmacol. 2002a;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insights from imaging studies. Neurobiol. Learn. Mem. 2002b;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Sparta DR, Hopf FW, Bowers MS, Melis M, Bonci A. Strain specific synaptic modifications on ventral tegmental area dopamine neurons after ethanol exposure. Biol. Psychiatry. 2009;65:646–653. doi: 10.1016/j.biopsych.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr. Opin. Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur. J. Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J. Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. doi: 10.1016/j.neuropharm.2009.10.001. in press. [DOI] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur. J. Neurosci. 2007;25:3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
- Young RL, Page AJ, O’Donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G501–511. doi: 10.1152/ajpgi.00353.2006. [DOI] [PubMed] [Google Scholar]