Abstract
Dysregulation of glutamate neurotransmission may play a role in cognitive deficits in schizophrenia. Manipulation of glutamate signaling using drugs acting at metabotropic glutamate receptors has been suggested as a novel approach to treating schizophrenia-related cognitive dysfunction. We examined how the metabotropic glutamate receptor 2/3 agonist LY379268 and the metabotropic glutamate receptor 2/3 antagonist LY341495 altered phencyclidine-induced disruptions in performance in the 5-choice serial reaction time task. This test assesses multiple cognitive modalities characteristically impaired in schizophrenia that are disrupted by phencyclidine administration. Acute LY379268 alone did not affect 5-choice serial reaction time task performance, except for nonspecific response suppression at high doses. Acute LY379268 administration exacerbated phencyclidine-induced disruption of attentional performance in this task, while acute LY341495 did not alter 5-choice serial reaction time task performance during phencyclidine exposure. Chronic LY341495 impaired attentional performance in the 5-choice serial reaction time task by itself, but attenuated phencyclidine -induced excessive timeout responding. The mixed effects of metabotropic glutamate receptor 2/3 agonism and antagonism on cognitive performance under baseline conditions and after disruption with phencyclidine demonstrate that different aspects of cognition may respond differently to a given pharmacological manipulation, indicating that potential antipsychotic or procognitive medications need to be tested for their effects on a range of cognitive modalities. Our findings also suggest that additional mechanisms, besides cortical glutamatergic transmission, may be involved in certain cognitive dysfunctions in schizophrenia.
Keywords: schizophrenia, phencyclidine, metabotropic glutamate receptors, LY379268, LY341495, cognition
1. Introduction
Cognitive dysfunction constitutes a core deficit in schizophrenia (Elvevåg and Goldberg, 2000) that is highly correlated with long-term disability (Green et al., 2004; McGurk and Meltzer, 2000; Sharma and Antonova, 2003). However, the neurobiological mechanisms underlying these deficits remain poorly understood, and currently available medications do not satisfactorily treat this symptom cluster (Mortimer, 1997; Sharma and Antonova, 2003).
Several studies reporting abnormalities in several components of the glutamate signaling system in schizophrenia patients (Deakin et al., 1989; Gao et al., 2000; Kim et al., 1980; Meador-Woodruff et al., 2001) raise the possibility that dysregulated glutamate transmission may be involved in schizophrenia pathology (Konradi and Heckers, 2003; Olney and Farber, 1995; Tamminga, 1998; Tsai and Coyle, 2002). Supporting this hypothesis, blockade of N-methyl-d-aspartate (NMDA) glutamate receptors with noncompetitive NMDA receptor antagonists, such as phencyclidine or its congeners, induces a psychosis-like state in healthy human subjects that replicates all major symptoms of schizophrenia (Halberstadt, 1995; Javitt and Zukin, 1991), including cognitive dysfunction (Krystal et al., 1994; Pradhan, 1984).
Modulation of glutamate transmission using compounds acting at metabotropic glutamate receptors offers a promising way to investigate the role of the glutamatergic system in cognitive schizophrenia symptoms, and may promote the development of novel treatments for these cognitive deficits. Group II metabotropic glutamate receptors, consisting of type 2 and 3 receptors, are predominantly located presynaptically and act as inhibitory autoreceptors (Schoepp, 2001). Metabotropic glutamate receptor 2/3 activation, therefore, decreases glutamate signaling, while metabotropic glutamate receptor 2/3 antagonism increases it. Preliminary clinical trials have demonstrated reductions in positive and negative schizophrenia symptoms after metabotropic glutamate receptor 2/3 agonist treatment, but the effects of metabotropic glutamate receptor 2/3 agonists on cognition in human schizophrenia patients remain unclear (Patil et al., 2007). Existing medications with significant effectiveness on other schizophrenia symptoms, such as the so-called positive symptoms including hallucinations and delusions, do not necessarily ameliorate, and can even aggravate, cognitive dysfunction in schizophrenia (Bilder et al., 1992; Medalia et al., 1988; Mortimer, 1997). Effectiveness on other schizophrenia symptoms therefore cannot be assumed to correlate with beneficial effects on cognition. Some studies in experimental animals reported attenuation of NMDA antagonist-induced cognitive deficits with metabotropic glutamate receptor 2/3 agonists (Moghaddam and Adams, 1998), suggesting that these compounds may represent a promising strategy for the effective treatment of cognitive dysfunction in schizophrenia (Moghaddam, 2004). However, findings have been inconsistent, with other studies finding no improvement of NMDA receptor antagonist-induced cognitive deficits after metabotropic glutamate receptor 2/3 agonist treatment (Ossowska et al., 2000; Schlumberger et al., 2009) or even impairment of baseline cognitive performance by metabotropic glutamate receptor 2/3 agonists (Higgins et al., 2004). Moreover, cognitive enhancement has been observed after treatment with metabotropic glutamate receptor 2/3 antagonists in some studies (Gargiulo et al., 2005; Higgins et al., 2004). The present study therefore further explored the effects of metabotropic glutamate receptor 2/3 manipulation on cognitive performance.
To this end, we examined how metabotropic glutamate receptor 2/3 agonism and antagonism affect performance of rats in the 5-choice serial reaction time task, a test of several cognitive modalities relevant to schizophrenia. The 5-choice serial reaction time task, originally developed as a test of attentional performance (Robbins, 2002), also measures disinhibition of inappropriate responding, allowing the assessment of impulsivity (Evenden 1999; Puumala and Sirviö, 1998). Additional measures provided by this task allow conclusions to be made about speed of processing and compulsivity or cognitive inflexibility (Robbins, 2002). We have developed a repeated phencyclidine administration regimen that induces robust and selective disruptions in 5-choice serial reaction time task performance. These disruptions include impaired attention, disinhibition of impulsive responding, slowed processing speed, and cognitive inflexibility (Amitai et al., 2007; Amitai and Markou 2008, 2009), all of which are cognitive deficits characterizing schizophrenia (Laurent et al., 1999; Morice, 1990; Nelson et al., 1990; Wykes et al., 2000). The disruptions induced in the 5-choice serial reaction time task by repeated phencyclidine administration are sensitive to attenuation with clozapine (Amitai et al., 2007), an atypical antipsychotic with partial effectiveness in cognitive dysfunction in schizophrenia (Meltzer and McGurk, 1999), indicating that this model has predictive validity. In the present study, we examined how the metabotropic glutamate receptor 2/3 agonist LY379268 affected performance in the 5-choice serial reaction time task under baseline conditions. Furthermore, we explored how LY379268 and LY341495, a metabotropic glutamate receptor 2/3 antagonist, affected disruptions in performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration.
2. Materials and methods
2.1 Subjects
One hundred eight male Wistar rats (Charles River Laboratories, Wilmington, MA) were housed two per cage on a 12 h:12 h reverse light-dark cycle (lights off at 8:00 am). Wistar rats were used because their behavior has been extensively characterized in our laboratory (e.g., Markou et al., 1992; Paterson and Markou, 2005) and elsewhere (e.g., Broersen and Uylings, 1999; Slawecki and Roth, 2005), including their performance in the 5-choice serial reaction time task (Amitai et al., 2007; Didriksen and Christensen, 1993).
All behavioral testing was conducted during the animals’ dark cycle. Rats were allowed to reach a body weight of at least 300 g before being restricted to 20 g of food per day (in addition to the food pellets earned during testing) and initiation of behavioral training. Water was available ad libitum at all times except during behavioral testing. Animals were treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council's Guide for the Care and Use of Laboratory Animals. All experiments were approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
2.2 Drugs
LY379268 [(−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate] was custom synthesized according to Monn et al. (1999) and purchased from ANAWA (Wangen, Switzerland). LY341495 [2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl)-3-(xanth-9-yl)propionic acid] was purchased from Tocris Cookson (Ellisville, MO). d-phencyclidine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD). LY379268 was dissolved in sterile water and LY341495 in 0.1 M sodium hydroxide; both drugs were then diluted with 0.9% saline solution. Matched vehicle solutions consisted of saline containing the same amounts of water or sodium hydroxide. Phencyclidine was dissolved in 0.9% saline solution. Drugs were administered by subcutaneous injection in a volume of 2 ml/kg. In the final experiment, LY341495 was administered chronically via subcutaneous osmotic minipumps. The final pH of the LY31495 solution was 8.5 for the solution injected i.p., and approximately 10 for the solution administered subcutaneously via the osmotic minipumps. The relatively high pH of the latter solution was mitigated by the fact that pump contents were released very slowly, exposing the animal to only very small volumes at any given time. Animals were inspected daily, and no signs of tissue damage or irritation was detected at the site of the pump opening. All drug doses are reported as the salt.
2.3 Osmotic minipump implantation and removal s
Rats were anesthetized (1–3% isoflurane/oxygen vapor mixture), and an osmotic minipump was inserted subcutaneously on the back of the animal parallel to the spine, with the flow moderator directed posteriorly. Rats received Alzet model 2ML1 14-day minipumps filled with LY341495 or vehicle. The wound was closed with surgical staples, and an antibacterial ointment was applied to the incision area. On day 14, the minipumps were removed under anesthesia.
2.4 Five-choice serial reaction time task
2.4.1 Apparatus
Training and testing were conducted in operant testing chambers enclosed in sound-attenuating chambers (Med Associates, St. Albans, VT). Each testing chamber contained a curved rear wall with nine contiguous apertures. Metal inserts covered every alternate hole, leaving open holes 1, 3, 5, 7, and 9. A photocell beam located at the entrance of each aperture detected nosepoke responses, and a 3 W stimulus light was located at the rear of each aperture. Food pellets could be delivered automatically into a magazine located in the opposite wall via a food dispenser; a photocell beam detected head entries into the magazine. A computer running MedPC software (Med Associates, St. Albans, VT) controlled the apparatus.
2.4.2 Five-choice serial reaction time task procedure
Animals were allowed to habituate to the operant testing chambers for 20 min on 2 consecutive days. During these sessions, each open aperture and the food magazine were baited with 5–10 food pellets to encourage the rats to explore them. Next, rats were trained to retrieve food rewards from the magazine in three daily 20 min sessions, during which a food pellet was delivered noncontingently into the magazine every 20 s. Preliminary training sessions followed, in which all apertures were illuminated until the rat made a nosepoke response into any aperture, resulting in lights being extinguished in all apertures and a food pellet being delivered into the magazine. A head entry into the magazine initiated an intertrial interval varying from 2–10 s, after which all apertures were illuminated again. Training on the 5-choice serial reaction time task began once rats retrieved >70 rewards on two consecutive sessions. The training procedure for the 5-choice serial reaction time task was based on the protocol established by Robbins and colleagues (Carli et al., 1983). Briefly, trials were initiated by head entry into the food magazine. An initial noncontingent food pellet was delivered into the magazine at the start of each session to facilitate initiation of the first trial. After a variable intertrial interval ranging from 2–10 s, a light stimulus was presented pseudorandomly in one of the response apertures. A nosepoke in this aperture within a limited hold period (correct response) resulted in the delivery of a food pellet into the magazine. Nosepokes in a wrong aperture (incorrect responses) or failures to respond within the limited hold period (omissions) were punished by a 5 s timeout, marked by extinction of the house light and no delivery of food reward. Nosepokes in any aperture made before presentation of the target stimulus (premature responses) likewise resulted in a timeout and no food reward. Nosepokes during the timeout period (timeout responses) reset the timeout period. Further responses into the apertures after the performance of a correct response, but before reward retrieval from the magazine/initiation of a new trial (perseverative responses), were recorded with no consequence for the animal. Each session lasted 30 min or until 100 trials had been completed, whichever occurred first. Duration of the light stimulus and limited hold period were initially set at 30 s and 60 s, respectively, and gradually decreased over the course of training to a 1 s stimulus duration and a 5 s limited hold. Rats were trained until they had achieved criterion performance (>70% accuracy and <30 omissions) and stable baselines (<10% variation in accuracy over 5 consecutive days). On average, 40 sessions were required for rats to attain criterion performance. The following measures were recorded to assess task performance:
Accuracy: the number of correct responses divided by the sum of correct and incorrect responses [# correct responses/(# correct responses + # incorrect responses) × 100]. Accuracy was only computed if correct + incorrect responses totaled 10 or more. Accuracy is the main measure of attentional performance in this task.
Percent Correct Responses: total number of correct responses divided by the total number of trials.
Percent Incorrect Responses: total number of incorrect responses divided by the total number of trials.
Percent Omissions: total number of omissions divided by total number of trials.
Premature Responses: total number of responses performed during the ITI, before presentation of the light stimulus. Premature responses reflect response disinhibition/impulsivity in this task.
Timeout Responses: total number of responses performed during a timeout period.
Perseverative Responses: nosepoke responses performed after a correct response but before collection of the reward. Perseverative responses are a measure of compulsivity/cognitive inflexibility in this task.
Latency to Correct Response: time from the onset of the light stimulus to the performance of a correct nosepoke response. Latency to correct response reflects processing speed in this task.
Latency to Incorrect Response: time from the onset of the light stimulus to the performance of a correct nosepoke response. This latency measure was assessed in all experiments but is not reported here because it reflected essentially the same drug effects as latency to correct response.
Latency to Reward Retrieval: time from the performance of a correct response to the retrieval of the food reward from the magazine.
Total Trials: total number of trials initiated during the session.
Because manipulations may affect the total number of trials completed, percent correct responses/incorrect responses/omissions are more unbiased measures of performance in the task than absolute number of correct responses, incorrect responses, and omissions.
2.5 Experimental design
2.5.1 Experiment 1: Effects of acute administration of the metabotropic glutamate receptor 2/3 agonist LY379268 on performance in the 5-choice serial reaction time task under baseline conditions
After establishment of stable baseline performance in the 5-choice serial reaction time task (<10% variation in accuracy over 5 consecutive days), 0.3, 1, or 3 mg/kg LY379268 or vehicle was administered to naive rats (n = 6) 30 min before testing in the 5-choice serial reaction time task. All rats received all drug doses according to a within-subjects Latin square design, with at least 4 drug-free days between injections. On injection-free days, rats were tested in the 5-choice serial reaction time task under baseline conditions. Additional drug doses were tested only after rats returned to baseline performance between injections.
2.5.2 Experiment 2: Effects of acute administration of the metabotropic glutamate receptor 2/3 agonist LY379268 on disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration (see Fig. 1A for a diagram of the experimental design)
Figure 1. Diagrams of experimental designs.
Injections and procedures in Experiments 2 and 3 (A) and Experiment 4 (B) are schematically represented; dark line denotes period of pump treatment. PCP: phencyclidine.
This experiment used rats from both Experiment 1 (which had undergone 2 months of drug-free washout before the initiation of Experiment 3; n = 6) and naive rats (n = 14) trained in the 5-choice serial reaction time task procedure until reaching stable baseline performance. Each experimental group contained equal numbers of previously drug-naive rats and rats previously exposed to LY379268. No significant differences were found between naive rats and rats from Experiment 1; therefore, data from the two groups were combined for data analyses. Rats received five consecutive daily injections of 2 mg/kg phencyclidine 30 min before testing in the 5-choice serial reaction time task. This protocol resembled the phencyclidine regimen developed previously, which induced robust and selective schizophrenia-like deficits in the 5-choice serial reaction time task after two phencyclidine injections, followed by 14 drug-free days and then followed by an additional five phencyclidine injections (Amitai et al., 2007). Preliminary studies showed that five consecutive daily phencyclidine injections were sufficient to produce comparable robust and selective cognitive deficits in this test (unpublished observations).
After the second phencyclidine injection, rats were assigned to two groups (n = 10 per group) that did not differ in accuracy, correct responses, incorrect responses, and premature responses both under baseline conditions and during phencyclidine exposure. This procedure was necessitated by the considerable variability in responsiveness to performance disruption with phencyclidine, which cannot be predicted from an animal’s baseline performance. Balancing the groups based on both their performance in the absence of phencyclidine and during phencyclidine exposure ensured that differential responsiveness to phencyclidine would not confound the results. A dose of 1 mg/kg LY379286 was administered to one group 5 min before the third, fourth, and fifth phencyclidine injections; the other group was given vehicle injections at the same time points. This LY379286 dose was chosen because it effectively counteracted behavioral effects of phencyclidine in several other studies (Cartmell et al., 1999; Clark et al., 2002; Galici et al., 2005; Greco et al., 2005; Imre et al., 2006; Lorrain et al., 2003b). After cessation of drug injections, rats were tested daily in the 5-choice serial reaction time task for 3 additional days to assess the return of performance to baseline.
2.5.3 Experiment 3: Effects of acute administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration
The design of this experiment was identical to that of Experiment 2, with the exception that naive rats (n = 10 per group) received 1 mg/kg LY341495 or vehicle before the third, fourth, and fifth phencyclidine administration. This dose of LY341495 was the highest dose that had induced no disruption of performance in the 5-choice serial reaction time task under baseline conditions (i.e., in the absence of phencyclidine exposure) in a previous study in our laboratory under conditions similar to those used here (Semenova and Markou, 2007).
2.5.4 Experiment 4: Effects of chronic administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration (see Fig. 1B for a diagram of the experimental design)
Naive rats were trained in the 5-choice serial reaction time task procedure until reaching stable baseline performance. Rats then received two initial injections of 2 mg/kg phencyclidine, 24 h apart. These two initial injections were necessary to allow balancing of groups based on both their performance in the absence of phencyclidine and during phencyclidine exposure before the start of chronic LY341495 treatment. The rats then were assigned to two groups (n = 11 per group) that were balanced as in Experiments 2 and 3. One group was prepared with 14-day osmotic minipumps delivering 2 mg/kg/day LY341495; the other group received pumps containing vehicle. This dose of LY341495 was chosen based on our previous experience in converting acute bolus doses of atypical antipsychotics to chronic daily doses infused continuously. Our previous studies with clozapine, whose serum half-life is similar to that of LY341495 (~40 min for clozapine and ~30 min for LY341495 after intravenous administration; Ornstein et al., 1998; Ma and Lau, 1998), indicated that a cumulative daily dose approximately twice as large as the highest acute bolus dose that does not induce disruptions in 5-choice serial reaction time task performance was successful in attenuating repeated phencyclidine effects without affecting performance by itself (Amitai et al., 2007). After four initial days of exposure to the LY341495/vehicle-containing minipumps, all rats received five consecutive daily saline injections 30 min before testing in the 5-choice serial reaction time task to habituate them to the injection procedure, followed by five consecutive daily injections with 2 mg/kg phencyclidine 30 min before testing in the task. As with the previous experiments, therefore, rats received two initial phencyclidine injections before the onset of metabotropic glutamate receptor treatment, followed by a number of further repeated phencyclidine administration coinciding with metabotropic glutamate receptor treatment. This design differed from the acute LY341495 experiment only in that the two initial and the later repeated phencyclidine administrations were separated by a longer time period, to allow for chronic LY341495 exposure. During this time period, phencyclidine injections were temporarily discontinued to avoid tolerance to the effects of phencyclidine, while still allowing the investigation of the effects of chronic treatments on the effects of phencyclidine in this task. This phencyclidine administration regimen also matched that used to induce robust, selective cognitive deficits in the 5-choice serial reaction time task in previous studies (Amitai et al., 2007; Amitai and Markou 2008, 2009). By examining the potential of a pharmacological manipulation to prevent phencyclidine effects, this design parallels the prevention of recurrence of a psychotic episode in schizophrenia by antipsychotic treatment. Overall, this design allowed for the comparison of the effects of LY341495 vs. vehicle (between-subjects factor), as well as of phencyclidine vs. saline (within-subjects factor), with all factorial combinations (vehicle/saline, vehicle/phencyclidine, LY341495/saline, LY341495/phencyclidine) explored. The mixed within/between-subjects design was necessitated by the long training times required by the task and the large numbers of animals needed in each group to reduce the considerable variation in the behavioral effects of phencyclidine that is commonly observed. Previous studies in our laboratory using repeated saline injections demonstrated that the repeated injection procedure alone does not induce any changes in any of the task parameters (Amitai et al., 2007; Amitai and Markou 2008, 2009). Pumps were removed on the 14th day of LY341495/vehicle exposure, and rats continued to be tested daily in the 5-choice serial reaction time task for 10 days to assess the return of performance to baseline.
2.6 Data analyses
Data from Experiment 1 were analyzed with one-way repeated-measures analyses of variance (ANOVA) with Drug Dose as the within-subjects factor. Data from Experiments 2–4 were analyzed using two-way mixed-design ANOVAs. In Experiments 2 and 3, average values from the 3 days preceding any drug treatment (“baseline days”) were compared with average values from the 3 days of LY379268/LY341495/vehicle+phencyclidine administration. Drug Challenge (baseline/phencyclidine) was the within-subjects factor, and Pretreatment (LY379268 or LY341495/vehicle) was the between-subjects factor. In Experiment 4, to assess the effects of chronic LY341495 by itself, average values from the 5 days preceding any drug treatment (“baseline days”) were compared with average values obtained during the 9 days of phencyclidine-free pump treatment. Presence of Pump (baseline/pump treatment) was the within-subjects factor, and Pump Content (LY341495/saline) was the between-subjects factor. To assess the effects of chronic LY341495 on performance disruption induced by repeated phencyclidine administration, average values from the 5 days of saline injection during pump treatment were compared with average values from the 5 days of phencyclidine administration during pump treatment. Drug Challenge (phencyclidine/saline) was the within-subjects factor, and Pump Content (LY341495/saline) was the between-subjects factor. The values for timeout responses were further analyzed using a two-way mixed-design ANCOVA, with Drug Challenge (phencyclidine/saline) as the within-subjects factor, Pump Content (LY341495/saline) as the between-subjects factor, and performance during the initial (pre-pump implantation) phencyclidine exposure as the covariate. Where statistically significant effects were found in the ANOVAs and ANCOVAs, post hoc comparisons among means were conducted using Bonferroni tests. The level of significance was set at 0.05. Data were analyzed using the GraphPad Prism statistical package (GraphPad, San Diego, CA).
3. Results
3.1 Experiment 1: Effects of acute administration of the metabotropic glutamate receptor 2/3 agonist LY379268 on performance in the 5-choice serial reaction time task under baseline conditions
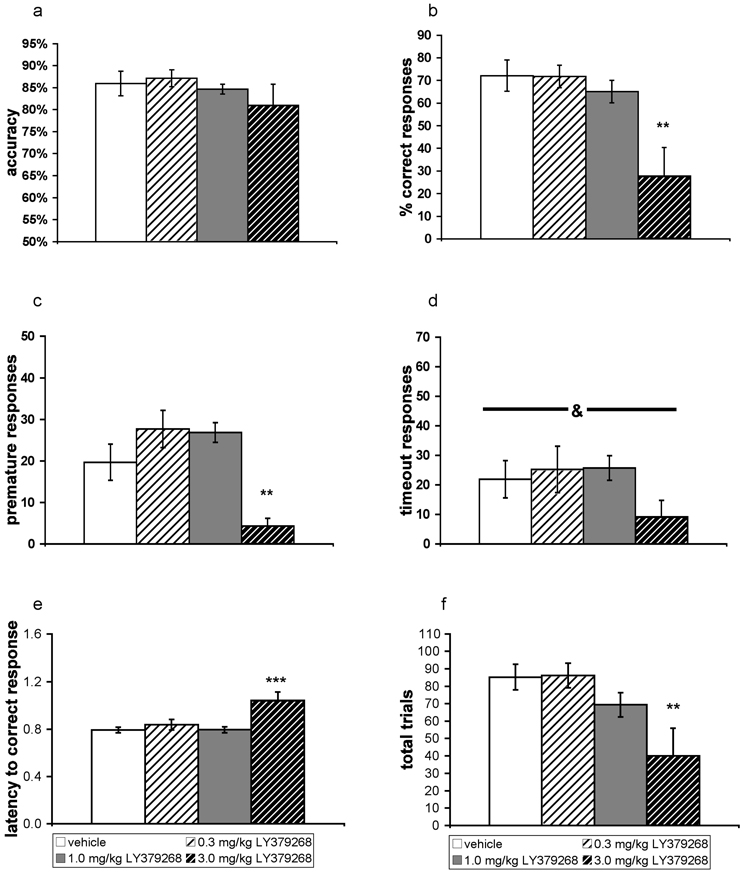
LY379268 did not alter accuracy in the 5-choice serial reaction time task at any dose (Fig. 2A). ANOVAs indicated that LY379268 significantly decreased percent correct responses (F(3,15) = 1.17, P < 0.01; Fig. 2B), premature responses (F(3,15) = 3.23, P < 0.0001; Fig. 2C), timeout responses (F(3,15) = 4.70, P < 0.05; Fig. 2D), and total number of trials initiated (F(3,15) = 1.17, P < 0.01; Fig. 2F). LY379268 also increased percent omissions (F(3,15) = 1.31, P < 0.01; data not shown) and latency to correct response (F(3,15) = 1.17, P < 0.0001; Fig. 3E). Post hoc tests showed that all of these measures were significantly altered only at the highest dose (3 mg/kg). For timeout responses, post hoc tests did not detect significant differences from the vehicle-treated group at any dose. Percent incorrect responses and latency to reward retrieval were unaffected by LY379268 (data not shown).
Figure 2. Effects of acute administration of the metabotropic glutamate receptor 2/3 agonist LY379268 on performance in the 5-choice serial reaction time task under baseline conditions.
Accuracy (A), percent correct responses (B), premature responses (C), timeout responses (D), latency to correct response (E), and total trials (F) are expressed as mean ± SEM. Asterisks (*P < 0.05; **P < 0.01; ***P < 0.001) denote statistically significant differences after LY379268 administration compared with performance after vehicle administration; ampersand (&P < 0.05) denotes significant main effect detected by ANOVA.
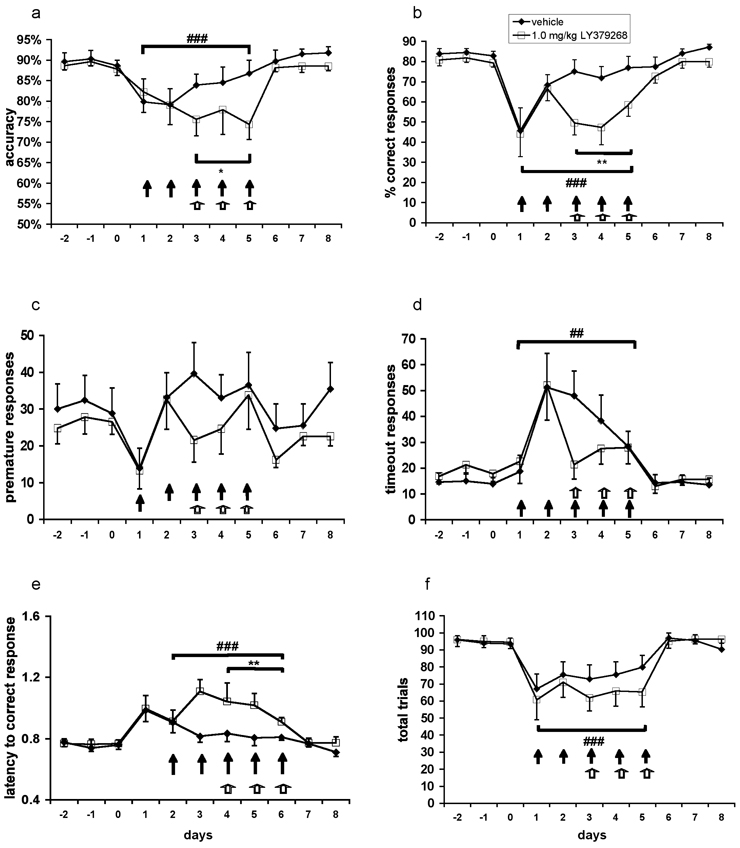
Figure 3. Effects of acute administration of the metabotropic glutamate receptor 2/3 agonist LY379268 on performance in the 5-choice serial reaction time task during repeated phencyclidine administration.
Accuracy (A), percent correct responses (B), premature responses (C), timeout responses (D), latency to correct response (E), and total trials (F) are expressed as mean ± SEM. Pound signs (##P < 0.01; ###P < 0.001) denote statistically significant difference during phencyclidine administration compared with baseline performance; asterisks (*P < 0.05; **P < 0.01) denote statistically significantly difference between the LY379268-treated group compared with the vehicle-treated group; closed arrow (↑) denotes a phencyclidine injection; open arrow (⇧) denotes an LY379268 injection.
Average trials completed were 85.17 ± 7.35 in the vehicle group, 86.17 ± 7.05 in the 0.3 mg/kg LY379268 group, 69.33 ± 6.87 in the 1 mg/kg LY379268 group, and 40.00 ± 15.99 in the 3 mg/kg LY379268 group.
3.2 Experiment 2: Effects of acute administration of the metabotropic glutamate receptor 2/3 agonist LY379268 on disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration (see Table 1 for summary of results)
Table 1.
Summary of effects of repeated phencyclidine and acute metabotropic glutamate receptor 2/3 agonist on performance in the 5-choice serial reaction time task.
| Task measure | Phencyclidine main effect |
LY379268 main effect |
Phencyclidine × LY379268 interaction |
Difference between LY379268 and vehicle groups during phencyclidine exposure (post hoc) |
|---|---|---|---|---|
| accuracy | ⬇ |
 (P = 0.057) (P = 0.057) |
trend (P = 0.053) | P < 0.05 |
| % correct responses | ⬇ | ⬇ | yes | P < 0.01 |
| % incorrect responses | ⬆ | ↔ | no | not significant |
| % omissions | ⬆ | ⬆ | trend (p = 0.051) | P < 0.01 |
| premature responses | ↔ | ↔ | no | not significant |
| timeout responses | ⬆ | ↔ | no | not significant |
| latency to correct response | ⬆ | ⬆ | yes | P < 0.01 |
| latency to reward retrieval | ↔ | ⬆ | no | P < 0.01 |
| total trials | ⬇ | ↔ | no | not significant |
The experimental groups did not differ significantly on any measure before phencyclidine, LY379268, or vehicle administration.
⬇: significant decrease;  : trend toward decrease; ⬆: significant increase; ↔: no change.
: trend toward decrease; ⬆: significant increase; ↔: no change.
Replicating previous work (Amitai et al., 2007; Amitai and Markou 2008, 2009), repeated phencyclidine administration disrupted performance in the 5-choice serial reaction time task in a number of measures. Repeated phencyclidine exposure decreased accuracy (main effect of Drug Challenge: F(1,18) = 16.59, P < 0.001; Fig. 3A), percent correct responses (F(1,18) = 19.26, P < 0.001; Fig. 3B), and total trials (F(1,18) = 26.31, P < 0.0001; Fig. 3F), and increased percent incorrect responses (F(1,18) = 8.65, P < 0.01; data not shown), percent omissions (F(1,18) = 14.45, P < 0.01; data not shown), and timeout responses (F(1,18) = 13.13, P < 0.01; Fig. 3D). Finally, repeated phencyclidine significantly increased latency to correct response (F(1,18) = 21.32, P < 0.001; Fig. 3E), but did not affect latency to reward retrieval (data not shown). The fact that latency to reward retrieval remained unaltered by repeated phencyclidine in this and the following experiments indicates that the observed increases in correct response latency were not due to nonspecific sedative, motivational, or locomotor-impairing effects, and reflected most likely a decrement in speed of processing.
LY379268 exacerbated the disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration in a number of measures. Strong trends toward a main effect of LY379268 (F(1,18) = 4.14, P = 0.057) and a Phencyclidine × LY379268 interaction (F(1,18) = 4.30, P = 0.053) suggest a further decrease in accuracy during phencyclidine treatment in animals pretreated with LY379268 compared with vehicle-pretreated animals. Post hoc tests indicated a significant decrease in accuracy in animals in the LY379268 group compared with those in the vehicle group during phencyclidine exposure only (P < 0.05; Fig. 3A). A significant main effect of LY379268 (F(1,18) = 7.90, P < 0.05) and a significant Phencyclidine × LY379268 interaction (F(1,18) = 4.73, P < 0.05) revealed that LY379268 further suppressed correct responding during phencyclidine exposure, a finding that was confirmed by post hoc tests (P < 0.01; Fig. 3B). A significant main effect of LY379268 to increase percent omissions (F(1,18) = 9.49, P < 0.01) and a strong trend towards a Phencyclidine × LY379268 interaction (F(1,18) = 4.39, P = 0.051) were also observed. Post hoc tests verified that LY379268 further elevated omissions after phencyclidine administration (P < 0.01; data not shown). Furthermore, LY379268 lengthened latency to correct response during phencyclidine exposure compared with vehicle-treated rats (main effect of LY379268: F(1,18) = 5.60, P < 0.05; Phencyclidine × LY379268 interaction: F(1,18) = 9.15, P < 0.01). Post hoc tests during phencyclidine exposure further confirmed these effects (P < 0.01; Fig. 3E). Finally, LY379268 had a significant main effect on latency to reward retrieval (F(1,18) = 8.52, P < 0.01). No Phencyclidine × LY379268 interaction was found, indicating that latencies to reward retrieval were slower in the LY379268 group than in the vehicle group already during drug-free baseline performance, as well as after drug treatment. However, post hoc tests showed that this difference reached significance only after LY379268 administration (P < 0.01), suggesting that LY379268 increased latency to reward retrieval (data not shown). Inspection of the data shows a tendency towards a lesser increase in premature and timeout responses during phencyclidine exposure in LY379268-treated rats compared to vehicle-treated rats; however, no significant main effect of LY379268 and no Phencyclidine × LY379268 interaction were found for these measures (Fig. 3C, D). No main effect of LY379268 and no Phencyclidine × LY379268 interaction were observed for percent incorrect responses or total trials (data not shown).
Average trials completed per group were 94.50 ± 2.95 during baseline and 76.10 ± 7.44 during PCP administration for vehicle-treated rats, and 95.23 ± 3.66 during baseline and 64.37 ± 7.39 during PCP administration for LY379268-treated rats.
3.3 Experiment 3: Effects of acute administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration (see Table 2 for summary of results)
Table 2.
Summary of effects of repeated phencyclidine and acute metabotropic glutamate receptor 2/3 antagonist on performance in the 5-choice serial reaction time task.
| Task measure | Phencyclidine main effect |
LY341495 main effect |
Phencyclidine × LY341495 interaction |
Difference between LY341495 and vehicle groups during phencyclidine exposure (post hoc) |
|---|---|---|---|---|
| accuracy | ⬇ | ↔ | no | not significant |
| % correct responses | ⬇ | ↔ | no | not significant |
| % incorrect responses | ↔ | ↔ | no | not significant |
| % omissions | ⬆ | ↔ | no | not significant |
| premature responses | ⬆ | ↔ | no | not significant |
| timeout responses | ⬆ | ↔ | no | not significant |
| latency to correct response |
 (P = 0.074) (P = 0.074) |
↔ | no | not significant |
| latency to reward retrieval | ↔ | ↔ | no | not significant |
| total trials | ⬇ | ↔ | no | not significant |
The experimental groups did not differ significantly on any measure before phencyclidine, LY341495, or vehicle administration.
⬇: significant decrease; ⬆: significant increase;  : trend toward increase; ↔: no change.
: trend toward increase; ↔: no change.
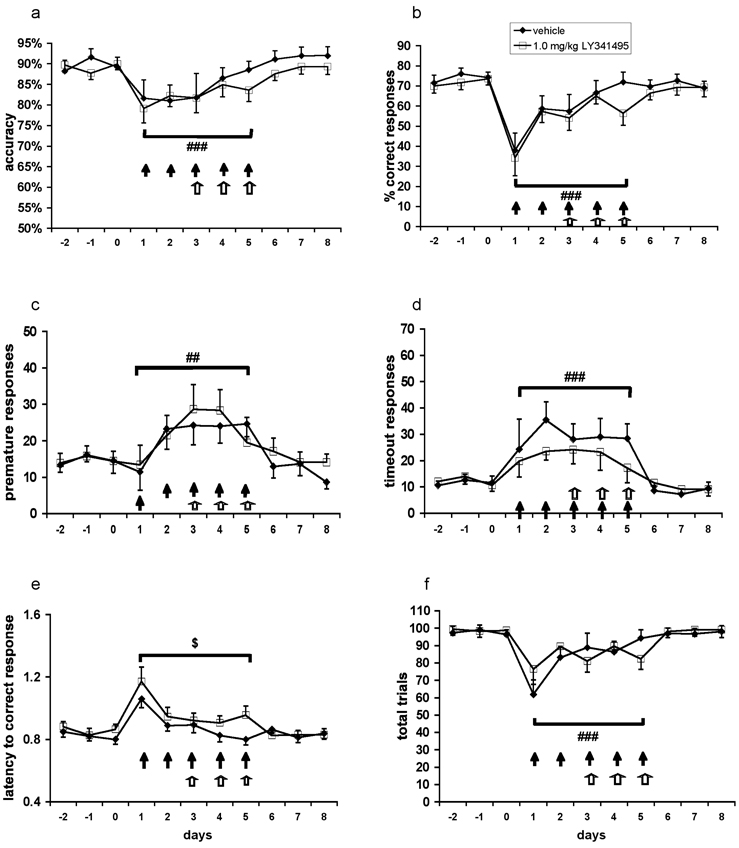
Given that pretreatment with a metabotropic glutamate receptor 2/3 agonist exacerbated phencyclidine-induced disruptions of performance in the 5-choice serial reaction time task, we investigated whether pretreatment with a metabotropic glutamate receptor 2/3 antagonist would ameliorate phencyclidine-induced cognitive deficits. This approach was further supported by reports of pro-cognitive effects of metabotropic glutamate receptor 2/3 antagonists in some studies (Gargiulo et al., 2005; Higgins et al., 2004). Repeated phencyclidine administration significantly decreased accuracy (F(1,18) = 18.64, P < 0.001; Fig. 4A) and percent correct responding (F(1,18) = 20.77, P < 0.001; Fig. 4B) and increased percent omissions (F(1,18) = 9.20, P < 0.01; data not shown), premature responses (F(1,18) = 13.70, P < 0.01; Fig. 4C), and timeout responses (F(1,18) = 18.87, P < 0.001; Fig. 4D). A trend toward increased latency to correct response with phencyclidine administration was also detected (F(1,18) = 3.61, P = 0.074; Fig. 4E). Repeated phencyclidine decreased total trials (F(1,18) = 20.83, P < 0.001; Fig. 4F). Percent incorrect responses and latency to reward retrieval were not affected by repeated phencyclidine administration (data not shown). No main effect of LY341495 and no Phencyclidine × LY341495 interaction were observed for any of the measures.
Figure 4. Effects of acute administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on performance in the 5-choice serial reaction time task during repeated phencyclidine administration.
Accuracy (A), percent correct responses (B), premature responses (C), timeout responses (D), latency to correct response (E), and total trials (F) are expressed as mean ± SEM. Pound signs (##P < 0.01; ###P < 0.001) denote statistically significant difference during phencyclidine administration compared with baseline performance; dollar signs ($P < 0.08) denote a trend toward differences during phencyclidine administration compared with baseline performance; closed arrow (↑) denotes a phencyclidine injection; open arrow (⇧) denotes an LY341495 injection.
Average trials completed per group were 97.57 ± 1.61 during baseline and 89.87 ± 3.93 during PCP administration for vehicle-treated rats, and 98.87 ± 0.69 during baseline and 84.40 ± 4.41 during PCP administration for LY341495-treated rats.
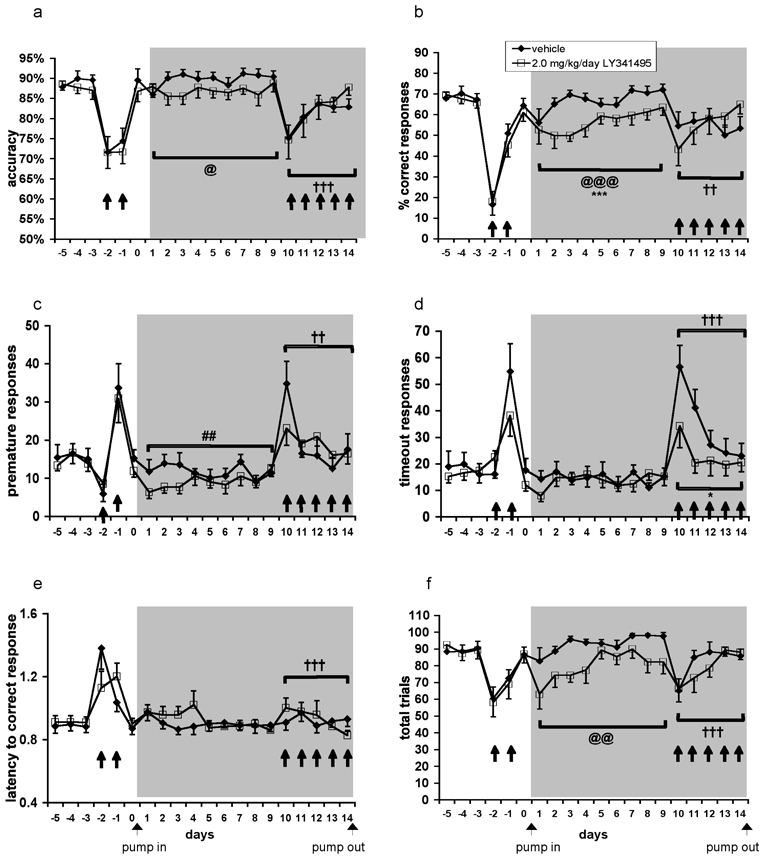
3.4 Experiment 4: Effects of chronic administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on disruptions of performance in the 5-choice serial reaction time task induced by repeated phencyclidine administration (see Table 3 for summary of results)
Table 3.
Summary of effects of chronic metabotropic glutamate receptor 2/3 antagonist on performance in the 5-choice serial reaction time task.
| Task measure | Pump Implantation main effect |
LY341495 main effect |
Implantation × LY341495 interaction |
Difference between LY341495 and vehicle groups during pump exposure in absence of phencyclidine (post-hoc) |
|---|---|---|---|---|
| accuracy | ↔ | ↔ | yes | not significant |
| % correct responses | ⬇ | ↔ | yes | P < 0.01 |
| % incorrect responses | ↔ | ↔ | no | not significant |
| % omissions | ⬆ | ↔ | yes | not significant |
| premature responses | ⬆ | ↔ | no | not significant |
| timeout responses | ↔ | ↔ | no | not significant |
| latency to correct response | ↔ | ↔ | no | not significant |
| latency to reward retrieval | ↔ | ↔ | no | not significant |
| total trials | ↔ | ↔ | yes | not significant |
The experimental groups did not differ significantly on any measure before minipump implantation.
⬇: significant decrease; ⬆: significant increase; ↔: no change.
Because acute pretreatment with a metabotropic glutamate receptor 2/3 antagonist had no effect on phencyclidine-induced disruptions of performance in the 5-choice serial reaction time task, we investigated whether chronic metabotropic glutamate receptor 2/3 antagonist treatment would be more effective at ameliorating phencyclidine-induced cognitive deficits. This possibility was suggested by studies in our laboratory that observed beneficial effects of antipsychotic medications only after chronic administration (Semenova and Markou, 2003). Similarly, the full clinical benefits of antipsychotic medications in schizophrenia are usually seen only after chronic administration (Beckmann et al., 1979; Gelder et al., 2000; Johnstone et al., 1978).
3.4.1 Effects of chronic administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on performance in the 5-choice serial reaction time task under baseline conditions (i.e., in the absence of phencyclidine treatment)
A number of 5-choice serial reaction time task measures reflected effects of Presence of Pump (i.e., performance before vs. after pump implantation) and Pump Content (vehicle or LY341495), suggesting disruption of attentional performance by LY341495 in the absence of phencyclidine exposure. Significant Presence of Pump × Pump Content interactions indicated that LY341495-treated rats had decreased accuracy (F(1,20) = 6.97, P < 0.05; Fig. 5A), percent correct responses (F(1,20) = 17.24, P < 0.001; Fig. 5B), and total trials (F(1,20) = 11.38, P < 0.01; Fig. 5F), as well as increased percent omissions (F(1,20) = 11.54, P < 0.01; data not shown) compared with vehicle-treated rats. Significant main effects of Presence of Pump were also found for percent correct responses (F(1,20) = 25.59, P < 0.0001) and percent omissions (F(1,20) = 28.15, P < 0.0001). Post hoc tests confirmed that LY341495-treated rats had lower percent correct responses than vehicle-treated rats only after pump implantation (Fig. 5B). In contrast, a main effect of Presence of Pump (F(1,20) = 14.97, P < 0.01), but no effect of Pump Content and no Presence of Pump × Pump Content interaction was observed for premature responses. This pattern of results indicates that premature responses decreased after pump implantation regardless of pump content, suggesting a possible effect of postsurgical recovery or continued learning that resulted in reductions in impulsive-like responding (Fig. 5C). No other parameters were altered by treatment with LY341495 or vehicle pumps alone.
Figure 5. Effects of chronic administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 on performance in the 5-choice serial reaction time task during repeated phencyclidine administration.
Accuracy (A), percent correct responses (B), premature responses (C), timeout responses (D), latency to correct response (E), and total trials (F) are expressed as mean ± SEM. Pound signs (##P < 0.01) denote statistically significant differences during phencyclidine administration compared with baseline performance; dagger signs (††P < 0.01; †††P < 0.001) denote statistically significant differences during phencyclidine administration compared with performance after saline injections; asterisks (*P < 0.05; ***P < 0.001) denote statistically significantly differences between the LY341495-treated group compared with the vehicle-treated group; “at” signs (@P < 0.05; @@P < 0.01; @@@P < 0.001) denote a statistically significant Presence of Pump × Pump Content interaction (see section 2.6 “Data analyses” for a definition of the factors analyzed); gray field denotes the period of pump treatment; closed arrow (↑) in the graphs denotes a phencyclidine injection; closed arrows (↑) below the abscissa denote pump implantation and removal.
Average trials completed per group were 88.22 ± 3.69 during baseline and 93.27 ± 1.63 during pump treatment for vehicle-treated rats, and 89.69 ± 4.08 during baseline and 80.44 ± 3.74 during pump treatment for LY341495-treated rats.
3.4.2 Effects of chronic administration of the metabotropic glutamate receptor 2/3 antagonist LY341495 and repeated phencyclidine administration on performance in the 5-choice serial reaction time task
Replicating previous work (Amitai et al., 2007; Amitai and Markou 2008, 2009), repeated phencyclidine significantly decreased accuracy (F(1,20) = 19.13, P < 0.001; Fig. 5A) and percent correct responses (F(1,20) = 11.45, P < 0.01; Fig. 5B), increased percent incorrect responses (F(1,20) = 11.45, P < 0.01; data not shown), percent omissions (F(1,20) = 5.16, P < 0.05; data not shown), premature responses (F(1,20) = 11.45, P < 0.01; Fig. 5C), and timeout responses (F(1,20) = 52.04, P < 0.0001; Fig. 5D), and decreased total trials (F(1,20) = 14.99, P < 0.001; Fig. 5F).
ANOVA and post hoc test (P < 0.05) detected fewer timeout responses in LY341494-treated rats (Phencyclidine × LY341495 interaction: F(1,20) = 7.56, P < 0.05) compared with vehicle-treated rats during phencyclidine exposure (Fig. 5D). Given that the LY341495 group exhibited lower levels of timeout responding compared to the vehicle-treated group even before pump treatment, the data was re-analyzed using a two-way mixed-design ANOCOVA using the performance during the initial phencyclidine exposure, before pump implantation, as a covariate. The Phencyclidine × LY341495 interaction remained significant (F(1,19) = 5.48, P < 0.05). No main effect of LY341495 and no Phencyclidine × LY341495 interaction were detected on any other measures.
Average trials completed per group were 95.65 ± 1.13 during saline administration and 82.33 ± 3.38 during phencyclidine administration for vehicle-treated rats, and 85.87 ± 4.36 during saline administration and 79.11 ± 4.72 during phencyclidine administration for LY341495-treated rats.
4. Discussion
Replicating previous findings (Amitai et al., 2007; Amitai and Markou 2008, 2009), phencyclidine-induced disruptions included decreased accuracy and percent correct responses and increased percent omissions (indicating impaired attention), increased premature responses (increased impulsivity), increased timeout responses (cognitive inflexibility), and increased latency to correct response (slowed processing speed) (Fig. 3–5) in the 5-choice serial reaction time task. The metabotropic glutamate receptor 2/3 agonist LY379268 coadministered with phencyclidine further decreased accuracy and percent correct responses (Fig. 3A, B) and augmented the increases in percent omissions and latency to correct response compared with those seen after phencyclidine administration alone (Fig. 3E).
The finding that the metabotropic glutamate receptor 2/3 agonist LY379268 exacerbated cognitive disruptions induced by repeated phencyclidine administration is surprising in light of previous studies showing that systemic administration of metabotropic glutamate receptor 2/3 agonists ameliorated NMDA receptor antagonist-induced behavioral disruptions (Cartmell et al., 1999, 2000; Chartoff et al., 2005; Clark et al., 2002; Galici et al., 2005; Imre et al., 2006; Lorrain et al., 2003b; Rorick-Kehn et al., 2007; Swanson and Schoepp, 2002; however, see Henry et al., 2002), including cognitive deficits (Greco et al., 2005; Krystal et al., 2005; Moghaddam and Adams, 1998). The disruptive effects of LY379268 observed in our study are unlikely to be due to excessively high doses of LY379268, given that a dose of at least 1 mg/kg was the minimal dosage that effectively counteracted other behavioral phencyclidine effects, such as hyperlocomotion, in other studies (Cartmell et al., 1999; Clark et al., 2002; Galici et al., 2005; Greco et al., 2005; Imre et al., 2006; Lorrain et al., 2003b; Schlumberger et al., 2009), and this dose did not disrupt performance in the 5-choice serial reaction time task by itself (Fig. 2). It is possible that metabotropic glutamate receptor 2/3 agonists may ameliorate NMDA receptor antagonist-induced disruption of some cognitive modalities, but not others. This hypothesis is supported by reports that metabotropic glutamate receptor 2/3 agonists improved working memory disrupted by NMDA receptor antagonists (Krystal et al., 2005; Moghaddam and Adams, 1998; but see Ossowska et al., 2000; Schlumberger et al., 2009) but not NMDA receptor antagonist-induced deficits in attention (Greco et al., 2005; present study), preattentional gating (Galici et al., 2005; Henry et al., 2002; Imre et al., 2006; Ossowska et al., 2000; Schlumberger et al., 2009; Schreiber et al., 2000), verbal memory (Krystal et al., 2005), or passive avoidance (Schlumberger et al., 2009). LY379268 also attenuated phencyclidine-induced increases in premature responding in the 5-choice serial reaction time task in mice (Greco et al., 2005) and tended (albeit not significantly) to decrease phencyclidine-induced increases in premature responding in the present study (Fig. 3C). Thus, schizophrenia-like impulsivity may be more responsive to attenuation by metabotropic glutamate receptor 2/3 agonists than phencyclidine-induced attentional deficits.
Nonspecific behavioral disruption induced by phencyclidine or LY379268, such as sedation, ataxia, or hyperlocomotion, may confound the results of cognitive tests. Acute phencyclidine administration can produce significant ataxia in animals; however, repeated administration results in profound reduction or even elimination of this ataxia (Amitai et al., 2007; Melnick et al., 2002; Podhorna and Didriksen, 2005). Indeed, while repeated phencyclidine increased the latency to correct response in our test (Fig. 3–5), it did not affect latency to reward retrieval (Table 1–Table 3), suggesting that the effects on correct latency were due to reduced processing speed, not a general locomotor impairment. Similarly, the pattern of results observed for response and retrieval latencies suggests that the effects of repeated phencyclidine on 5-choice serial reaction time task performance were not simply due to phencyclidine-induced hyperlocomotion. While the number of trials completed was reduced by repeated phencyclidine (Fig. 3–5), the unchanged latencies to reward retrieval suggest that this effect is unlikely to reflect a significant loss of motivation. Instead, the significant increase in premature and timeout responses during phencyclidine exposure, which greatly increased the time it took the animals to complete a given trial, may explain the smaller number of trials completed during the test sessions. Moreover, the finding that phencyclidine significantly decreased accuracy means that correct responses were reduced significantly more than incorrect responses. This selective effect on correct responding indicates that specific quantifiable cognitive deficits were induced by phencyclidine, even if some nonspecific behavioral disruptions were present at the same time. LY379268 alone reduced the number of trials completed and increased latency to reward retrieval only at the highest dose (3 mg/kg; Fig. 2F). When co-administered with phencyclidine, a lower dose of LY379268 (1 mg/kg) tended to increase latency to reward retrieval (Table 2). While this dose did not affect reward retrieval latencies by itself, it is possible that interactions between LY379268 and phencyclidine may have produced some locomotor disruption, which may have contributed to the effects of LY379268 during phencyclidine exposure.
A possible mechanism that may underlie cognitive deficits induced by NMDA antagonists is their action on glutamate transmission in the prefrontal cortex. Profound increases in extracellular glutamate levels in the prefrontal cortex have been observed after NMDA antagonist administration (Abekawa et al., 2003; Adams and Moghaddam, 1998, 2001; Moghaddam et al., 1997), including the phencyclidine regimen used in this study (Amitai et al., 2008). Despite the presence of noncompetitive NMDA receptor blockade, these increased glutamate levels may result in increased glutamate neurotransmission through non-NMDA glutamate receptors in the prefrontal cortex (Mathe et al., 1998; Moghaddam et al., 1997). Given the crucial involvement of the prefrontal cortex in many aspects of cognition (Fuster, 1973; Robbins, 1996), including cognitive deficits in schizophrenia patients (Weinberger et al., 1986), these changes in prefrontal glutamate transmission have been suggested to underlie the schizophrenia-like cognitive deficits induced by phencyclidine (Anand et al., 2000; Moghaddam et al., 1997). Systemic treatment with metabotropic glutamate receptor 2/3 agonists attenuates NMDA receptor antagonist-induced increases in prefrontal cortex glutamate (Lorrain et al., 2003a; Moghaddam and Adams, 1998). Therefore, treatment with a metabotropic glutamate receptor 2/3 agonist might be expected to attenuate schizophrenia-like cognitive deficits. However, our observation that LY379268 aggravates some NMDA receptor antagonist-induced cognitive deficits suggests that additional mechanisms may be involved in NMDA receptor antagonist-induced cognitive dysfunction. It should be noted that the effects of the drug treatments used in this study on glutamate transmission are highly complex. The administration of phencyclidine reduces transmission through NMDA receptors, but increases glutamate release in the prefrontal cortex, thus increasing transmission through non-NMDA glutamate receptors. The administration of LY379268 increases the activity of metabotropic glutamate receptors type 2/3, but decreases overall glutamate levels, and thus transmission through the remaining glutamate receptors. It is possible that metabotropic glutamate receptor 2/3 agonists will show effectiveness against schizophrenia-like cognitive disruptions if such deficits are induced by a different inducing condition than NMDA receptor blockade.
Secondary changes in dopamine neurotransmission have been widely suggested as a primary mechanism mediating the schizophrenia-like effects of NMDA receptor blockade (Carlsson and Carlsson, 1990; Kornhuber et al., 1990; Steinpreis et al., 1996; but see Adams and Moghaddam, 1998). Moreover, phencyclidine may directly interact with dopamine receptors (Kapur and Seeman, 2002). NMDA receptor antagonists significantly increase dopamine efflux in several brain areas, including the prefrontal cortex, striatum, hippocampus, and amygdala (Deutch et al., 1987; Hertel et al., 1995; Hondo et al., 1994; Rao et al., 1990; Whitton et al., 1992a,b). Excessive dopamine levels in the prefrontal cortex disrupt cognition (Arnsten and Goldman-Rakic, 1998; Murphy et al., 1996). Metabotropic glutamate receptor 2/3 agonists do not affect (Lorrain et al., 2003a; Moghaddam and Adams, 1998) or exacerbate (Maeda et al., 2003) NMDA receptor antagonist-induced dopamine increases in the prefrontal cortex. This phenomenon may explain why pretreatment with LY379268 aggravated phencyclidine-induced attentional deficits in the present study. Moreover, excessive dopamine levels induced by NMDA receptor blockade in other brain areas besides the prefrontal cortex, such as the striatum, may also be involved in the effects of phencyclidine. Given that studies found no increase in glutamate levels in the striatum after phencyclidine administration (Lillrank et al., 1994a,b; Yamamoto et al., 1999), any role this brain area plays in phencyclidine-induced cognitive disruptions is likely to be primarily mediated by alterations in dopamine, not glutamate, transmission at this site. Selective manipulation of glutamatergic transmission by metabotropic glutamate receptor 2/3 agonists may therefore not be able to attenuate these deficits.
NMDA receptor antagonists also strongly elevate extracellular serotonin levels in the prefrontal cortex (Martin et al., 1998). Because excessive serotonin efflux in the prefrontal cortex can disrupt cognition (Santucci et al., 1996), this phenomenon may constitute a further mechanism mediating phencyclidine-induced cognitive deficits. The effects of metabotropic glutamate receptor 2/3 agonists on NMDA receptor antagonist-induced increases in serotonin in the prefrontal cortex are not currently known. If selective manipulation of metabotropic glutamate receptors with LY379268 does not attenuate phencyclidine-induced serotonin efflux, such lack of an effect on serotonin may also explain the lack of ameliorative effects observed in this study.
Selective tolerance and sensitization may play a role in the development of the profile of disruptions induced by repeated phencyclidine administration. For example, the ataxia induced by acute phencyclidine exposure is reduced or even eliminated after repeated administration, while cognitive impairments such as memory deficits or attentional disruption are preserved (Amitai et al., 2007; Melnick et al., 2002; Podhorna and Didriksen, 2005). Conversely, we observed increases in premature and timeout responses in the 5-choice serial reaction time test only after repeated phencyclidine administration; the initial phencyclidine injection usually did not alter these measures or even tended to suppress these types of responses (Fig. 3C, D; Fig. 4 C, D; Fig 5 C, D; Amitai et al., 2007; Amitai and Markou 2008, 2009). The development of selective tolerance of certain phencyclidine-induced disruptions may depend on repeated exposure to phencyclidine-induced neural changes, such as increases in prefrontal glutamate release. By blocking these neural changes, administration of a metabotropic glutamate receptor 2/3 agonist may preclude the development of selective tolerance. Inspection of our data suggests that the attentional deficits induced by phencyclidine in Experiment 2 lessened during the later injections (Fig. 3A, B); the exacerbation of these symptoms by LY379268 may be due to prevention of tolerance against phencyclidine-induced attentional disruption.
In contrast to the exacerbation of phencyclidine-induced deficits with LY379268, acute pretreatment with the metabotropic glutamate receptor 2/3 antagonist LY341495 did not affect phencyclidine-induced disruptions in performance in the 5-choice serial reaction time task (Fig. 4). However, chronic treatment with LY341495 decreased accuracy and percent correct responses under baseline conditions (Fig. 5A, B) and increased percent omissions. LY341495 did not affect the number of trials completed or the latency to reward retrieval at any dose, whether given acutely or chronically. It is therefore unlikely that nonspecific locomotor disruption produced any of the observed effects of LY341495.
Metabotropic glutamate receptor 2/3 antagonists increase glutamate efflux and prefrontal cortex serotonin levels (Kawashima et al., 2005), effects that may underlie the cognitive deficits induced by these compounds. However, other studies reported that metabotropic glutamate receptor 2/3 antagonists improved acquisition of a spatial memory task and visual discrimination (Gargiulo et al., 2005; Higgins et al., 2004), again showing that different cognitive modalities are differentially affected by a given compound. Previous studies have reported exacerbation of phencyclidine-induced hyperlocomotion by LY341495 (Rorick-Kehn et al., 2007), but few studies have examined the effects of LY341495 or other metabotropic glutamate receptor 2/3 antagonists on cognitive disruptions induced by phencyclidine or other NMDA receptor antagonists. Remarkably, we found that chronic LY341495 partially, but significantly, attenuated the increase in timeout responses during exposure to repeated phencyclidine (Fig. 5D). The brain areas involved in the control of timeout responding in the 5-choice serial reaction time task are not yet known. In brain areas where no increased glutamate release after phencyclidine administration has been found, such as the striatum (Lillrank et al., 1994a,b; Yamamoto et al., 1999), the predominant effect of phencyclidine may be the reduction of NMDA glutamate transmission. Increased glutamate release induced by LY341495 could serve to overcome this reduction in NMDA glutamate transmission through actions at non-NMDA glutamate receptors, and thus beneficially affect cognitive functions controlled by these other brain sites. Furthermore, although LY341495 is predominantly selective for metabotropic glutamate receptors 2/3, it also interacts with all other metabotropic glutamate receptors at higher doses, including antagonism of metabotropic glutamate receptor 8 at submicromolar doses (Kingston et al., 1998). Interactions with other metabotropic glutamate receptors, therefore, may also play a role in the effects of LY341495 observed in the present study. The fact that chronic LY341495 attenuated phencyclidine-induced increases in timeout, but not premature, responding in the 5-choice serial reaction time task is particularly interesting when considering our previous finding that chronic clozapine attenuated phencyclidine-induced increases in premature, but not timeout, responses (Amitai et al., 2007). This double dissociation suggests that these different types of disinhibition of inappropriate responding can be differentially sensitive to pharmacological treatment and may be attributable to different underlying mechanisms.
Notably, the compounds investigated in the present study exhibited differing and even opposite effects on different cognitive modalities, such as attention and impulsivity. These findings suggest that compounds cannot simply be described as either “procognitive” or “cognitive-disruptive” and that cognitive performance level (normal or disrupted), as well as the specific cognitive modality investigated, may greatly influence what effect a given pharmacological manipulation will have. This consideration suggests that potential novel medications need to be evaluated carefully for their effects on a range of cognitive measures, both at baseline and after performance disruption, and that testing of any single cognitive modality will likely not suffice to properly assess the therapeutic potential of a compound.
4.1 Conclusions
Neither agonism nor antagonism at metabotropic glutamate receptors 2/3 improved cognitive performance under baseline conditions in our study. While pretreatment with a metabotropic glutamate receptor 2/3 agonist exacerbated phencyclidine-induced schizophrenialike cognitive deficits, chronic treatment with a metabotropic glutamate receptor 2/3 antagonist produced a complex profile characterized by disruption of some cognitive measures under baseline conditions, but attenuation of phencyclidine disruption of other measures. These findings indicate that the effects of prefrontal cortex glutamate signaling alone cannot explain all of the cognitive disruptions induced by phencyclidine. Other mechanisms involved in NMDA receptor antagonist-induced cognitive dysfunction may include prefrontal cortex dopamine and serotonin transmission, as well as glutamate and monoamine transmission in subcortical brain sites.
Table 4.
Summary of effects of repeated phencyclidine and chronic metabotropic glutamate receptor 2/3 antagonist on performance in the 5-choice serial reaction time task.
| Task measure | Phencyclidine main effect |
LY341495 main effect |
Phencyclidine × LY341495 interaction |
Difference between LY341495 and vehicle groups during phencyclidine exposure (post hoc) |
|---|---|---|---|---|
| accuracy | ⬇ | ↔ | no | not significant |
| % correct responses | ⬇ | ↔ | no | not significant |
| % incorrect responses | ⬆ | ↔ | no | not significant |
| % omissions | ⬆ | ↔ | no | not significant |
| premature responses | ⬆ | ↔ | no | not significant |
| timeout responses | ⬆ | ↔ | yes | P < 0.05 |
| latency to correct response | ↔ | ↔ | no | not significant |
| latency to reward retrieval | ↔ | ↔ | no | not significant |
| total trials | ⬇ | ↔ | no | not significant |
The experimental groups did not differ significantly on any measure before minipump implantation and phencyclidine administration.
⬇: significant decrease; ⬆: significant increase; ↔: no change.
Acknowledgments
The authors would like to thank Mr. Michael Arends for excellent editorial assistance.
Role of the funding source
Supported by National Institute of Mental Health grant R01MH062527 to AM and Tobacco-Related Disease Research Program (TRDRP) Individual Pre-doctoral Fellowship 15DT-0048 from the State of California to NA. The funding sources had no input on the research design, data analyses or interpretation, or the writing of the manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abekawa T, Honda M, Ito K, Koyama T. Effects of NRA0045, a novel potent antagonist at dopamine D4, 5-HT2A, and α1 adrenaline receptors, and NRA0160, a selective D4 receptor antagonist, on phencyclidine-induced behavior and glutamate release in rats. Psychopharmacology (Berl.) 2003;169:247–256. doi: 10.1007/s00213-003-1517-8. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol. Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Increased impulsivity and disrupted attention induced by repeated phencyclidine are not attenuated by chronic quetiapine treatment. Pharmacol. Biochem. Behav. 2008 doi: 10.1016/j.pbb.2008.08.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology (Berl.) 2009;202:275–286. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl.) 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Amitai N, Kuczenski R, Markou A. Role of glutamate and metabotropic glutamate receptors in schizophrenia-like cognitive deficits induced by phencyclidine in rats. Neuropharmacology. 2008;55:585–586. [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-d-aspartate receptor antagonists. Arch. Gen. Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Beckmann B, Hippius H, Ruther E. Treatment of schizophrenia. Prog. Neuropsychopharmacol. 1979;3:47–52. doi: 10.1016/0364-7722(79)90068-7. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Lieberman JA, Kim Y, Alvir JM, Reiter G. Methylphenidate and neuroleptic effects on oral word production in schizophrenia. Neuropsychiatry Neuropsychol. Behav. Neurol. 1992;5:262–271. [Google Scholar]
- Broersen LM, Uylings HB. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 1999;94:47–57. doi: 10.1016/s0306-4522(99)00312-7. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats: implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J. Pharmacol. Exp. Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Attenuation of specific phencyclidine-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology (Berl.) 2000;148:423–429. doi: 10.1007/s002130050072. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Clark M, Johnson BG, Wright RA, Monn JA, Schoepp DD. Effects of the mGlu2/3 receptor agonist LY379268 on motor activity in phencyclidine-sensitized rats. Pharmacol. Biochem. Behav. 2002;73:339–346. doi: 10.1016/s0091-3057(02)00848-1. [DOI] [PubMed] [Google Scholar]
- Deakin JFW, Slater P, Simpson MDC, Gilchrist AC, Skan WJ, Royston MC, Reynolds GP, Cross AJ. Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J. Neurochem. 1989;52:1781–1786. doi: 10.1111/j.1471-4159.1989.tb07257.x. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Tam SY, Freeman AS, Bowers MB, Jr, Roth RH. Mesolimbic and mesocortical dopamine activation induced by phencyclidine: contrasting pattern to striatal response. Eur. J. Pharmacol. 1987;134:257–264. doi: 10.1016/0014-2999(87)90356-6. [DOI] [PubMed] [Google Scholar]
- Didriksen M, Christensen AV. Differences in performance in three strains of rats in a 5-choice serial reaction time task. Pharmacol. Toxicol. 1993;72:66–68. doi: 10.1111/j.1600-0773.1993.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl.) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J. Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J. Pharmacol. Exp. Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-d-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am. J. Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Gargiulo PA, Acerbo MJ, Krug I, Delius JD. Cognitive effects of dopaminergic and glutamatergic blockade in nucleus accumbens in pigeons. Pharmacol. Biochem. Behav. 2005;81:732–739. doi: 10.1016/j.pbb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Gelder MG, López-Ibor JJ, Andreasen N. New Oxford textbook of psychiatry. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Greco B, Invernizzi RW, Carli M. Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology (Berl.) 2005;179:68–76. doi: 10.1007/s00213-004-2127-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. The phencyclidine-glutamate model of schizophrenia. Clin. Neuropharmacol. 1995;18:237–249. doi: 10.1097/00002826-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments phencyclidine effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–1209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Hertel P, Mathé JM, Nomikos GG, Iurlo M, Mathé AA, Svensson TH. Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav. Brain Res. 1995;72:103–114. doi: 10.1016/0166-4328(96)00138-6. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, Adam G, Woltering T, Nakanishi S, Mutel V. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46:907–917. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, Tashiro N. Effect of phencyclidine on dopamine release in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 1994;633:337–342. doi: 10.1016/0006-8993(94)91558-x. [DOI] [PubMed] [Google Scholar]
- Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol. Biochem. Behav. 2006;84:392–399. doi: 10.1016/j.pbb.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry. 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Carney MW, Price JS. Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. Lancet. 1978;1:848–851. doi: 10.1016/s0140-6736(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol. Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Karasawa J, Shimazaki T, Chaki S, Okuyama S, Yasuhara A, Nakazato A. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci. Lett. 2005;378:131–134. doi: 10.1016/j.neulet.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmüller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol. Ther. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Riederer P, Beckmann H. Neuropsychopharmacology. Berlin: Springer; 1990. The dopaminergic and glutamatergic systems in schizophrenia; pp. 714–720. [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl.) 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, O'Connor WT, Oja SS, Ungerstedt U. Systemic phencyclidine administration is associated with increased dopamine, GABA, and 5-HIAA levels in the dorsolateral striatum of conscious rats: an in vivo microdialysis study. J. Neural Transm. Gen. Sect. 1994a;95:145–155. doi: 10.1007/BF01276433. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, O'Connor WT, Saransaari P, Ungerstedt U. In vivo effects of local and systemic phencyclidine on the extracellular levels of catecholamines and transmitter amino acids in the dorsolateral striatum of anaesthetized rats. Acta Physiol. Scand. 1994b;150:109–115. doi: 10.1111/j.1748-1716.1994.tb09667.x. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-d-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003a;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Schaffhauser H, Campbell UC, Baccei CS, Correa LD, Rowe B, Rodriguez DE, Anderson JJ, Varney MA, Pinkerton AB, Vernier JM, Bristow LJ. Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology. 2003b;28:1622–1632. doi: 10.1038/sj.npp.1300238. [DOI] [PubMed] [Google Scholar]
- Ma F, Lau CE. Determination of clozapine and its metabolite, N-desmethylclozapine, in serum microsamples by high-performance liquid chromatography and its application to pharmacokinetics in rats. J. Chromatogr. B. Biomed. Sci. Appl. 1998;712:193–198. doi: 10.1016/s0378-4347(98)00148-0. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Okauchi T, Semba J. Different roles of group I and group II metabotropic glutamate receptors on phencyclidine-induced dopamine release in the rat prefrontal cortex. Neurosci. Lett. 2003;336:171–174. doi: 10.1016/s0304-3940(02)01261-2. [DOI] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology (Berl.) 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- Martin P, Carlsson ML, Hjorth S. Systemic phencyclidine treatment elevates brain extracellular 5-HT: a microdialysis study in awake rats. Neuroreport. 1998;9:2985–2988. doi: 10.1097/00001756-199809140-00012. [DOI] [PubMed] [Google Scholar]
- Mathé JM, Nomikos GG, Schilström B, Svensson TH. Non-NMDA excitatory amino acid receptors in the ventral tegmental area mediate systemic dizocilpine (MK-801) induced hyperlocomotion and dopamine release in the nucleus accumbens. J. Neurosci. Research. 1998;51:583–592. doi: 10.1002/(SICI)1097-4547(19980301)51:5<583::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr. Res. 2000;45:175–184. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Davis KL, Haroutunian V. Abnormal kainate receptor expression in prefrontal cortex in schizophrenia. Neuropsychopharmacology. 2001;24:545–552. doi: 10.1016/S0893-133X(00)00189-5. [DOI] [PubMed] [Google Scholar]
- Medalia A, Gold JM, Merriam A. The effects of neuroleptics on neuropsychological test results of schizophrenics. Arch. Clin. Neuropsychol. 1988;3:249–271. [PubMed] [Google Scholar]
- Melnick SM, Rodriguez JS, Bernardi RE, Ettenberg A. A simple procedure for assessing ataxia in rats: effects of phencyclidine. Pharmacol. Biochem. Behav. 2002;72:125–130. doi: 10.1016/s0091-3057(01)00727-4. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr. Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl.) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr, Wright RA, Johnson BG, Andis SL, Kindston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid ( LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J. Med. Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br. J. Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- Mortimer AM. Cognitive function in schizophrenia: do neuroleptics make a difference? Pharmacol. Biochem. Behav. 1997;56:789–795. doi: 10.1016/s0091-3057(96)00425-x. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TRE. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol. Med. 1990;20:357–365. doi: 10.1017/s0033291700017670. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Ornstein PL, Bleisch TJ, Arnold MB, Kennedy JH, Wright RA, Johnson BG, Tizzano JP, Helton DR, Kallman MJ, Schoepp DD, Hérin M. 2-substituted (2SR)-2-amino-2-((1SR,2SR)-2-carboxycycloprop-1-yl)glycines as potent and selective antagonists of group II metabotropic glutamate receptors. 2. Effects of aromatic substitution, pharmacological characterization, and bioavailability. J. Med. Chem. 1998;41:358–378. doi: 10.1021/jm970498o. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Pietraszek M, Wardas J, Nowak G, Zajaczkowski W, Wolfarth S, Pilc A. The role of glutamate receptors in antipsychotic drug action. Amino Acids. 2000;19:87–94. doi: 10.1007/s007260070037. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl.) 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. Performance of male C57BL/6J mice and Wistar rats in the water maze following various schedules of phencyclidine treatment. Behav. Pharmacol. 2005;16:25–34. doi: 10.1097/00008877-200502000-00003. [DOI] [PubMed] [Google Scholar]
- Pradhan SN. Phencyclidine (phencyclidine): some human studies. Neurosci. Biobehav. Rev. 1984;8:493–501. doi: 10.1016/0149-7634(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Puumala T, Sirviö J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- Rao TS, Kim HS, Lehmann J, Martin LL, Wood PL. Interactions of phencyclidine receptor agonist MK-801 with dopaminergic system: regional studies in the rat. J. Neurochem. 1990;54:1157–1162. doi: 10.1111/j.1471-4159.1990.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. discussion 1470–1471. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl.) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl.) 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Knott PJ, Haroutunian V. Excessive serotonin release, not depletion, leads to memory impairments in rats. Eur. J. Pharmacol. 1996;295:7–17. doi: 10.1016/0014-2999(95)00629-x. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schäfer D, Barberi C, Morè L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav. Pharmacol. 2009;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schreiber R, Lowe D, Voerste A, De Vry J. LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine. Eur. J. Pharmacol. 2000;388:R3–R4. doi: 10.1016/s0014-2999(99)00844-4. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol. Psychiatry. 2003;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 LY341495 on rats' performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia: deficits, functional consequences, and future treatment. Psychiatr. Clin. North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Assessment of sustained attention in ad libitum fed Wistar rats: effects of MK-801. Physiol. Behav. 2005;85:346–353. doi: 10.1016/j.physbeh.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Steinpreis R. The behavioral and neurochemical effects of phencyclidine in humans and animals: some implications for modeling psychosis. Behav. Brain Res. 1996;74:45–55. doi: 10.1016/0166-4328(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Schoepp DD. The group II metabotropic glutamate receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate ( LY379268) and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. J. Pharmacol. Exp. Ther. 2002;303:919–927. doi: 10.1124/jpet.102.038422. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit. Rev. Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Whitton PS, Biggs CS, Pearce BR, Fowler LJ. MK-801 increases extracellular 5-hydroxytryptamine in rat hippocampus and striatum in vivo. J. Neurochem. 1992a;58:1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- Whitton PS, Biggs CS, Pearce BR, Fowler LJ. Regional effects of MK-801 on dopamine and its metabolites studied by in vivo microdialysis. Neurosci. Lett. 1992b;142:5–8. doi: 10.1016/0304-3940(92)90607-9. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Corner J. The prevalence and stability of an executive processing deficit, response inhibition, in people with chronic schizophrenia. Schizophr. Res. 2000;46:241–253. doi: 10.1016/s0920-9964(99)00233-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kakigi T, Maeda K. Intra-striatal phencyclidine inhibits N-methyl-d-aspartic acid-stimulated increase in glutamate levels of freely moving rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23:161–174. doi: 10.1016/s0278-5846(98)00085-2. [DOI] [PubMed] [Google Scholar]