Abstract
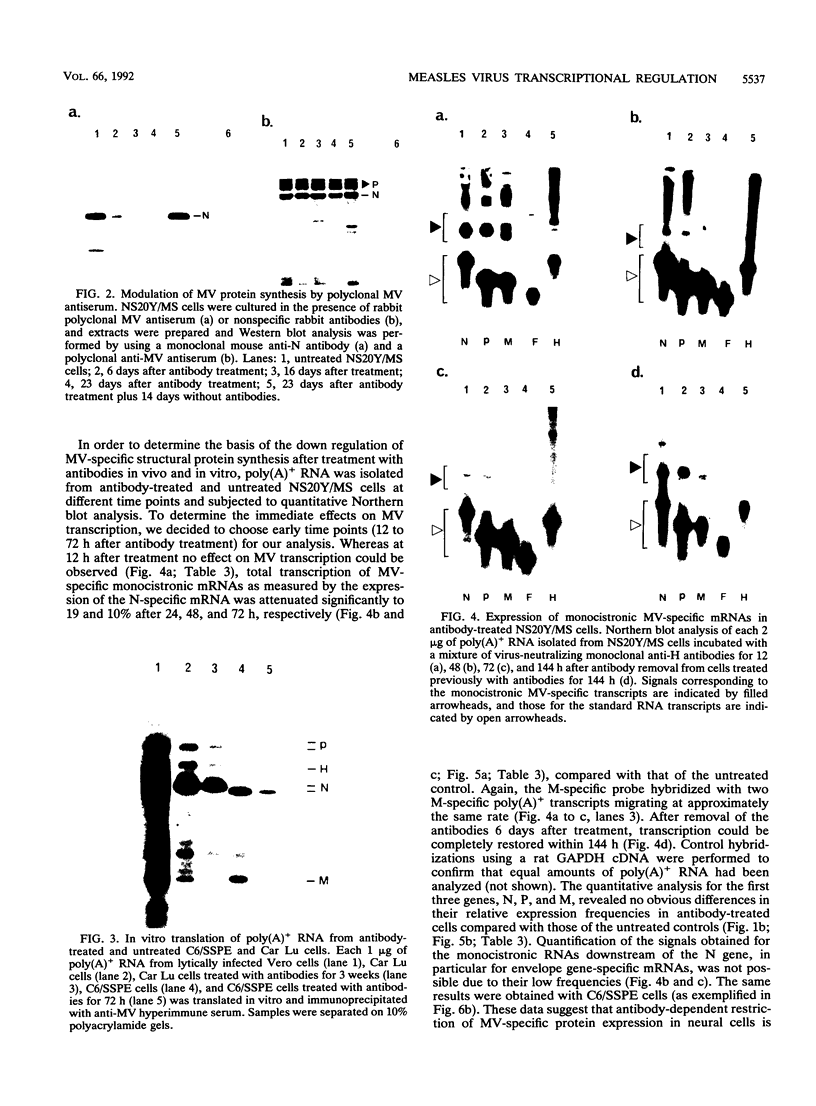
Application of neutralizing anti-hemagglutinin antibodies to mouse neuroblastoma cells (NS20Y/MS) persistently infected with measles virus (MV) leads to a significant reduction of viral structural proteins within 6 days. While the transcriptional gradient for MV-specific mRNAs remained unaffected upon antibody treatment, the total amount of MV-specific transcripts dropped by 80% after 24 h. The expression of genomic RNA was affected similarly, with slightly slower time kinetics. Both transcription and expression of the viral structural proteins could be completely reactivated when viral antibodies were removed from the tissue culture. The same findings could be obtained in rat glioma cells persistently infected with subacute sclerosing panencephalitis virus (C6/SSPE) but not in cells of nonneural origin. The data indicate that antibody-induced antigenic modulation affects the early stages of viral transcription within a few hours after the addition of antibodies and leads to an almost complete repression of viral gene expression in cells of neural origin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Larsen S., Cohn A., Uttenthal A., Race R. E., Aasted B., Hansen M., Bloom M. E. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J Virol. 1989 Jan;63(1):9–17. doi: 10.1128/jvi.63.1.9-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Cattaneo R., Billeter M. A., Roos R. P., ter Meulen V. Restriction of measles virus gene expression in measles inclusion body encephalitis. J Infect Dis. 1988 Jul;158(1):144–150. doi: 10.1093/infdis/158.1.144. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. N., Koschel K., Carter M., ter Meulen V. Effect of measles virus antibodies on a measles SSPE virus persistently infected C6 rat glioma cell line. J Gen Virol. 1985 Jul;66(Pt 7):1411–1421. doi: 10.1099/0022-1317-66-7-1411. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H., Cattaneo R., Billeter M. A. Biased hypermutation of viral RNA genomes could be due to unwinding/modification of double-stranded RNA. Cell. 1989 Feb 10;56(3):331–331. doi: 10.1016/0092-8674(89)90234-1. [DOI] [PubMed] [Google Scholar]
- Birrer M. J., Bloom B. R., Udem S. Characterization of measles polypeptides by monoclonal antibodies. Virology. 1981 Jan 30;108(2):381–390. doi: 10.1016/0042-6822(81)90446-3. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Talbot P. J., Knobler R. L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984 Jan 30;132(2):261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., ter Meulen V. Relationships between monoclonal antibody-binding sites on the measles virus haemagglutinin. J Gen Virol. 1982 Nov;63(Pt 1):113–120. doi: 10.1099/0022-1317-63-1-113. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Baczko K., ter Meulen V., Billeter M. A. Altered ratios of measles virus transcripts in diseased human brains. Virology. 1987 Oct;160(2):523–526. doi: 10.1016/0042-6822(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Schmid A., Baczko K., ter Meulen V., Billeter M. A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987 Mar;6(3):681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Mink M. A., Stec D. S. Rescue of synthetic analogs of respiratory syncytial virus genomic RNA and effect of truncations and mutations on the expression of a foreign reporter gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9663–9667. doi: 10.1073/pnas.88.21.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries R., ter Meulen V. Specificity of IgM antibodies in acute human coxsackievirus B infections, analysed by indirect solid phase enzyme immunoassay and immunoblot technique. J Gen Virol. 1983 Jan;64(Pt 1):159–167. doi: 10.1099/0022-1317-64-1-159. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980 Jul;125(1):78–85. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Halbach M., Koschel K. Impairment of hormone dependent signal transfer by chronic SSPE virus infection. J Gen Virol. 1979 Mar;42(3):615–619. doi: 10.1099/0022-1317-42-3-615. [DOI] [PubMed] [Google Scholar]
- Horikami S. M., Moyer S. A. Synthesis of leader RNA and editing of the P mRNA during transcription by purified measles virus. J Virol. 1991 Oct;65(10):5342–5347. doi: 10.1128/jvi.65.10.5342-5347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Udem S., Rager-Zisman B., Bloom B. R. Isolation of a heterogeneous population of temperature-sensitive mutants of measles virus from persistently infected human lymphoblastoid cell lines. J Exp Med. 1978 Jun 1;147(6):1637–1652. doi: 10.1084/jem.147.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobune K., Kobune F., Yamanouchi K., Nagashima K., Yoshikawa Y., Hayami M. Neurovirulence of rat brain-adapted measles virus. Jpn J Exp Med. 1983 Jun;53(3):177–180. [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991 Nov 8;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., Schneider-Schaulies S., Baczko K., ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990 Feb;64(2):706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet G., Curran J., Roux L. Intracellular stability of nonreplicating paramyxovirus nucleocapsids. Virology. 1990 May;176(1):1–7. doi: 10.1016/0042-6822(90)90223-e. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Horikami S. M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990 Apr;71(Pt 4):775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- Norrby E. A carrier cell line of measles virus in Lu 106 cells. Arch Gesamte Virusforsch. 1967;20(2):215–224. doi: 10.1007/BF01241275. [DOI] [PubMed] [Google Scholar]
- Norrby E., Kristensson K., Brzosko W. J., Kapsenberg J. G. Measles virus matrix protein detected by immune fluorescence with monoclonal antibodies in the brain of patients with subacute sclerosing panencephalitis. J Virol. 1985 Oct;56(1):337–340. doi: 10.1128/jvi.56.1.337-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. H., Huang T., Correia F. F., Krystal M. Rescue of a foreign gene by Sendai virus. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5537–5541. doi: 10.1073/pnas.88.13.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D., Schild G. C., Dimmock N. J. Studies on the mechanism of neutralization of influenza virus by antibody: evidence that neutralizing antibody (anti-haemagglutinin) inactivates influenza virus in vivo by inhibiting virion transcriptase activity. J Gen Virol. 1982 Feb;58(Pt 2):373–386. doi: 10.1099/0022-1317-58-2-373. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Egan J. E., Kress Y., Bloom B. R. Isolation of cold-sensitive mutants of measles virus from persistently infected murine neuroblastoma cells. J Virol. 1984 Sep;51(3):845–855. doi: 10.1128/jvi.51.3.845-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., McFarlin D. E. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981 Apr 16;290(5807):588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- Rataul S. M., Hirano A., Wong T. C. Irreversible modification of measles virus RNA in vitro by nuclear RNA-unwinding activity in human neuroblastoma cells. J Virol. 1992 Mar;66(3):1769–1773. doi: 10.1128/jvi.66.3.1769-1773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima B. K., Lappin S. A., Roberts M. W., Martin S. J. A study of phosphorylation of the measles membrane protein. J Gen Virol. 1981 Oct;56(Pt 2):447–450. doi: 10.1099/0022-1317-56-2-447. [DOI] [PubMed] [Google Scholar]
- Rima B. K. The proteins of morbilliviruses. J Gen Virol. 1983 Jun;64(Pt 6):1205–1219. doi: 10.1099/0022-1317-64-6-1205. [DOI] [PubMed] [Google Scholar]
- Robbins S. J., Rapp F. Inhibition of measles virus replication by cyclic AMP. Virology. 1980 Oct 30;106(2):317–326. doi: 10.1016/0042-6822(80)90255-x. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Baczko K., Cattaneo R., Billeter M., ter Meulen V. Restriction of measles virus gene expression in acute and subacute encephalitis of Lewis rats. Virology. 1989 Aug;171(2):525–534. doi: 10.1016/0042-6822(89)90622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Baczko K., ter Meulen V. Restricted expression of measles virus in primary rat astroglial cells. Virology. 1990 Aug;177(2):802–806. doi: 10.1016/0042-6822(90)90553-4. [DOI] [PubMed] [Google Scholar]
- Vainionpä R., Hyypiä T., Akerman K. E. Early signal transduction in measles virus-infected lymphocytes is unaltered, but second messengers activate virus replication. J Virol. 1991 Dec;65(12):6743–6748. doi: 10.1128/jvi.65.12.6743-6748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann-Dorsch C., Koschel K. Coupling of viral membrane proteins to phosphatidylinositide signalling system. FEBS Lett. 1989 Apr 24;247(2):185–188. doi: 10.1016/0014-5793(89)81330-4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y., Yamanouchi K. Effect of papaverine treatment on replication of measles virus in human neural and nonneural cells. J Virol. 1984 May;50(2):489–496. doi: 10.1128/jvi.50.2.489-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]