Abstract
Bacillus anthracis remains a serious bioterrorism concern, and the currently licensed vaccine remains an incomplete solution for population protection from inhalation anthrax and has been associated with concerns regarding efficacy and safety. Thus, understanding how to generate long lasting protective immunity with reduced immunizations or providing protection through post exposure immunotherapeutics are long sought goals. Through evaluation of a large military cohort, we characterized the levels of antibodies against protective antigen and found that over half of anthrax vaccinees had low levels of in vitro toxin neutralization capacity in their sera. Using solid phase epitope mapping and confirmatory assays, we identified several neutralization-associated humoral epitopes and demonstrated that select anti-peptide responses mediated protection in vitro. Finally, passively transferred antibodies specific for select epitopes provided protection in an in vivo lethal toxin mouse model. Identification of these antigenic regions has important implications for vaccine design and the development of directed immunotherapeutics.
Keywords: anthrax, vaccination, antibodies, protective antigen
Introduction
Bacillus anthracis has been used for over sixty years as a biological weapon. Relative ease of obtaining and growing the bacterium, spore stability, and accidental or deliberate release of anthrax causing human infection and death all make this a high-priority, NIAID category A pathogen [1]. Even with aggressive anti-microbial treatment, inhalation anthrax results in 45-90% mortality [1]. This high mortality rate is likely related both to overwhelming bacterial infection and the effects of the tripartite toxin.
Anthrax toxin is composed of three proteins: protective antigen (PA), lethal factor (LF), and edema factor (EF). Cleavage of PA by a furin-like endoprotease promotes oligomerization and binding of EF and/or LF [1-3]. Lethal toxin (LT) is a zinc-dependent protease that causes macrophage lysis and death in animal models [1, 4]. Edema toxin (ET) is an adenylate cyclase that is also lethal to animals [5] and is able to increase cAMP and impair macrophage phagocytosis [1, 6]. PA serves as a crucial component of both LT and ET and antibodies to PA can provide protection from disease in animals [7, 8]. Indeed, passive transfer of antibodies against the major toxin proteins (PA, LF, and EF) can provide protection against anthrax challenge [7-12].
The current US vaccine (anthrax vaccine absorbed, AVA) is a cell-free filtrate of an attenuated bovine isolate [1, 13, 14], with an onerous immunization schedule until recent evidence that dose reductions were not associated with significant quantitative reductions in anti-PA levels [1, 14, 15]. Animal models have shown that AVA vaccination protects against challenge with nonencapsulated strains [1] but not against fully virulent strains of B. anthracis [14, 16]. Human AVA vaccination results primarily in antibodies to PA [1, 15, 17, 18], but the degree of protection offered by these antibodies, the fine specificity the protective anti-PA response, and the humoral responses generated in real-world vaccination programs have not been fully elucidated.
This study addresses the protective aspects of human humoral immune responses to AVA vaccination. The neutralizing capacity of sera from AVA-vaccinated participants is dissected to determine the extent of active protection and to characterize antibody specificities that represent effective immunity. Anti-PA epitope target specificities are identified and correlated to in vitro neutralization. Additionally, select human anti-peptide responses are characterized as protective via both in vitro and in vivo assays. By identifying the crucial elements of protective anti-PA responses, this work provides insights necessary for the generation of directed immunotherapeutics and refined vaccinations to enhance protective immunity to anthrax. The potential identification of a limited spectrum of antibody specificities for protection may enable more efficient and cost-effective production of passive immunization products, necessary for emergency protection of immunocompromised populations as well as post-exposure treatment scenarios.
Methods
Human Subjects
Vaccinated individuals (n=200) with at least three AVA immunizations participated. Volunteers provided informed consent and information about vaccination, gender, age, and race. One hundred non-vaccinated individuals served as controls. Institutional Review Board approval was obtained from OMRF, OUHSC, and Walter Reed Army Medical Center. Serum and plasma was collected and stored at -20°C.
Standard and peptide-specific ELISAs
Ninety-six well plates were coated with 1 μg/well of rPA (BEI Resources, Manassas, VA) or ≥ 95% pure peptide (GenScript Corporation, Piscataway, NJ). The peptide sequences were: 193NSRKKRSTSAGPTVPDRDN211, 259FESDPYSDFEKVTGRIDKNVSPE281, and 637EADESVVKEAHREVINSST655. Using a standard ELISA, diluted sera was added followed by an anti-human IgG and substrate with appropriate washing between steps. The optical density (OD) was detected and endpoint titer calculated (titer = average OD + 2*SD for controls). The concentration of antibodies to PA was calculated using reference sera AVR801 (BEI Resources, Manassas, VA) containing 109.4 μg/ml of anti-PA serially diluted two-fold [19]. Serum samples were tested at 1:100 and samples that could not be interpolated at this dilution were repeated at 1:10 or 1:1,000.
Lethal Toxin Neutralization
Inhibition of LT activity was performed [20] with a few modifications. RAW264.7 macrophages were plated into flat bottom tissue culture plates and cultured overnight. Serum was diluted 1:100 and incubated with LT (50ng of PA and 50ng of LF). For neutralization with column absorbed fractions, fractions were diluted 1:5. Next 100 μl of the serum/toxin mixture was added. Cells alone, PA only, LF only, or LT served as controls. After a 2 hour incubation, 10 μl of WST-8 (Cell Counting Kit 8, CCK8, Dojindo, Rockville, MD) was added and incubated for 3 hours.
Percent viability was calculated by the formula: (cells incubated with toxin OD ÷ cells incubated without toxin OD) × 100. Sera was considered low if the average viability from three independent experiments was below the neutralization cut-off as determined by the average viability plus two times the standard deviation of the unvaccinated controls (12% viability).
Solid-Phase Peptide Assay
Peptides that were 10 amino acids in length, overlapping by 8, spanning the length of the PA protein (Pub Med: AAA22637) were covalently synthesized onto polyethylene rods [21]. Peptides were incubated with sera and conjugate using a modified ELISA protocol [21] and OD determined. A region was defined as commonly antigenic when the OD for the anti-peptide response was greater than the control group average peptide OD plus two times the SD (average cut-off value: 0.316± 0.063) and more than 50% of the vaccinated individuals were positive.
Column Absorption
Peptides from the furin cleavage, ligand binding, and receptor binding sites were synthesized as multiple antigenic peptides (MAP) by the OUHSC Molecular Biology Core Facility and bound to cyanogen-preactivated Sepharose 4B [21]. To absorb peptide-specific antibodies, sera were re-circulated six times over the beads, eluted with glycine after each passage, and concentrated to a 1 mL volume. Column-absorbed antibodies were tested for reactivity to rPA and the peptides of interest by ELISA and for in vitro toxin neutralization.
Toxin challenge
Peptide-specific antibodies were enriched via column absorption. The amount of IgG was quantified via Nanodrop [spectroscopy at A280] and tested for reactivity with rPA and peptides. Next, 30 μg of antibody was i.p. injected into 6-week-old female A/J mice. Control mice received saline or 30 μg of IgG enriched from a non-vaccinated control. The mice were challenged three hours later with 3 × LD50 of LT (300 μg PA + 125 μg LF, experimentally determined) injected i.p and mortality was recorded. Survival curves and percent survival were generated by using GraphPad Prism. All animal procedures were reviewed and approved by the OMRF IACUC.
Statistics
Non-parametric correlations (Spearman's correlation) tested the significance of the relationship between anti-PA titer and toxin neutralization, age, number of vaccinations, and years post vaccination. An unpaired Student's t test compared the toxin neutralization activity in recently vaccinated individuals to that in individuals more than three years post vaccination. Chi-square tests determined the relationship between the toxin neutralization activity and the presence of peptide-specific antibodies. The effect of pre-incubation with peptide-specific antibodies on in vitro toxin neutralization was evaluated with a paired Student's t test. A Mann-Whitney t test was used to determine the impact of passive transfer upon survival after toxin challenge.
Results
High titers of antibodies to protective antigen follow AVA vaccination
Two hundred human subjects who received the currently licensed anthrax vaccine and consented to have serum samples drawn were used in this study. Number of vaccinations received, average time since the last vaccine dose, and demographics are presented (Table 1). All individuals had received at least three vaccinations as previously deemed necessary for protective immunity [22].
Table 1.
Demographics of AVA vaccinated individuals
| Characteristic | AVA-vaccinated participants (n = 200) |
|---|---|
| Age: | |
| Average (SD) | 33.4 (8.1) |
| Range | 21-60 |
| Gender, No. (%): | |
| Male | 153 (76.5) |
| Female | 47 (23.5) |
| Race, No. (%): | |
| European American | 129 (64.5) |
| African American | 45 (22.5) |
| Hispanic | 12 (6.0) |
| Asian | 8 (4.0) |
| Other | 6 (3.0) |
| Number of vaccinations: | |
| Average (SD) | 5.2 (1.6) |
| Median | 5 |
| Range | 3 - 12 |
| Years post vaccination: | |
| Average (SD) | 2.3 (1.4) |
| Median | 2.3 |
| Range | 7 days – 7 years |
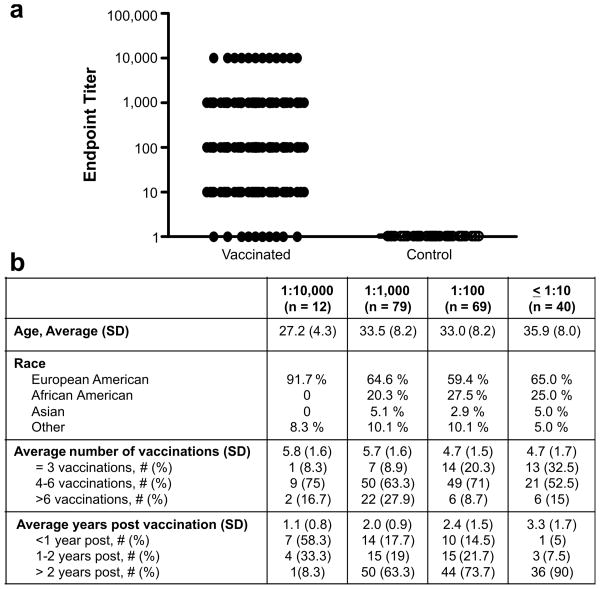
Of the vaccinated individuals, 95% had detectable antibodies to PA (Fig.1a) and were divided into groups based upon titer (≤1:10, 1:100, 1:1,000, and ≥1:10,000). The antibody level was also quantified in each titer defined group using the reference standard AVR801 [19]: the average microgram content in each titer group was 7.6 ± 0.7 μg/ml for ≤ 1:10, 21.1 ± 3.3 μg/ml for 1:100, 52.9 ± 9.8 μg/ml for 1:1,000, and 180.4 ± 27.65 μg/ml for 1:10,000. In our study, the level of antibodies to PA correlated with number of vaccinations received but not age (Fig. 1b, p<0.0001 and p=0.22, respectively). Additionally, those participants with the highest titers of antibodies were most recently vaccinated (1.1 ± 0.8 years post vaccination, p < 0.001, Fig. 1b). These data are consistent with previous reports which show that anti-PA titers decline over time [17, 23, 24].
Figure 1. AVA vaccination results in high titers of antibodies to PA.
The endpoint titer or last dilution at which a sample was positive for antibodies to PA (a). Each symbol represents one participant and the line shows the mean titer (1:100) of all individuals. The demographics of the participants at the indicated endpoint titer for anti-protective antigen antibodies are listed (b).
Over half of vaccinated individuals generate only low levels of antibodies capable of in vitro lethal toxin neutralization
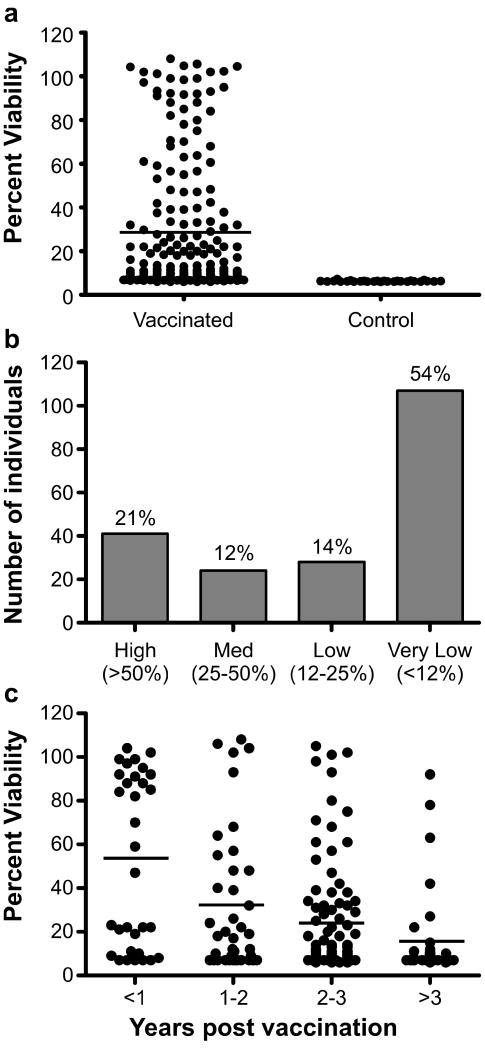
The ability of antibodies to PA from AVA vaccinated individuals to inhibit lethal toxin activity, as measured by in vitro toxin neutralization, is shown in Fig. 2a. Participants were classified into four groups based upon their serum responses in this assay: high (51-100%), medium (26-50%), low (12-25%), and very low responders (less than 12%). For comparison, the average and standard deviation of a group of 100 non-vaccinated control individuals was 8.06 ± 1.02 percent. On the basis of these criteria, 54% of those who were immunized were very low responders and thus did not have increased neutralizing capacity compared to individuals who were not immunized (Fig. 2b). Of the 200 participants, only 41 sera (20.5%) had greater than 50% in vitro neutralization by this assay. Those participants (52%) who were vaccinated within the year preceding enrollment had significantly higher toxin neutralization activity than those (16%) who were not vaccinated within three years of enrollment (Fig. 2c, p<0.0001). Most importantly, fifteen (7.5%) participants immunized within the last two years had titers of anti-PA antibodies greater than or equal to 1:1,000 but were not able to neutralize LT in vitro.
Figure 2. Over 50% of AVA vaccinated individuals fail to generate antibodies with detectable in vitro toxin neutralization activity.
The percent viability of macrophages in response to lethal toxin (LT) neutralization by sera from all vaccinated and non-vaccinated individuals is shown (a). Each symbol represents the average viability from three independent experiments using sera from each individual and the horizontal line indicates the overall median viability. The number and percent of individuals who were considered high, medium, low, or very low responders are shown (b). The percent viability of macrophages in response to LT neutralization is shown for each individual and is grouped based upon the number of years post vaccination for each participant (c). Each symbol represents the average viability from three independent experiments for each individual and the horizontal lines indicate the overall median viability of macrophages for each group.
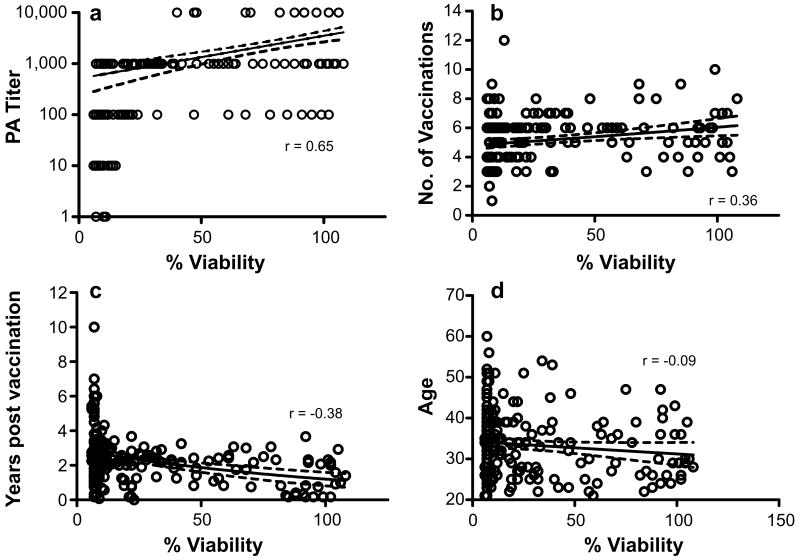
Despite the lack of 100% concordance between anti-PA titer and neutralization in some subjects, the most significant correlation with LT neutralization was anti-PA titer (Fig. 3, p<0.0001, r = 0.65), indicating that antibodies to PA tracked with protection in this assay. A direct correlation was found between the percent viability of macrophages and the number of vaccinations received (Fig. 3, p<0.0001, r = 0.36). However, an inverse correlation was found between the percent neutralization and the number of years post vaccination (Fig. 3, p<0.0001, r = -0.36). Thus, the sera of participants who were most recently vaccinated had higher neutralization activity. These data indicate that in most individuals repeated immunization results in higher titers of PA antibodies and that these antibodies correlate with in vitro neutralizing ability. No correlation was seen between the in vitro neutralizing activity and other demographic features, such as age, race, or gender in this cohort.
Figure 3. In vitro LT neutralization correlates with anti-PA titer and the number of years post vaccination.
Antibody neutralization of the toxin was correlated with PA titer (p<0.0001; a), number of vaccinations (p<0.0001; b), and number of years post last vaccination (p<0.0001; c), but not with age (p = 0.22; d) as determined by using a Spearman' correlation coefficient. Each symbol represents one individual.
Select epitopes are associated with in vitro neutralization
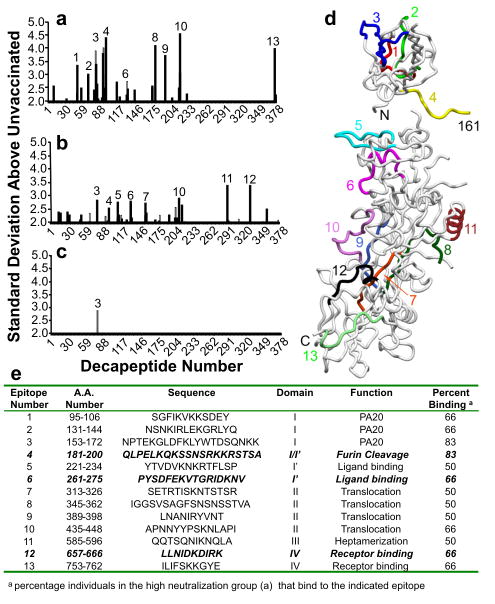
Since the highest correlation with protection in the in vitro LT neutralization assay was anti-PA titer, we analyzed the anti-PA fine specificity response through solid phase peptide binding assays. Antigenic regions were identified for the antibodies of those participants who had high or moderate levels of neutralizing antibodies and those who had antibodies to PA but very low levels of LT neutralization (Fig. 4). Based upon previous reports [1, 25-29], we identified the location of each epitope on the PA crystal structure, the domain locations and proposed function of each epitope (Fig. 4).
Figure 4. Thirteen common antigenic regions, spanning all four domains of PA, were identified using solid phase epitope mapping.
Sera were used in a standard solid-phase peptide assay and compared to sera from non-vaccinated controls. Shown are serum antibody responses from individuals with high neutralization (n=6; a), moderate neutralization (n=12; b), and low neutralization (n=12; c). The locations of the indicated antigenic regions mapped onto the crystal structure of PA are shown (d). The table shows the amino acid number, sequence, and functional domain for each epitope.
Since we were most interested in antibodies that are potentially neutralizing, we focused on epitopes identified in participants with high titers of neutralizing antibodies. The most prominent epitope identified in sera from participants with high LT neutralization activity was epitope number 4 (aa181-200), which was identified as the region containing the furin cleavage site [1, 28]. In fact, 83% of the individuals with high titers of neutralizing antibodies had responses directed against this antigenic region. An epitope within the ligand-binding region (identified as epitope 6, aa261-275, Fig. 4) was of particular interest because mutagenesis in this region was previously shown to be critical for ligand binding and LT activity [28]. A third potentially important epitope was identified in 66% of the individuals with high neutralization and is within the receptor-binding domain of PA (epitope 12, Fig. 4).
To confirm antibodies directed against these epitopes of interest using a second method, we synthesized these epitopes as soluble peptides and used them in peptide-specific ELISAs. We identified 28 different study participants (14%) whose sera contained antibodies directed to the furin cleavage site epitope, 30 participants (15%) with antibodies directed to the ligand-binding domain epitope, and 29 participants (14.5%) with antibodies directed to the receptor-binding domain epitope. Peptide antibodies directed against the furin cleavage and receptor-binding sites were more common in participants whose sera had high neutralizing activity (p = 0.04), but there was no relationship between neutralizing activity and the anti-ligand binding domain antibodies.
Select anti-peptide antibodies provide in vitro lethal toxin neutralization
Once these unique antigenic regions had been identified and confirmed, we enriched human sera for peptide-specific antibodies by column absorption. The absorption resulted in 78.8% depletion of the furin-cleavage site specific antibodies, 90.5% of the ligand-binding site antibodies, and 91% of the receptor-binding site antibodies in the un-retained fractions. Each of these enriched samples retained the ability to bind to whole recombinant PA.
These column-absorbed samples were then used in the in vitro LT neutralization assay to test the ability of these peptide-specific antibodies to mediate protection. Whole sera from vaccinated individuals or affinity purified antibodies directed against the three specific epitopes significantly increased macrophage cell survival in the LT neutralization assay (Fig. 5a, p = 0.03). In contrast, depletion of these peptide-specific antibodies resulted in loss of toxin neutralization (average viability of 12%). IgG affinity purified from non-vaccinated controls showed no ability to neutralize LT activity, indicating that this protection was not due to inhibition via non-specific interactions.
Figure 5. Peptide-specific antibodies mediate protection against in vitro and in vivo LT challenge.
Plasma samples from AVA-vaccinated participants with high titers of peptide-specific antibodies were passaged and eluted six times over the corresponding peptide-specific column. Following column-enrichment and –depletion, the samples were used in an in vitro neutralization assay to determine the neutralization activity of the specific peptides. Mean neutralization (±SD) values are shown for 4 individuals in each group (a). Six-week-old A/J mice (n= 5 mice/group) were injected intraperitoneally with 30 μg of peptide-specific antibodies and then were challenged three hours later with 3 × LD50 of LT. Control mice received either saline or IgG purified from the serum of a non-vaccinated control. Shown is the combined percent survival of the mice from two independent experiments (b).
Select anti-peptide antibodies provide protection against lethal toxin challenge in vivo
Antibodies specific for each of the three epitopes of interest were column enriched and then passively transferred to A/J mice to measure in vivo protection. Following passive transfer of anti-peptide enriched samples or sham antibody controls, mice were challenged with 3 × LD50 of LT and mortality was recorded (Fig. 5b). Sixty percent of mice receiving receptor-binding site epitope antibodies survived the LT challenge (p = 0.0018). In contrast, only 30% of mice receiving ligand-binding site antibodies and 10% of mice receiving furin cleavage site-specific antibodies survived LT challenge (p = 0.05 and p =0.36 respectively). All control mice, those receiving no antibodies or non-specific IgG, succumbed to death by day 2 post challenge. These data indicate that select PA peptide-specific antibodies provide protection from LT challenge in vivo.
Discussion
The US anthrax vaccine is predicated on evidence that antibodies directed against protective antigen provide protection against challenge. Work using animal models, including rabbits and guinea pigs, show that while antibodies to PA and in vitro toxin neutralization titers predict survival in an aerosol spore challenge model, this prediction is imperfect [16, 30]. Since this time, several groups have performed limited characterization of the immune response in individuals receiving the AVA vaccine. These studies have shown that, as with animals, humans vaccinated with AVA produce high titers of antibodies to PA and that antibody titer and in vitro neutralization are correlated [23, 24, 31, 32]. However, in each of these studies, there were individuals that generated high titers of antibodies to PA but were not able to neutralize toxin. Therefore, it is probable that antibody qualities such as epitope specificity are important factors for effective toxin neutralization. Results from this study provide a biologic explanation for observations of discordance between high concentrations of antibodies but low neutralizing capacities.
In our cohort of AVA immunized subjects, ninety-five percent had measurable antibodies to PA and lower concentrations were associated with longer time since last vaccination and lower total number of doses. Thus, those participants with the highest titers of antibodies received more vaccinations (4-6) and were among those most recently vaccinated (1.1 ± 0.8 years post). These data are consistent with previous reports which show that anti-PA titers decline over time [17, 23, 24].
Toxin neutralization is a critical component of protection [17, 23, 24, 31] and neutralizing antibodies are the best surrogate marker for protective immunity found to date [30]. Over half of the vaccinated individuals (54%) had little in vitro neutralizing activity above that seen in unvaccinated controls and only twenty-one percent had 50% or greater neutralization at a 1:100 dilution. While it is unclear whether these individuals would be at risk following exposure, a recent report used sera from AVA vaccinated individuals to determine the concentration of PA-specific antibodies necessary for protection. Using a Sterne strain challenge in mice, they found that a toxin neutralization titer of 280 μg/ml or higher (corresponding to 50% neutralization at a 1:112 dilution) provided 79% protection [33]. These data highlight the need for improved understanding of the variables that determine the quality of vaccine response, potential for different vaccine design and dosing strategies, as well as the desirable properties of specific antibodies used for passive immunization after spore exposure.
Since the highest correlation with in vitro LT neutralization was anti-PA titer, we analyzed the fine specificity of the anti-PA response and identified neutralization-associated humoral epitopes. While there are reports detailing the levels of antigen-specific antibodies following AVA vaccination [34-37], the humoral epitope-binding patterns and protective capacity of select specificities have not been well characterized.
We identified 13 unique antibody binding domains after AVA vaccination within all four PA domains [1, 28]. Three of the antigenic regions were in the PA20 domain and recent evidence indicates that antibodies to this domain can prolong survival of mice challenged with toxin [38]. An epitope that was identified within sera of participants with high toxin neutralization activity was within the furin cleavage site. Several reports have demonstrated that antibodies or small molecule inhibitors that prevent or slow furin cleavage can provide protection in vivo [39-42]. Indeed, Abboud et al [43] generated a neutralizing monoclonal antibody in mice that bound to a linear epitope within the furin cleavage site (QKSSN) which is contained within our epitope number 4.
Two epitopes were identified in the ligand-binding domain and mutants in this region have been shown to reduce LF binding and inhibit toxin activity [26]. The two epitopes found in domain II contain amino acids that are necessary for toxin translocation [28], as well as sequence homology to the antigenic region (SKNLAPI) bound by a murine monoclonal antibody that provides protection from Sterne strain challenge [39]. We also identified an antigenic region in the critical receptor-binding domain. Mutations of amino acids within this region result in partial or complete loss of toxicity [29], and murine monoclonal antibodies to this region neutralize toxin activity in vitro and in vivo [25, 44, 45]. The antigenic regions within the receptor binding domain that we identified did not overlap with those found by others [25, 44]. However, Kaur et al immunized mice with an antigenic region (ID-II), which contained our epitope #12 (LLNIDKDIRK), and demonstrated both in vitro and in vivo protection.
A recent report by Gubbins et al [46] demonstrated that AVA-vaccinated individuals generate antibodies to the translocation domain (SFFD), but it is not clear if the antibodies in that study mediate protection. Additionally, others have demonstrated that antibodies from vaccinated individuals directed against the receptor-binding and the ligand-binding regions can provide in vitro protection [47]. However, the ability of these antibodies to protect against in vivo challenge has not been tested.
We enriched for peptide-specific antibodies against the furin cleavage, ligand-binding, and receptor-binding regions and demonstrated that these antibodies are capable of in vitro neutralization. The neutralizing activity in the un-retained samples is likely due to either low level of antibodies directed against these three regions which were not removed by absorption or to antibodies specific for other peptides since these antibodies account for only three of the antigenic regions identified in the high responders.
Surprisingly, we found that while the antibodies directed to the furin cleavage site mediated the best protection in vitro, the receptor-binding site antibodies resulted in the most robust protection in vivo, despite their apparently equivalent affinity. This may be due in part to rapid furin cleavage and binding of LF to PA before the epitope-specific antibody can bind its target. Pre-incubation of PA with the anti-furin cleavage site antibody probably decreases toxin activity, but this scenario is unlikely to occur naturally. The receptor binding antibody, however, would be able to bind the cellular receptors before the toxin was administered and could result in decreased toxin activity. This is consistent with a previous report showing that monoclonal antibodies to the receptor binding region have neutralizing activity [25]. Thus, we have demonstrated that epitope-specific antibodies derived from AVA vaccinated individuals can provide protection against lethal toxin challenge.
This is the first report to perform a systematic characterization of the humoral fine-specificity response following human AVA vaccination. We demonstrate that select peptide-specific antibodies enriched from the sera of vaccinated individuals can provide in vivo protection against lethal toxin challenge. The new anthrax vaccination schedule based on quantitative levels of anti-PA following doses may need to be evaluated in the context of antibody specificities that are functionally effective in LT neutralization and critical to survival from challenge. Additionally, the data presented suggest that it may be feasible to create a limited number of monoclonal mixtures that can provide highly effective passive immunity in a post exposure scenario.
Acknowledgments
We thank the Walter Reed Army Medical Center Research Team: Limone C. Collins, MD, Michael R. Nelson, MD, Mary Klote, MD, Jeannette Williams, Laurie Duran, Mary Minor, Christina Spooner, Kaureen Langlie, Cathy Rowe, Sadie Massey, and Denece Shelton. We also thank the staff of the regional Vaccine Healthcare Centers Fort Bragg Research Team: Nancy Blacker, Gary Robinson, MD, Nora Rachels, Amy McCoart, Rebecca Bernacki, Tammi Griggs, and Joseph Weagraff. Thanks to J. Donald Capra, MD for scientific and manuscript input. The purified toxin used in the passive transfer studies and the LD50 was provided by Melissa Nguyen. Additionally, we thank Wendy Klein, Timothy Gross, Clay Nelson, and Donyelle Weston for technical assistance. Multi-antigenic peptides were constructed by the Molecular Biology and Proteomics Core Facility at Oklahoma University Health Sciences Center. Recombinant Protective Antigen (NR-140) and human anti-AVA reference serum (AVR801, NR-719) was obtained through the NIH Biodefense and Emerging Infections Research Repository, NIAID, NIH.
This work was supported by funds from the National Institute of Allergy and Infectious Diseases (NIAID) through grant U19AI062629 and NCRR grant P20RR15577. Local protocol development and management was supported by Walter Reed Army Medical Center Vaccine Healthcare Centers Network/Allergy-Immunology Department. The opinions and assertions contained herein are private views of the authors and are not to be construed as official or as reflecting the official views of the Department of Defense, Department of the Army, National Institutes of Health, or other government agencies.
Footnotes
The authors have no commercial or other associations that might pose a conflict of interest.
Select features of this work were presented at the Association of Immunologists Annual meeting held in Seattle, Washington in May 2009 (abstract # 129.16).
All affiliations and addresses are current.
References
- 1.Mourez M. Anthrax toxins. Rev Physiol Biochem Pharmacol. 2004;152:135–64. doi: 10.1007/s10254-004-0028-2. [DOI] [PubMed] [Google Scholar]
- 2.Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–7. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, Collier RJ. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc Natl Acad Sci U S A. 2004;101:13147–51. doi: 10.1073/pnas.0405405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starnbach MN, Collier RJ. Anthrax delivers a lethal blow to host immunity. Nat Med. 2003;9:996–7. doi: 10.1038/nm0803-996. [DOI] [PubMed] [Google Scholar]
- 5.Firoved AM, Miller GF, Moayeri M, et al. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–20. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P, Ahuja N, Bhatnagar R. Anthrax edema toxin requires influx of calcium for inducing cyclic AMP toxicity in target cells. Infect Immun. 2002;70:4997–5007. doi: 10.1128/IAI.70.9.4997-5007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little SF, Ivins BE, Fellows PF, Friedlander AM. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect Immun. 1997;65:5171–5. doi: 10.1128/iai.65.12.5171-5175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little SF, Leppla SH, Cora E. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect Immun. 1988;56:1807–13. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little SF, Leppla SH, Friedlander AM. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect Immun. 1990;58:1606–13. doi: 10.21236/ada216203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price BM, Liner AL, Park S, Leppla SH, Mateczun A, Galloway DR. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect Immun. 2001;69:4509–15. doi: 10.1128/IAI.69.7.4509-4515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology. 2001;147:1677–85. doi: 10.1099/00221287-147-6-1677. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen ML, Crowe SR, Kurella S, et al. Sequential B-cell epitopes of Bacillus anthracis lethal factor bind lethal toxin-neutralizing antibodies. Infect Immun. 2009;77:162–9. doi: 10.1128/IAI.00788-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn CP, Dull PM, Semenova V, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–36. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander AM, Pittman PR, Parker GW. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA. 1999;282:2104–6. doi: 10.1001/jama.282.22.2104. [DOI] [PubMed] [Google Scholar]
- 15.Marano N, Plikaytis BD, Martin SW, et al. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA. 2008;300:1532–43. doi: 10.1001/jama.300.13.1532. [DOI] [PubMed] [Google Scholar]
- 16.Pitt ML, Little S, Ivins BE, et al. In vitro correlate of immunity in an animal model of inhalational anthrax. J Appl Microbiol. 1999;87:304. doi: 10.1046/j.1365-2672.1999.00897.x. [DOI] [PubMed] [Google Scholar]
- 17.Baillie L, Townend T, Walker N, Eriksson U, Williamson D. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol Med Microbiol. 2004;42:267–70. doi: 10.1016/j.femsim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Baillie LW, Fowler K, Turnbull PC. Human immune responses to the UK human anthrax vaccine. J Appl Microbiol. 1999;87:306–8. doi: 10.1046/j.1365-2672.1999.00899.x. [DOI] [PubMed] [Google Scholar]
- 19.Quinn CP, Semenova VA, Elie CM, et al. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis. 2002;8:1103–10. doi: 10.3201/eid0810.020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed N, Li J, Ferreira CS, et al. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect Immun. 2004;72:3276–83. doi: 10.1128/IAI.72.6.3276-3283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–9. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 22.Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices. J Toxicol Clin Toxicol. 2001;39:85–100. doi: 10.1081/clt-100102886. [DOI] [PubMed] [Google Scholar]
- 23.Pittman PR, Leitman SF, Oro JG, et al. Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis. Clin Diagn Lab Immunol. 2005;12:713–21. doi: 10.1128/CDLI.12.6.713-721.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittman PR, Norris SL, Barrera Oro JG, Bedwell D, Cannon TL, McKee KT., Jr Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine. 2006;24:3654–60. doi: 10.1016/j.vaccine.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Moayeri M, Zhou YH, et al. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J Infect Dis. 2006;193:625–33. doi: 10.1086/500148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham K, Lacy DB, Mogridge J, Collier RJ. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc Natl Acad Sci U S A. 2002;99:7049–53. doi: 10.1073/pnas.062160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flick-Smith HC, Walker NJ, Gibson P, et al. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect Immun. 2002;70:1653–6. doi: 10.1128/IAI.70.3.1653-1656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourez M, Yan M, Lacy DB, et al. Mapping dominant-negative mutations of anthrax protective antigen by scanning mutagenesis. Proc Natl Acad Sci U S A. 2003;100:13803–8. doi: 10.1073/pnas.2436299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varughese M, Teixeira AV, Liu S, Leppla SH. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect Immun. 1999;67:1860–5. doi: 10.1128/iai.67.4.1860-1865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuveny S, White MD, Adar YY, et al. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69:2888–93. doi: 10.1128/IAI.69.5.2888-2893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson JF, Taft SC, Weiss AA. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin Vaccine Immunol. 2006;13:208–13. doi: 10.1128/CVI.13.2.208-213.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunow R, Porsch-Ozcurumez M, Splettstoesser W, et al. Monitoring of ELISA-reactive antibodies against anthrax protective antigen (PA), lethal factor (LF), and toxin-neutralising antibodies in serum of individuals vaccinated against anthrax with the PA-based UK anthrax vaccine. Vaccine. 2007;25:3679–83. doi: 10.1016/j.vaccine.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 33.Hewetson JF, Little SF, Ivins BE, et al. An in vivo passive protection assay for the evaluation of immunity in AVA-vaccinated individuals. Vaccine. 2008;26:4262–6. doi: 10.1016/j.vaccine.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Ruther P, Jiang I, et al. Human monoclonal antibodies that neutralize anthrax toxin by inhibiting heptamer assembly. Hum Antibodies. 2004;13:105–10. [PubMed] [Google Scholar]
- 35.Sawada-Hirai R, Jiang I, Wang F, et al. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J Immune Based Ther Vaccines. 2004;2:5. doi: 10.1186/1476-8518-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albrecht MT, Li H, Williamson ED, et al. Human Monoclonal Antibodies against Anthrax Lethal Factor and Protective Antigen Act Independently To Protect against Bacillus anthracis Infection and Enhance Endogenous Immunity to Anthrax. Infect Immun. 2007;75:5425–33. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson JW, Comer JE, Noffsinger DM, et al. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect Immun. 2006;74:1016–24. doi: 10.1128/IAI.74.2.1016-1024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera J, Nakouzi A, Abboud N, et al. A monoclonal antibody to Bacillus anthracis protective antigen defines a neutralizing epitope in domain 1. Infect Immun. 2006;74:4149–56. doi: 10.1128/IAI.00150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brossier F, Levy M, Landier A, Lafaye P, Mock M. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect Immun. 2004;72:6313–7. doi: 10.1128/IAI.72.11.6313-6317.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao GS, Cregar L, Wang J, et al. Synthetic small molecule furin inhibitors derived from 2,5-dideoxystreptamine. Proc Natl Acad Sci U S A. 2006;103:19707–12. doi: 10.1073/pnas.0606555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarac MS, Peinado JR, Leppla SH, Lindberg I. Protection against anthrax toxemia by hexa-D-arginine in vitro and in vivo. Infect Immun. 2004;72:602–5. doi: 10.1128/IAI.72.1.602-605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brossier F, Weber-Levy M, Mock M, Sirard JC. Role of toxin functional domains in anthrax pathogenesis. Infect Immun. 2000;68:1781–6. doi: 10.1128/iai.68.4.1781-1786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abboud N, De Jesus M, Nakouzi A, et al. Identification of linear epitopes in bacillus anthracis protective antigen bound by neutralizing antibodies. J Biol Chem. 2009 doi: 10.1074/jbc.M109.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly-Cirino CD, Mantis NJ. Neutralizing monoclonal antibodies directed against defined linear epitopes on domain 4 of anthrax protective antigen. Infect Immun. 2009;77:4859–67. doi: 10.1128/IAI.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur M, Chug H, Singh H, et al. Identification and characterization of immunodominant B-cell epitope of the C-terminus of protective antigen of Bacillus anthracis. Mol Immunol. 2009;46:2107–15. doi: 10.1016/j.molimm.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 46.Gubbins MJ, Schmidt L, Tsang RS, Berry JD, Kabani A, Stewart DI. Development of a competitive enzyme linked immunosorbent assay to identify epitope specific antibodies in recipients of the U.S. licensed anthrax vaccine. J Immunoassay Immunochem. 2007;28:213–25. doi: 10.1080/15321810701454706. [DOI] [PubMed] [Google Scholar]
- 47.Zhou B, Carney C, Janda KD. Selection and characterization of human antibodies neutralizing Bacillus anthracis toxin. Bioorg Med Chem. 2008;16:1903–13. doi: 10.1016/j.bmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]