Abstract
Following the results of the Women’s Health Initiative, many women now decline estrogen replacement at the time of menopause and seek natural remedies that would treat menopausal symptoms and prevent bone loss and other long-term consequences of estrogen deficiency, but without adverse effects on the breast, uterus, and cardiovascular system. The results of most soy studies in this population have had limitations because of poor design, small sample size, or short duration. This report describes the study rationale, design, and procedures of the Soy Phytoestrogens As Replacement Estrogen (SPARE) study, which was designed to determine the efficacy of soy isoflavones in preventing spinal bone loss and menopausal symptoms in the initial years of menopause.
Women ages 45 to 60 without osteoporosis and within five years from menopause were randomized to receive soy isoflavones 200 mg daily or placebo for 2 years. Participants have yearly measurements of spine and hip bone density, urinary phytoestrogens, and serum lipids, thyroid stimulating hormone, and estradiol. Menopausal symptoms, mood changes, depression, and quality of life are assessed annually.
The SPARE study recruited 283 women, 66.1% were Hispanic white. With a large cohort, long duration, and large isoflavone dose, this trial will provide important, relevant, and currently unavailable information on the benefits of purified soy isoflavones in the prevention of bone loss and menopausal symptoms in the first five years of menopause. Given the high proportion of Hispanics participating in the study, the results of this trial will also be applicable to this minority group.
Keywords: menopause, bone health, osteoporosis, soy, isoflavones
1. Introduction
The cessation of ovarian function at the time of menopause and resulting hormonal changes are associated with specific health conditions that are unrelated to those typically attributed to aging. Estrogen deficiency plays a major role in menopausal bone loss, hot flashes, and vaginal epithelium atrophy, thus until recently menopausal symptoms and the prevention of bone loss had been primarily managed with hormone replacement [1].
The findings of increased risk of breast cancer and cardiovascular complications by the Women’s Health Initiative Study (WHI) resulted in the early termination of its estrogen/progestin arm. [2] In response to these well publicized results many menopausal women discontinued or did not start hormonal replacement therapy [3-5]. The belief that products containing “natural” estrogens would provide all of the benefits but none of the risks of prescription hormones has resulted in a vast increase in the use of herbal products containing phytoestrogens by women seeking to alleviate menopausal symptoms and prevent osteoporosis.
Although results of randomized trials suggest that isoflavones, the phytoestrogens found in soy, may prevent bone loss and help with menopausal symptoms, these studies have had serious limitations. Most have enrolled a small number of women [6-16], included women in a wide-range of ages and years from menopause [17-19], had high drop out rates [20], were of a short duration [6,9,11-13,21,22], or used preparations with low isoflavone content [6-11,21-24]. Additionally, many of these trials have tested soy-containing foods [8,21,22,24,25], which vary widely in phytoestrogen source, type, and dose. Finally, only few have correlated outcomes with serum or urinary concentrations of isoflavone metabolites [6,14-16,25] and none have assessed these products in the US Hispanic population. Despite the lack of clear evidence of its effectiveness and safety, millions of menopausal women continue to self-medicate with soy products.
The Soy Phytoestrogen as Replacement Estrogen (SPARE) study was designed to address these issues and provide comprehensive information about the benefits and risks of soy isoflavones in recently menopausal women. This report details the SPARE study design, rationale, and the procedures used to assess the effects of isoflavones on various target tissues. This article will ascertain any significant differences among the women who were screened and those who were randomized and identify any participation bias among those women who qualified for the study but chose not to participate.
2. Study design and methods
2.1. Objectives
The overall aim of the Soy Phytoestrogen as Replacement Estrogen (SPARE) study is to establish the long-term effectiveness of a high-dose of purified soy isoflavones in sparing the complications and multiple symptoms associated with declining estrogen levels in the first years of menopause: the prevention of spinal bone loss and the improvement of biological and other emotional changes of menopause. The primary objective of the study is to determine the extent to which purified soy isoflavones administered daily over two years prevents bone loss in recently menopausal women. This will be assessed by serial measurements of bone mineral density (BMD) in the lumbar spine and proximal femur and of urinary N-telopeptide of type I bone collagen (NTx), a marker of bone resorption [26]. Secondary objectives will establish the estrogen-agonist effects of soy isoflavones tablets in estrogen-sensitive tissues. These include changes in hot flashes, vaginal cytology, thyroid function, and mood.
2.2 Study site and overall design
The SPARE study differs in many ways from previous soy trials as it enrolled a larger number of participants, has a longer duration, utilizes a larger isoflavone dose, provides isoflavones in tablet form, evaluates multiple outcomes in the same population, and will correlate these outcomes to concentrations of urinary isoflavone metabolites. This single-center, double-blind, placebo-controlled, randomized clinical trial is conducted at the University of Miami Miller School of Medicine, Miami, Florida, USA. Menopausal women were recruited for a 2-year participation that requires 10 clinic visits. The SPARE study was approved by the University of Miami’s Institutional Review Board.
2.3. Study population
The SPARE study population consists of a multiethnic group of community-dwelling women aged 45 to 60 years. They were eligible to participate if they met the following inclusion criteria: (1) did not consume soy products regularly, (2) had a BMD T-score in the lumbar spine or total hip < −2.0, and (3) had their last menstrual period at least 12 months before enrolling or at least 6 months before enrolling and a follicle stimulating hormone (FSH) >40 IU/liter. Exclusions were designed to identify women who had osteoporosis, were receiving medications or had medical conditions that would influence rates of bone loss. The complete inclusion and exclusion criteria are listed in Table 1.
Table 1. Eligibility Criteria for participation in the SPARE study.
Inclusion criteria
|
Exclusion criteria
|
2.4. Recruitment strategies
The plan for participant recruitment was designed to result in the enrollment of a group of women representative of the local community. The effort initially relied on self-referral of University of Miami employees volunteering for the study, in addition to referrals from the medical center’s physicians. Posters and brochures advertising the study were placed in the entrances of buildings, waiting areas, and bulletin boards. After very slow recruitment in the initial months of the study, the initial plan was revised and several other strategies were employed to extend recruitment activities to the community at large. Thereafter, many women were enrolled from outreach activities such as health fairs and community groups, but mostly from community-based mailings. Using a list provided by the Florida Department of Motor Vehicle, multiple mailings of bilingual postcards were sent to women ages 45-60 residing in South Florida. In addition, posters were placed in local commuting trains, community doctor’s offices, and other public locations. The study employed bilingual staff who made special efforts to recruit minorities by attending health fairs and community events. Study staff also worked with local newspapers and radio and television stations to produce stories about the study.
2.5. Study events and timeline
2.5.1. Screening process
Potential participants followed a two-stage screening process. The first stage consisted of a brief telephone screen designed to determine if the woman was within the study age range, was menopausal, did not take hormone replacement therapy and was not obese, defined as Body Mass Index (BMI) >32 kg/cm2. It also provided the opportunity to exclude women who included soy products in their daily diet and were not willing to discontinue them for the study duration. Candidates who successfully completed the telephone screening interview were scheduled for a screening clinic visit at the University of Miami. Those who declined or did not appear at their scheduled clinic appointment were classified as having declined to participate.
The second phase of the screening process was the screening visit. After signing an informed consent form, all study candidates went through the testing procedures described in Table 2. Women meeting all eligibility criteria were assigned at their second study visit to a one-month, single-blind, placebo run-in period designed to identify and exclude those women who would not comply with study requirements or be lost to follow-up early on.
Table 2. Overview of the SPARE study visits and measurements.
| Time from Randomization in Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S a | -1 b | 0 c | 2 | 4 | 8 | 12 | 16 | 20 | 24 | |
| Informed consent | X | |||||||||
| Inclusion/exclusion criteria | X | |||||||||
| Bone mineral density | X | X | X | X | ||||||
| Body Composition | X | X | X | |||||||
| Mammogram | X | X | X | |||||||
| Vital Signs | X | X | X | X | X | X | X | X | X | X |
| Yale Physical Activity Survey | X | X | X | |||||||
| Dietary assessment | X | X | X | |||||||
| Vaginal cytology | X | X | X | |||||||
| Women’s Health Questionnaire | X | X | X | |||||||
| SF-36 | X | X | X | |||||||
| Profile Mood of States | X | X | X | |||||||
| Beck Depression Inventory | X | X | X | |||||||
| Hot Flash Diary | X | X | X | X | ||||||
| CBC d | X | X | X | |||||||
| Serum chemistries | X | X | X | |||||||
| TSH e | X | X | X | |||||||
| Lipids | X | X | X | |||||||
| NTx f | X | X | X | |||||||
| Urinary isoflavones | X | X | X | |||||||
| Adverse events | X | X | X | X | X | X | X | X | X | |
| Compliance | X | X | X | X | X | X | X | X | ||
Screening visit
Enrollment and start of run-in phase of the study
Randomization
Complete blood cell count
Thyroid stimulating hormone
N-telopeptide of Type I bone collagen
2.5.2. Randomization
Those participants who met eligibility criteria and completed the run-in period were assigned in equal proportions to the soy or placebo group following the randomization sequence created by the study biostatistician. Participants were randomized in blocks of ten to ensure equal allocation to the control or treatment group. All study personnel remained blinded to the subject treatment assignment.
2.5.3. Study medication rationale
The selected dose of 200 mg/day is expected to produce isoflavone blood levels slightly above those observed in individuals on an Asian diet but not higher than those observed in infants consuming soy formula [27]. Randomized subjects were assigned to receive either purified aglycone soy isoflavones, 200 mg daily, or a similarly-appearing placebo. Each daily dose was provided by four 50 mg tablets. The tablets were manufactured by Atrium, Inc. (Wautoma, WI) with purified soy isoflavones purchased from Archer Daniels Midland, Decatur, IL, which reported a total isoflavone content (aglycone equivalent) in each tablet of 52.9 mg, or 105% of the stated amount. Testing of the tablets at the FDA’s National Center for Toxicological Research, Jefferson, AR using previously published methodology [28], showed that each tablet contained 48.47 ± 2.18 mg of total isoflavones, or 97% of the stated amount (daidzein 25.68 ± 1.08 mg and genistein 22.79 ± 1.21 mg). The placebo tablets were similarly analyzed and did not contain isoflavones. Participants were instructed to take four tablets in the morning, on an empty stomach.
Based on their initial responses to the dietary assessment [29] and in order to provide sufficient calcium intake to all participants, subjects were provided tablets of calcium carbonate 500 mg plus vitamin D 200 IU. Participants received two tablets per day if their daily calcium intake was less than 500 mg or one tablet per day if their daily calcium intake was between 500 and 1,000 mg.
2.5.4. Compliance with study medication
Drug compliance is calculated for each subject at every visit using the following formula: (number of tablets dispensed – number of tablets returned / number of tablets expected to take) × 100. In addition, to validate compliance results obtained by pill-count, urinary isoflavone levels will be determined (see below) for each subject at baseline and at each annual visit thereafter.
2.6. Outcomes, procedures and schedule of study visits
Each subject makes 10 visits over a period of 2 years. All endpoints are measured at baseline, and after 12 and 24 months, with the exception of lipids that are assessed after a 12-hour fast at baseline and month 12. A complete physical exam was performed in all participants before randomization. Vital signs (heart rate, blood pressure, weight, height, and waist and hip circumference) are obtained at every visit. Questionnaires are administered in either English or Spanish, depending upon the participants’ preference [30]. An overview of the schedule of study visits and assessments is shown in Table 2.
2.6.1. Primary outcomes
Main outcomes include 2-year changes in lumbar spine BMD and in urinary NTx. BMD is measured by dual x-ray absorptiometry (DXA) using a Lunar Prodigy densitometer (Lunar, Madison, WI). Scans of the lumbar vertebrae L1-L4 and the proximal left femur are performed and analyzed following the manufacturer procedure manual. Using the same equipment, a total body scan for analysis of body composition is performed in those participants who agreed. NTx is tested in a fasting second-morning urine sample by an enhanced chemiluminescence assay (Quest Labs, San Juan de Capistrano, CA).
2.6.2. Secondary outcomes
Secondary outcomes consist of changes in the vaginal Maturation Index and Maturation Value to determine the degree of estrogenization of the vaginal epithelium, and changes in thyroid stimulation hormone, thyroid peroxidase antibodies, serum lipids, menopausal symptoms, mood, depression, and quality of life.
Vaginal cytology was performed to determine the degree of estrogenization of the vaginal epithelium from samples obtained by gently brushing vaginal side walls with a Rover’s Cervex-Brush™ and processed with the Surepath™ preparation system for gynecologic cytology. The vaginal Maturation Index (MI) is expressed as MI: % parabasal cells: % intermediate cells: % superficial cells [31]. The vaginal Maturation Value (MV) is calculated as MV: 0.2 (number of parabasal cells) + 0.6 (number of intermediate cells) + number of superficial cells [32]. Serum ultrasensitive estradiol and the total isoflavones, genistein, daidzein, and equol, from a fasting spot urine sample are tested at baseline and at each annual visit by using validated LC/MS/MS-isotope dilution methodology; these levels will be correlated to study outcomes. All samples are stored frozen and will be tested by liquid chromatography/mass spectrometry at the end of the study, as previously described [28]. Thyroid stimulating hormone (TSH), thyroid peroxidase antibodies (TPO), and serum lipids are measured to assess the effects of soy isoflavones on thyroid function, risk of developing autoimmune thyroid disease, and lipid profile, respectively [33]. Calcium and vitamin D intake are estimated using the Block Dietary Data Systems [29]. Physical activity is evaluated using the Yale Physical Activity Survey (YPAS) [34]. Menopausal symptoms are assessed using the Women’s Health Questionnaire (WHQ), which contains 35 items designed to measure mid-aged women’s perceptions of a range of physical and emotional symptoms [35]. Participants are also asked to complete a Hot Flashes Diary during the week preceding each study visit. Quality of life is assessed using the Short Form of 36 questions (SF-36) [36]. Depression is measured by the Beck Depression Inventory (BDI) [37] and mood is assessed by the Profile Mood of States (POMS) [38].
2.6.3. Data and Participant Safety
Safety measures include a serum chemistry panel, complete cell blood count, and mammogram at baseline and at each yearly visit. Study design, recruitment strategies, and participant and data safety are monitored semi-annually by a Data Safety and Monitoring Board (DSMB) established for this purpose by the funding agency, the National Institutes of Arthritis, Musculoskeletal and Skin Diseases (NIAMS). The DSMB reviews study data concerning recruitment, treatment effects, and adverse events by group on six monthly basis for the duration of the study and makes recommendations concerning continuation of the trial. Participants are notified of abnormal laboratory, BMD, and mammogram results of clinical significance.
Excessive bone loss is defined as >8% loss since baseline or since the previous study, or reaching a T-score of −2.5 or below. Bone loss greater than 8% requires that the participant be repositioned and rescanned. The average of the two scans is then used to recalculate the rate of change. Participants who experience excessive bone loss or non-traumatic fractures are asked to permanently discontinue study medication but continue with scheduled study visits and are referred to their physician for medical management.
2.7. Statistical Analyses
The sample size/power calculation is based on testing the primary hypothesis that soy isoflavones tablets prevent bone loss among women in the first years of menopause. Bone loss will be measured by BMD of the lumbar spine. The trial will test the hypothesis that treatment with soy isoflavones tablets may maintain bone density values at two years (no additional bone loss) as compared to a placebo control. Based on the data provided by Lunar (Lunar, Madison, WI), the mean BMD in women aged 50 years without intervention is 1.149 g/cm2 with standard deviation = 0.120 g/cm2. It is well documented that women are expected to experience 4-7% bone loss in the first two years of menopause [39]. Thus, with a sample size of 130 in each group (two-tailed, α = 0.05), the study has >80% power to detect a 4% or greater difference in BMD of the lumbar spine with the assumption that the mean BMD in the control group will lose 4-5% bone mass. Assuming a 15% attrition rate, the target total sample size is 306.
Univariate descriptive statistics, including boxplots and graphs, will be used to describe patterns of data to a) ensure that the scales have distributional properties that do not violate assumptions underlying statistical procedures, b) determine missing data, and c) detect outliers. For variables that are not approximately normally distributed, transformations (log, square root) will be tried first to test the normality; otherwise nonparametric techniques will be used for analysis of these variables. For outliers, original data will be checked to exclude any data entry errors. If needed, a description of the data will provide for the appropriate use for transformations of outliers.
To test the hypothesis that soy isoflavones decrease hot flash incidents in menopausal women, generalized linear models will be fitted to the data. Also random effect models will be used. These models which represent a general approach to the problem of modeling repeated measurements with fairly general error structures, can allow for missing observations, serial correlations, time-varying covariates, and irregular measurement occasions. The number of hot flashes will be treated as the dependent variable in the model. The treatment vs. placebo will be included in the model as an indicator variable. Demographic variables as well as pertinent clinical variables including (e.g., use of antidepressants and/or sleep aids) will be included in the model as covariates. For vaginal cytology, chi-square tests will be used to compare the percentage of basal, intermediate, and superficial squamous cells or MI between treatment group and controls at a given point of time. For the description of the data, the association of covariates with MI at a given point of time will be assessed using logistic regression models.
A random effects model will be used to analyze the impact of the treatment on lipid level including total cholesterol, total triglycerides, HDL-cholesterol, LDL, and VLDL. Potential confounding factors and effect modifiers discussed above will be adjusted for in the models.
Repeated measures analysis of variance will be performed to determine whether soy isoflavones tablets improve quality of life (measured by SF-36) and mental (measured by BDI) and emotional health (measured by POMS). The analysis is essentially the same as the analysis for BMD and NTx using a Group x Time analysis of variance after controlling for covariates. Post hoc analyses will be tested if Group x Time interaction is significant. Because during menopause and during the trial many subjects may be prescribed antidepressants, lipid-lower agents, sleeping aids, or other medication, each of these will have an impact on quality of life, mental and/or emotional health, these variables will be treated as covariates and controlled for in the repeated measures ANOVA.
3. Results
3.1. Study Recruitment
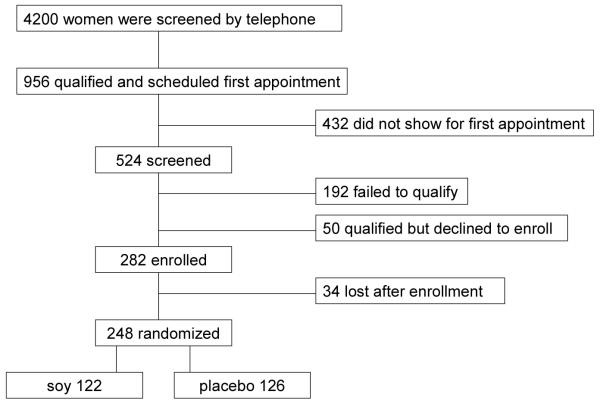
All data in this report represent the baseline information collected at the time of initial consent for the women who were screened and for those who were later randomized to soy isoflavone or placebo tablets. The recruitment, enrollment, and randomization study scheme is presented in Figure 1. Approximately 4,200 women participated in the initial telephone screening process. The main reasons for disqualifying at this stage were frequent use of soy products, recent or current hormone replacement, BMI >32 kg/cm2, and treatment with bisphosphonates. The 956 women who qualified were scheduled for a screening visit, of which 524 attended the visit and consented to participate. Among the 192 women who did not qualify for participation after the screening procedures, 88% had only one reason for not qualifying and 11% had two or more reasons. The main reasons for not qualifying were low BMD (26%), abnormal mammogram (22%), incomplete screening visit (8%), and a high BMI (7%). Of the 333 women who qualified for participation after the screening visit, 50 chose not to enroll in the study and all of the 283 who enrolled received placebo for four weeks. After the placebo run-in phase, 87% of study candidates had >80% compliance with study medication. Citing mostly either lack of interest or refusal to comply with study requirements, 34 women declined randomization. The remaining 248 participants were randomized to tablets containing soy isoflavones.
Figure 1.
Participant flow in the Soy Phytoestrogen As Replacement Estrogen (SPARE) Study
3.2. Demographic Characteristics of the Screened and Randomized Population
Table 3 summarizes baseline demographic characteristics, presented as frequency and mean distributions ± Standard Deviation, for all the women who were screened, the women who did not qualify, those who qualified but declined randomization, and for those who were randomized.
Table 3. Demographic characteristics of the women who were screened, those who failed to qualify, qualified but did not agree to be randomized, and qualified and were randomized to the SPARE Study.
| Characteristic | Screened (n= 524) |
Failed to Qualify (n= 192) |
Qualified but not Randomized (n= 84) |
Qualified and Randomized (n= 248) |
|---|---|---|---|---|
| Age (years), n (%) | ||||
| 45-49 | 116 (22.1) | 45 (23.4) | 21 (25.0) | 50 (20.2) |
| 50-54 | 263 (50.2) | 96 (50.0) | 38 (45.2) | 129 (52.0) |
| 55-60 | 145 (27.7) | 51 (26.6) | 25 (29.8) | 69 (27.8) |
| Age, years, mean ± SD | 52.3 ± 3.4 | 52.1 ± 3.3 | 52.5 ± 3.3 | 52.5 ± 3.3 |
| Race/ethnicity, n (%) | ||||
| White Hispanic | 366 (69.8) | 139 (72.4) | 63 (75.0) | 164 (66.1) |
| White non-Hispanic | 104 (19.8) | 33 (17.2) | 15 (17.9 | 56 (22.6) |
| Black | 45 (8.6) | 17 (8.9) | 4 (4.8) | 24 (9.7) |
| Asian | 9 (1.7) | 3 (1.6) | 2 (2.4) | 4 (1.6) |
| Education, n (%) | ||||
| Less than high school | 84 (16.0) | 33 (17.2) | 16 (19.0) | 35 (14.1) |
| High school | 117 (22.4) | 51 (26.6) | 16 (19.0) | 50 (20.2) |
| Part College | 117 (22.3) | 33 (17.2) | 20 (23.9) | 64 (25.8) |
| College | 109 (20.8) | 34 (17.7) | 16 (19.0) | 59 (23.8) |
| Post College | 86 (16.4) | 30 (15.6) | 16 (19.0) | 40 (16.0) |
| Unknown | 11 (2.1) | 11 (5.7) | 0 | 0 |
| Education, years, mean ± SD | 14.3 ± 3.4 | 13.9 ± 3.6 | 14.3 ± 3.2 | 14.5 ± 3.3 |
| Marital status, n (%) | ||||
| Married or Significant other | 297 (56.7) | 97 (50.5) | 47 (56.0) | 153 (61.7) |
| Divorced/Separated | 156 (29.8) | 62 (32.3) | 29 (34.5) | 65 (26.2) |
| Widowed | 17 (3.2) | 5 (2.6) | 3 (3.6) | 9 (3.6) |
| Never Married | 44 (8.4) | 18 (9.4) | 5 (6.0) | 21 (8.5) |
| Unknown | 10 (1.9) | 10 (5.2) | 0 | 0 |
Demographic baseline characteristics did not differ among the various subgroups. The age of the study candidates who were screened was 52.3 ± 3.4 years; 69.8% were white Hispanic, 19.8% white non-Hispanic, 8.6% black, and 1.7% Asian, reflecting the current make-up of the local population as reported by the US Census Bureau, which is 61.3% white Hispanic, 18.3% white non-Hispanic, and 20.0% black. Among the screened women, the mean number of years of education was 14.3 ± 3.4; 84% had at least a high school education and 37% a college degree or higher. The women who were randomized had comparable characteristics; their age was 52.5 ± 3.3, 66.1% were Hispanic, 22.6% white non-Hispanic, 9.7% black, and 1.6% Asian. Among those randomized, the mean number of years of education was 14.5 ± 3.3; 86 % had at least a high school education and 40% a college degree or higher.
3.3. Clinical Characteristics of the Screened and Randomized Population
Table 4 summarizes baseline clinical characteristics, also presented as frequency and mean distributions ± Standard Deviation, for all the women who were screened, the women who did not qualify, those who qualified but declined randomization, and for those who were randomized.
Table 4. Clinical characteristics of the women who were screened, those who failed to qualify, qualified but did not agree to be randomized, and qualified and were randomized to the SPARE Study.
| Characteristic | Screened (n= 524) |
Failed to Qualify (n= 192) |
Qualified but not Randomized (n= 84) |
Qualified and Randomized (n= 248) |
|---|---|---|---|---|
| BMI, n (%) | ||||
| Underweight (BMI <18.5) | 2 (0.4) | 2 (1.0) | 0 | 0 |
| Normal (BMI ≥18.5 - 24.9) | 190 (36.3) | 73 (38.0) | 29 (34.5) | 90 (35.8) |
| Overweight (BMI 25-29) | 231 (44.1) | 74 (38.5) | 41 (48.8) | 116 (47.2) |
| Obese (BMI 30-32) | 90 (17.2) | 34 (17.7) | 14 (16.7) | 42 (17.1) |
| Unknown | 9 (1.7) (2.1) | 9 (4.7) | 0 | 0 |
| BMI, kg/m2, mean ± SD | 26.3 ± 3.5 | 26.2 ± 4.0 | 26.3 ± 3.2 | 26.3 ± 3.2 |
| Waist to hip ratio | 0.835 ± 0.4 | 0.825 ± 0.1 | 0.816 ± 0.1 | 0.85 ± 0.5 |
| BP (mmHg), mean ± SD) (n= 511) | ||||
| Mean systolic BP | 125.9 ± 18.3 | 127.8 ± 19.2 | 123.4 ± 17.5 | 125.3 ± 17.8 |
| Mean diastolic BP | 78.4 ± 9.4 | 78.7 ± 9.7 | 78.5 ± 9.6 | 78.1 ± 9.1 |
| Blood pressure status, n (%) | ||||
| Normal blood pressurea | 194 (37.0) | 63 (32.8) | 32 (38.1) | 99 (39.9) |
| Pre-hypertensiveb | 199 (38.0) | 76 (39.6) | 38 (45.2) | 85 (34.3) |
| Hypertensionc | 118 (22.5) | 42 (21.9) | 14 (16.7) | 62 (25.0) |
| Unknown | 13 (2.5) | 11 (5.7) | 0 | 2 (0.8) |
| BMD (T-score), mean ± SD (n= 491) | ||||
| Lumbar spine | −0.7 ± 1.2 | −1.2 ± 1.3 | −0.6 ± 0.9 | −0.3 ± 1.0 |
| Total hip | −0.4 ± 0.9 | −0.7 ± 1.2 | −0.3 ± 0.9 | −0.2 ± 0.9 |
| Femoral neck | −0.9 ± 0.9 | −1.1 ± 0.9 | −0.9 ± 0.8 | −0.7 ± 0.8 |
| BMD category, n (%) | ||||
| Met inclusion criteria (T-score ≥−2.0) | 430 (88.0) | 102 (53.1) | 84 (100) | 248 (100) |
| Normal (T-score ≥−1.0) | 219 (41.8) | 51 (26.6) | 37 (44.0) | 134 (54.0) |
| Osteopenia (T-score <−1.0 to >−2.5) | 237 (45.2) | 76 (36.9) | 47 (56.0) | 114 (46.0) |
| Osteoporosis (T-score ≤−2.5) | 34 (6.5) | 34 (17.7) | - - - | - - - |
| Unknown (BMD not done) | 34 (6.5) | 31 (16.1) | - - - | - - - |
| Number of Menopausal Symptoms | 6.3 ± 2.8 | 6.28 ±2.9 | 6.51 ± 2.75* | 6.20 ± 2.84 |
BMI: Body Mass Index; BP: blood pressure; BMD: bone mineral density
Normal blood pressure: systolic BP (SBP) <120 and diastolic BP (DBP) < 80
Pre-hypertensive: SBP ≥120 to <140 or DBP ≥80 to <90
Hypertension: SBP ≥140 or DBP ≥90.
As expected, mean BMD was higher in the population that qualified for the study than in the screened population, which included several women with osteoporosis who were subsequently excluded from participation. None of the participants had osteoporosis (T-score ≤ −2.5), compared to 17.7% of those who did not qualify. Other clinical characteristics were similar among the women who were screened and those who qualified for the study, as shown in Table 4.
Despite all attempts to identify obese women during the initial telephone screen, 17.2% study candidates who attended a screening visit were obese (BMI >30). Mean BMI in all the women who were screened was 26.3 ± 3.5 kg/m2; only 36% of the screened women had normal BMI (18.5 to 24.9), 44% were overweight (BMI 25 to 29), and 17% were obese (BMI 30-32). BMI in participants who were randomized was 26.3 ± 3.2 kg/m2, 35% had normal BMI, 47% were overweight, and 17% were obese.
Blood pressure was normal in 37% of the screened candidates, 38% were pre-hypertensive (systolic blood pressure (SBP) ≥120 or <140, or diastolic blood pressure (DBP) ≥ 80 or <90 mmHg), and 22% were hypertensive (SBP ≥ 140 or DBP ≥ 90 mmHg). Among the randomized women, 40% had normal blood pressure, 34% were pre-hypertensive, and 25% hypertensive.
In response to questions regarding menopausal symptoms, 81% of the screened participants reported hot flashes and almost 50% vaginal dryness. Regardless of the subgroup, women reported approximately 6 menopausal symptoms at the time of screening. Among the women who were randomized, 46% had low but measurable isoflavone levels in urine at baseline.
3.4. Participant Bias
In order to ascertain the potential of participation bias, demographic and clinical characteristics of participants who qualified and were randomized were compared to those of women who qualified but chose not to continue into the treatment phase (Tables 3 and 4). No significant differences between the two groups were detected for any of the demographic variables. However, participants who were randomized had significantly higher lumbar spine BMD than those who qualified but chose not to participate (p=0.028). Results of the screening tests were shared with all study participants, thus suggesting that although none had osteoporosis, a lower BMD might have prompted some of them to seek medical treatment.
3.5. Randomized group by race and ethnicity
Although we randomized a smaller percentage of blacks than expected, our study group adequately reflects the high proportion of Hispanics in the South Florida population. Because of the small number of Asian participants, this group is excluded from further statistical analyses. There were significant differences among the three main race/ethnic groups (Table 5). White non-Hispanics reported more years of education than did both white Hispanics (p= 0.001) and blacks (p= 0.04). White non-Hispanic participants had significantly lower mean BMI than black participants (25.6 ± 3.2 vs. 27.8 ± 2.9, p= 0.024). In addition, black women had a significantly higher mean systolic blood pressure than both white Hispanic and white non-Hispanic women (p= 0.001, p= 0.003, respectively), and significantly higher bone density than either group (p= 0.006 and p= 0.016, respectively). White non-Hispanic participants reported significantly higher daily calcium intake at baseline than black participants (1,080 ± 734 mg vs. 692 ± 451 mg, p= 0.016).
Table 5. Clinical characteristics of participants randomized in the SPARE Study by race and ethnicity.
| Total (n= 244)a |
White Hispanic (n= 164) |
White non- Hispanic (n= 56) |
Black (n= 24) |
|
|---|---|---|---|---|
| Age, mean ± SD | 52.5 ± 3.3 | 52.2 ± 3.2 | 53.2 ± 3.4 | 52.4 ± 3.4 |
| Education, mean ± SD | 14.5 ± 3.3 | 13.9+ ± 3.4 b | 16.1 ± 2.2 | 14.6 ± 3.1 |
| Menopausal symptoms, mean ± SD | 6.2 ± 2.8 | 6.1 ± 2.9 | 6.5 ± 2.9 | 5.7 ± 2.8 |
| BMI (kg/m2), mean ± SD | 26.3 ± 3.2 | 26.3 ± 3.2 | 25.7 ± 3.2c | 27.4 ± 2.9 |
| Systolic BP (mmHg), mean ± SD | 125.3 ± 17.9 | 124.1 ± 17.4 | 123.8 ± 17.3 | 136.8 ± 18.9 d |
| Diastolic BP (mmHg), mean ± SD | 78.1 ± 9.2 | 77.8 ± 9.1 | 77.6 ± 8.9 | 81.5 ± 9.8 |
| Normal blood pressure, n (%) | 99 (40.9) | 73 (44.8) | 234 (41.8) | 3 (12.5) |
| Pre–Hypertension, n (%) | 81 (33.5) | 49 (30.1) | 21 (38.2) | 11 (45.8) e |
| Hypertension, n (%) | 62 (25.6) | 41 (25.2) | 11 (20.0) | 10 (41.7) e |
| BMD spine (T-score), mean ± SD | −0.346 ± 1.04 | −0.461 ± 1.06 | −0.305 ± 0.866 | 0.345 ± 1.07 |
| BMD femoral neck (T-score), mean ± SD | −0.711 ± 0.80 | −0.787 ± 0.83 | −0.664 ± 0.667 | −0.300 ± 0.78f |
| BMD total hip (T-score), mean ± SD | −0.162 ± 0.85 | −0.213 ± 0.87 | −0.205 ± 0.760 | 0.292 ± 0.81f |
| Normal BMDg, n (%) | 132 (54.1) | 76 (46.3) | 36 (64.3) | 20 (83.3) |
Four Asian participants not included in this analysis
White Hispanics significantly have fewer years of education than White non-Hispanics (p=0.00)
White non-Hispanic participants have significantly lower BMI than blacks (p= 0.024)
Black women have significantly higher systolic blood pressure (BP) than both white Hispanics and white non-Hispanic participants (p=0.04)
A higher proportion of black women are both pre-hypertensive and hypertensive than white Hispanic (p= 0.001) and white non-Hispanic women (p=0.003)
Black women have significantly higher T-scores at all skeletal sites than both white Hispanic and white non-Hispanic participants (p= 0.006 and p= 0.016, respectively)
Normal BMD according to WHO definition (T-score ≥ −1.0)
4. Discussion
The main purpose of the SPARE study is to determine the effectiveness of soy isoflavones 200 mg daily in attenuating or preventing the rapid bone loss that usually occurs in the first years of menopause. A total of the 248 women were randomized into this trial, which includes a large proportion of white Hispanic women. A limitation of the study is the relatively high BMI of the study population which could impact the rate of bone loss over the course of the trial [39].
Although persons volunteering for a clinical trial could represent a self-selected group, we believe the characteristics of our participants represent those of the general local population. Clinical characteristics found during the screening of this multiethnic group of recently menopausal women anticipate potential medical problems that might arise as they age. Remarkably, almost 18% of the women who failed the screening process did so because of osteoporosis; 61% of the women screened were either overweight or obese, and 25% were hypertensive. In addition, 47% of the women participating in the study had measurable isoflavone levels in urine at baseline. Although the levels were low, they reflect the ubiquitous presence of isoflavones in the food supply [40].
It is usually women in the initial years of menopause who seek to prevent the consequences of estrogen depletion, including bone loss and menopausal symptoms. The WHI results stopped many menopausal women from starting or continuing hormone replacement and many resorted to soy isoflavone products expecting to obtain the benefits but none of the adverse effects of estrogens. Previous soy studies assessing the effects of soy isoflavones in menopausal women have had multiple limitations and reported conflicting results [18,19].
It is estimated that about 40% of all women will suffer an osteoporotic fracture in their lifetime [41]. Earlier studies have reported up to 50% trabecular bone loss and 30% loss of cortical bone loss during a woman’s lifetime [42]. Women experience the most severe menopausal symptoms immediately following the last menses and more than 10% of their bone mass can be lost in the initial 5 years of menopause [39,43,44]. Thus, until recently estrogen replacement was the first-line treatment for prevention of menopausal symptoms and early postmenopausal bone loss. The beneficial skeletal effects of estrogens have been extensively documented in observational studies and in prospective studies assessing surrogate markers for fracture risk, such as BMD and markers of bone turnover. The WHI was the first large-scale prospective clinical trial to assess the effectiveness of estrogen replacement, with and without progestin, in preventing fractures. Although the WHI did not address specifically early postmenopausal bone loss, the study showed a 24% decreased risk of fractures in menopausal women on estrogen therapy, with or without progestin [45]. After a mean use of hormone therapy for 5.2 years, the trial also demonstrated a 37% decreased risk of colon cancer, but a 26% increased risk of breast cancer and a 22% increase in cardiovascular events. Because of these concerns, many menopausal women stopped using estrogens and searched for natural alternatives that would provide skeletal benefits and relief from menopausal symptoms as estrogens but none of the potential adverse effects of estrogens.
Soy products have been actively sought by menopausal women because of their isoflavone content. In addition to protein, the soy bean contains isoflavones, natural phytoestrogens that activate estrogen receptors. The two major isoflavone glucosides in the soybean are genistin and daidzin. Once ingested, isoflavone glucosides are metabolized in the bowel by β-glucosidases of microbial and intestinal origin into the aglycones genistein and daidzein, respectively [46]. These compounds are absorbed as free isoflavones and also metabolized by the intestinal flora into other metabolites, such as equol which is derived from daidzein and is the most potent soy metabolite [47]. The metabolism of isoflavones in the intestine varies significantly among individuals, depending largely on the microflora, diet, and the use of antibiotics. Only about one third of the population has the ability to produce equol. The isoflavone molecules resemble that of 17-beta estradiol and bind to the estrogen receptor (ER) alpha and beta, exerting partial or full agonistic or antagonistic effects. Isoflavones have a weaker effect than estradiol. The relative affinities of these compounds for the ER vary and are lower than for estradiol, i.e., genistein has greater affinity for ER-beta than ER-alpha and equol has 10- to 100-fold greater affinity to ERs than daidzein [48,49].
It is still not clear how isoflavones influence bone remodeling in humans and evidence suggests that it might be through different mechanisms than estradiol [50]. At the time of menopause, low estrogen levels result in increased osteoclastic activity and rapid bone loss. Estrogen replacement protects from the rapid bone loss that accompanies menopause by decreasing the function and life span and inducing apoptosis of the osteoclast. Studies suggest that isoflavones act on both osteoclasts and osteoblasts [51,52]. Genistein appears to have an anabolic effect on bone, by acting directly on osteoblasts and some of these effects might not be through the ER [50].
Epidemiological studies strongly suggest that phytoestrogens have a beneficial skeletal effect. Among Chinese women, those on diets with higher isoflavone content have higher BMD and lower markers of bone turnover [53]. A prospective population-based study of Chinese women reported an inverse relationship between the consumption of soy foods and fracture risk [54]. Postmenopausal Japanese women and Japanese-American premenopausal women also show a significant positive association between isoflavone intake and BMD [30,55]. The consumption of soy products among women in the US varies significantly and is generally very low. Not surprisingly, a study of peri- and postmenopausal women in California did not find any association between soy intake and BMD [56].
Although animal studies demonstrate a clear skeletal benefit, prospective trials of soy isoflavones on bone in postmenopausal women, whether using soy foods or soy supplements, show conflicting results. Problems with most of these trials include their study design, small sample size, short duration (12 months or less), use of low doses, variation in isoflavone formulation (soy foods, soy supplements, isolated isoflavones), and high drop-out rates [20]. In addition, some trials have enrolled women who had recent treatment with bone-active drugs and most included women in a wide age range or who were several years into menopause, not women in the initial postmenopausal years, the most likely users of soy products [7,20]. In contrast, the SPARE study includes a large sample size, it enrolled multiethnic group of women in the first 5 postmenopausal years, has a 2-year duration, utilizes a large dose of isoflavones of a defined composition, and measures isoflavone metabolites, including equol, to correlate each participant’s biological response to her serum estradiol in addition to serve as an objective indicator of compliance. The results of the SPARE study will provide a wide range of information on the effectiveness of soy that is particularly important to a growing number of women, a number that continues to rise as the “baby boom” generation reaches menopause.
Based on the large sample size and multiethnic study population, the trial will be able to evaluate differences and similarities in the response to soy isoflavones among several racial and ethnic groups. In addition, the SPARE study is the first long-term prospective clinical trial simultaneously examining the effect of soy on multiple other outcomes associated to menopausal changes.
Conclusions
Soy supplements are increasingly popular among women who expect these products to prevent bone loss and symptoms associated with menopause. Although animal and human studies suggest a protective role of soy isoflavones, these studies have had several limitations, precluding evidence-based treatment recommendations. The SPARE Study is a double-blind randomized trial that recruited 248 women for a 2-year intervention with 200 mg of soy isoflavones or placebo. Given its large sample size and long duration, this study will provide much needed information about the skeletal and other benefits of soy isoflavone supplementation in the first five years of menopause.
Table 6. Demographic characteristics of participants randomized in the SPARE Study by treatment arm.
| Characteristic | All (n= 248) |
Soy (n= 122) |
Placebo (n= 126) |
|---|---|---|---|
| Age (years), n (%) | |||
| 45-49 | 50 (20.2) | 23 (18.9) | 27 (21.4) |
| 50-54 | 129 (52.0) | 60 (49.2) | 69 (54.8) |
| 55-60 | 69 (27.8) | 39 (32.0) | 30 (23.8) |
| Age, years, mean ± SD | 52.5 ± 3.3 | 52.9 ± 3.3 | 52.1 ± 3.3* |
| Race/ethnicity, n (%) | |||
| White Hispanic | 164 (66.1) | 76 (62.3) | (88 (69.8) |
| White non-Hispanic | 56 (22.6) | 33 (27.0) | 23 (18.3) |
| African American | 24 (9.7) | 12 (9.8) | 12 (9.5) |
| Asian | 4 (1.6) | 1 (0.8) | 3 (2.4) |
| Education, n (%) | |||
| Less than high school | 35 (14.1) | 12 (9.8) | 23 (18.3) |
| High school | 114 (46.0) | 54 (44.3) | 60 (47.6) |
| College | 59 (23.8) | 33 (27.1) | 26 (20.6) |
| Post-graduate | 40 (16.1) | 23 (18.8) | 17 (13.5) |
| Education, years, mean ± SD | 14.5 ± 3.3 | 1 4.8 ± 3.1 | 14.3 ± 3.4 |
| Marital status, n (%) | |||
| Married or Significant other | 153 (61.7) | 72 (59.0) | 81 (64.3) |
| Divorced/Separated | 65 (26.2) | 33 (27.1) | 32 (25.4) |
| Widowed | 9 (3.6) | 5 (4.1) | 4 (3.2) |
| Never Married | 21 (8.5) | 12 (9.8) | 9 (7.1) |
| Unknown | 0 | 0 | 0 |
p = 0.03
Table 7. Clinical and laboratory characteristics of participants in the SPARE Study by treatment arm.
| Characteristic | All (n= 248) |
Soy (n= 122) |
Placebo (n= 126) |
|---|---|---|---|
| BMI, n (%) | |||
| Underweight (BMI <18.5) | 1 (0.4) | - - - | 1 (0.8) |
| Normal (BMI ≥18.5 - 24.9) | 89 (35.9) | 46 (37.7) | 43 (34.1) |
| Overweight (BMI 25-29) | 116 (46.8) | 60 (49.2) | 56 (44.4) |
| Obese (BMI 30-32) | 42 (16.9) | 16 (13.1) | 26 (20.6) |
| BMI (kg/m2), mean ± SD | 26.29 ± 3.2 | 25.99 ± 3.2 | 26.57 ± 3.2 |
| Waist to hip ratio | 0.848 ± 0.5 | 0.82 ± 0.1 | 0.82 ± 0.1 |
| BP (mm Hg), mean ± SD | |||
| Mean systolic BP | 125.3 ± 17.8 | 121.4 ± 16.8 | 118.6 ± 14.9 |
| Mean diastolic BP | 78.1 ± 9.1 | 76.4 ± 9.5 | 75.5 ± 8.8 |
| Blood pressure status, n (%) | |||
| Normal blood pressure1 | 99 (39.9) | 44 (36.1) | 55 (43.7) |
| Pre-hypertensive2 | 85 (34.3) | 44 (36.1) | 41 (32.5) |
| Hypertension3 | 62 (25.0) | 33 (27.0) | 29 (23.0) |
| BMD (g/cm2), mean ± SD | |||
| Lumbar spine | 1.135 ± 0.126 | 1.146 ± 0.125 | 1.132 ± 0.124 |
| Total hip | 0.984 ± 0.107 | 0.990 ± 0.110 | 0.982 ± 0.104 |
| Femoral neck | 0.936 ± 0.108 | 0.940 ± 0.119 | 0.937 ± 0.100 |
| BMD category n (%) | |||
| Normal (T-score ≥−1.0) | 133 (53.8) | 66 (54.1) | 68 (54.0) |
| Osteopenia (T-score <−1.0 to >−2.0) | 114 (46.2) | 56 (45.9) | 58 (46.0) |
| Menopausal symptoms, mean ± SD | 12.58 ± 6.87 | 12.15 ± 6.61 | 12.95 ± 7.10 |
| Vaginal Maturation Value, mean ± SD | 41.7 ± 19.0 | 40. 7 ± 18.9 | 43.0 ± 19.3 |
| Estradiol (pg/mL), mean ± SD | 16.22 ± 25.79 | 13.85 ± 21.08 | 18.95 ± 30.22 |
| Genistein, urine (pmol/μL), mean ± SD | 0.43 ± 1.40 | 0.53 ± 1.84 | 0.32 ± 0.59 |
| Daidzein, urine (pmol/μL), mean ± SD | 0.94 ± 3.28 | 1.23 ± 4.42 | 0.62 ± 0.94 |
| Equol, urine (pmol/μL), mean ± SD | 0.19 ± 0.95 | 0.25 ± 1.27 | 0.12 ± 0.33 |
| TSH (μIU/L), mean ± SD | 2.35 ± 4.82 | 2.01 ± 1.3 | 2.74 ± 7.0 |
| Positive TPO Ab (%) 4 | 13.19 | 10.10 | 16.87 |
| Total cholesterol (mg/dL), mean ± SD | 214.0 ± 36.7 | 215.4 ± 38.4 | 212.5 ± 34.8 |
| HDL cholesterol (mg/dL), mean ± SD | 56.3 ± 14.6 | 56.9 ± 15.5 | 55.5 ± 13.7 |
| LDL cholesterol (mg/dL), mean ± SD | 132.4 ± 33.0 | 132.3 ± 35.9 | 132.5 ± 29.6 |
| Triglycerides(mg/dL), mean ± SD | 128.3 ± 76.5 | 133.5 ± 89.7 | 122.1 ± 57.4 |
BMI: Body Mass Index; BP: blood pressure; BMD: bone mineral density;
Normal blood pressure: systolic BP (SBP) <120 and diastolic BP (DBP) < 80
Pre-hypertensive: SBP ≥120 to <140 orDBP ≥80 to <90
Hyypertension: SBP ≥140 or DBP ≥90
Positive TPO antibodies: >35 IU/mL
Acknowledgements
This work is supported by a grant (R01 AR48932-01A1) from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases. The views expressed do not necessarily reflect those of the U.S. Food and Drug Administration. The authors report no competing financial interests.
We thank Ann Herrin RN, Sylvia Morales RN, Patricia Bryan RN, for their excellent clinical care of study participants, Iliana Perez for bone densitometry services, Aileen Saraceno, Rafael Franjul, and Concepcion Ramos for their assistance in the recruitment of participants, Emilio Weiss and Rand Fingles for their outstanding research pharmacy services, and Lisa Rafkin-Mervis for her assistance in the initial planning stages of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Utian WH. The place of oestriol therapy after menopause. Acta Endocrinol Suppl (Copenh) 1980;233:51–6. [PubMed] [Google Scholar]
- 2.Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–3. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Buist DS, Newton KM, Miglioretti DL, Beverly K, Connelly MT, Andrade SE, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–50. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 4.Newton KM, Buist DS, Hartsfield CL, Andrade SE, Wei F, Connelly MT, et al. Hormone therapy initiation after the Women’s Health Initiative. Menopause. 2009;15:487–93. doi: 10.1097/gme.0b013e318154b9a5. [DOI] [PubMed] [Google Scholar]
- 5.Wei F, Miglioretti DL, Connelly MT, Andrade SE, Newton KM, Hartsfield CL, et al. Changes in women’s use of hormones after the Women’s Health Initiative estrogen and progestin trial by race, education, and income. J Natl Cancer Inst Monogr. 2005;35:106–12. doi: 10.1093/jncimonographs/lgi047. [DOI] [PubMed] [Google Scholar]
- 6.Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone mineral metabolism and serum lipid in postmenopausal Japanese women: a four-week study. J Am Coll Nutr. 2002;21:97–102. doi: 10.1080/07315724.2002.10719200. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004;11:290–8. doi: 10.1097/01.gme.0000097845.95550.71. [DOI] [PubMed] [Google Scholar]
- 8.Lydeking-Olsen E, Beck-Jensen JE, Setchell KD, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss – a 2-year randomized, placebo-controlled trial. Eur J Nutr. 2005;43:246–57. doi: 10.1007/s00394-004-0497-8. [DOI] [PubMed] [Google Scholar]
- 9.Potter SM, Baum JA, Teng H, et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68(6 Suppl):1375S–9S. doi: 10.1093/ajcn/68.6.1375S. [DOI] [PubMed] [Google Scholar]
- 10.Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–12. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- 11.Yamori Y, Moriguchi EH, Teramoto T, Miura A, Fukui Y, Honda KI, et al. Soybean isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: a ten-week study. J Am Coll Nutr. 2002;21:560–3. doi: 10.1080/07315724.2002.10719255. [DOI] [PubMed] [Google Scholar]
- 12.Harkness LS, Fiedler K, Sehgal AR, Oravec D, Lerner E, et al. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J Women’s Health. 2004;13:1000–7. doi: 10.1089/jwh.2004.13.1000. [DOI] [PubMed] [Google Scholar]
- 13.Wangen KE, Duncan AM, Mertz-Demlow BE, Xu X, Marcus R, Phipps WR, et al. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85:3043–8. doi: 10.1210/jcem.85.9.6787. [DOI] [PubMed] [Google Scholar]
- 14.Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, Haschke F, et al. Effect of a nutritional supplementation on bone health in Chilean elderly subjects with femoral osteoporosis. J Am Coll Nutr. 2006;25:170–7. doi: 10.1080/07315724.2006.10719529. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006;55:423–33. doi: 10.1016/j.metabol.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Kenny AM, Mangano KM, Abourizk RH, Bruno RS, Anamani DE, Kleppinger A, et al. Soy proteins and isoflavones affect bone mineral density in older women: a randomized controlled trial. Am J Clin Nutr. 2009;90:234–42. doi: 10.3945/ajcn.2009.27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. Effects of phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women. Ann Int Med. 2007;146:839–47. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: Meta-analysis of randomized controlled trials. Clin Nutr. 2008;27:57–64. doi: 10.1016/j.clnu.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone. 2009;44:948–53. doi: 10.1016/j.bone.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Vupadhyayula PM, Gallagher JC, Templin T, Logsdon SM, Smith LM. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women – a 2-year randomized, double-blind, placebo-controlled trial. Menopause. 2009;16:320–8. doi: 10.1097/gme.0b013e3181844893. [DOI] [PubMed] [Google Scholar]
- 21.Chiechi LM, Secreto G, D’Amore M, Fanelli M, Venturelli E, Cantatore F, et al. Efficacy of soy rich diet in preventing postmenopausal osteoporosis: the Menfis randomized trial. Maturitas. 2002;42:295–300. doi: 10.1016/s0378-5122(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 22.Dalais FS, Rice GE, Wahlqvist ML, Grehan M, Murkies AL, Medley G, et al. Effects of dietary phytoestrogens in postmenopausal women. Climacteric. 1998;1:124–9. doi: 10.3109/13697139809085527. [DOI] [PubMed] [Google Scholar]
- 23.Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4740–7. doi: 10.1210/jc.2003-030290. [DOI] [PubMed] [Google Scholar]
- 24.Kreijkemp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lample JW, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F, PHYTOS Investigators Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr. 2008;87:761–70. doi: 10.1093/ajcn/87.3.761. [DOI] [PubMed] [Google Scholar]
- 26.Eastell R, Mallinak N, Weiss S, Ettinger M, Petinger M, Cain D, Fressland K, Chestnut C., III Biological variability of serum and urinary N-telopeptides of type I collagen in postmenopausal women. J Bone Miner Res. 2000;15:594–8. doi: 10.1359/jbmr.2000.15.3.594. [DOI] [PubMed] [Google Scholar]
- 27.Adlercreutz H, Markhanen H, Watanabe S. Plasma concentration of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–10. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 28.Doerge DR, Chang HC. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J Chromatogr B Biomed Appl J Chrom B. 2002;777:245–55. doi: 10.1016/s1570-0232(02)00214-3. [DOI] [PubMed] [Google Scholar]
- 29.Block G. Dietary assessment issues related to cancer for NHANES III. Vital Health Stat. 1992;4:24–31. [PubMed] [Google Scholar]
- 30.Perczek RE, Carver CS, Price AA, Pozo-Kaderman C. Coping, mood, and aspects of personality in Spanish translation and evidence of convergence with English versions. J Pers Assess. 2000;74:63–87. doi: 10.1207/S15327752JPA740105. [DOI] [PubMed] [Google Scholar]
- 31.Greendale GA, Zibecchi L, Petersen L, Ouslander JG, Kahn B, Ganz PA. Development and validation of a physical examination scale to assess vaginal atrophy and inflammation. Climacteric. 1999;2:197–204. doi: 10.3109/13697139909038062. [DOI] [PubMed] [Google Scholar]
- 32.Meisels A. The maturation value. Acta Cytol. 1967;11:249. [PubMed] [Google Scholar]
- 33.Doerge DR, Chang HC, Holder CL, Churchwell MI. Enzymatic conjugation of the soy isoflavones, genistein and daidzein, and analysis in human blood using liquid chromatography and mass spectrometry. Drug Metab Dispos. 2000;28:298–307. [PubMed] [Google Scholar]
- 34.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25:628–42. [PubMed] [Google Scholar]
- 35.Wilkund I, Holst J, Karlberg J, Mattson LA, Samsioe G, Sandin K, et al. A new methodological approach to the evaluation of quality of life in postmenopausal women. Maturitas. 1992;14:211–24. doi: 10.1016/0378-5122(92)90116-l. [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 37.Beck AT, Steer RA, Garrison R. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 38.McNair DM, Lorr M, Droppleman LF. Profile of Mood of States (POMS) Manual. Educational and Industrial Testing Service; San Diego, CA: p. 1071. [Google Scholar]
- 39.Recker RR, Davies KM, Dowd K, Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000;15:1965–73. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 40.Kardinaal AF, Morton MS, Brüggemann-Rotgans IE, van Beresteijn EC. Phytooestrogen excretion and rate of bone loss in postmenopausal women. Eur J Clin Nutr. 1998;52:850–5. doi: 10.1038/sj.ejcn.1600659. [DOI] [PubMed] [Google Scholar]
- 41.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 42.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–8. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahlborg HG, Johnell O, Nilsson BE, Jeppsson S, Rannevik G, Karlsson MK. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28:327–31. doi: 10.1016/s8756-3282(00)00451-8. [DOI] [PubMed] [Google Scholar]
- 44.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–7. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 45.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberb C, Stefanick ML, et al. Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 46.Vincent A, Fitzpatrick LA. Soy isoflavones: are they useful in menopause? Mayo Clin Proc. 2000;75:1174–84. doi: 10.4065/75.11.1174. [DOI] [PubMed] [Google Scholar]
- 47.Joannou GE, Kelly GE, Reeder AY, Waring M, Nelson C. A urinary profile study of dietary phytoestrogens: the identification and mode of metabolism of new isoflavonoids. J Steroid Biochem Mol Biol. 1995;54:167–84. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 48.Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, et al. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem Res Toxicol. 2001;14:280–95. doi: 10.1021/tx000208y. [DOI] [PubMed] [Google Scholar]
- 49.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 50.Poulsen RC, Kruger MC. Soy phytoestrogens: impact on postmenopausal bone loss and mechanism of action. Nutr Rev. 2008;66:359–74. doi: 10.1111/j.1753-4887.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 51.Yamagishi T, Otsuka E, Hagiwara H. Reciprocal control of expression of mRNAs for osteoclast differentiation factor and OPG in osteogenic stromal cells by genistein: evidence for the involvement of topoisomerase II in osteoclastogenesis. Endocrinology. 2001;142:3632–7. doi: 10.1210/endo.142.8.8310. [DOI] [PubMed] [Google Scholar]
- 52.Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. 2002;295:417–22. doi: 10.1016/s0006-291x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- 53.Mei C, Yeung SSC, Kung AWC. High dietary phytoestrogen intake is associated with hiher bone mieral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001;86:5217–21. doi: 10.1210/jcem.86.11.8040. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Int Med. 2005;165:1890–5. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 55.Horiuchi T, Onouchi T, Takahashi M, Ito H, Orimi H. Effect of soy protein on bone metabolism in postmenopausal Japanese women. Osteoporos Int. 2000;11:721–4. doi: 10.1007/s001980070072. [DOI] [PubMed] [Google Scholar]
- 56.Kritz-Silverstein D, Goodman-Gruen DL. Usual dietary isoflavone intake, bone mineral density, and bone metabolism in postmenopausal women. J Womens Health Gend Based Med. 2002;11:69–78. doi: 10.1089/152460902753473480. [DOI] [PubMed] [Google Scholar]