Abstract
Background
The first step in infection by human parainfluenza viruses (HPIVs) is binding to the surface of respiratory epithelial cells via interaction between viral receptor-binding molecules and sialic acid-containing receptors. DAS181, a recombinant sialidase protein containing the catalytic domain of A. viscosus sialidase, removes cell surface sialic acid, and we proposed that it would inhibit HPIV infection.
Methods
Depletion of sialic acid receptors by DAS181 was evaluated by lectin binding assays. Anti-HPIV activity in cultured cell lines and in human airway epithelium (HAE) was assessed by the reduction in viral genomes and/or plaque forming units (PFU) upon treatment. In vivo efficacy of intranasally administered DAS181 was assessed using a cotton rat model.
Results
DAS181-mediated desialylation led to anti-HPIV activity in cell lines and HAE. Intranasal DAS181 in cotton rats, a model for human disease, significantly curtailed infection.
Conclusions
Enzymatic removal of the sialic acid moiety of HPIV receptors inhibits infection with all tested HPIV strains, both in vitro and in cotton rats. Enzyme-mediated removal of sialic acid receptors represents a novel antiviral strategy for HPIV. The results of this study raise the possibility of a broad spectrum antiviral agent for influenza virus and HPIVs.
Keywords: Parainfluenza, LLC-MK, CV-1, wdHAE, human airway epithelium, cotton rat, DAS181, sialidase
Introduction
Human parainfluenza viruses (HPIVs) are a group of respiratory viruses that cause human diseases including bronchitis, bronchiolitis and pneumonia in infants, children or immunocompromised individuals [1], and increasingly recognized as an etiology of lower respiratory disease in people of all ages[2, 3]. HPIVs, together with two other respiratory viruses, influenza virus (IFV) and respiratory syncytial virus (RSV), are responsible for the majority of respiratory viral infections that require medical attention. Currently there are no effective vaccines or specific treatments for HPIV infection [4, 5]. It is difficult to distinguish clinically between the lower respiratory tract illnesses caused by IFV, HPIV, RSV, human metapneumovirus (hMPV) and other viruses, and specific viral diagnosis is typically unavailable. Therefore, it is frequently not possible to determine quickly the etiologic agent in an individual with acute onset of lower respiratory tract disease. Nonetheless, the available antiviral therapies are effective only when started as early as possible after the onset of symptoms. A broad spectrum therapeutic approach against multiple respiratory pathogens, that could be initiated before the etiologic agent is identified, may therefore be of clinical use.
The first step in infection of a cell by HPIV is binding to the target cell surface, via interaction of the viral receptor-binding molecule (hemagglutinin-neuraminidase, HN) with sialic acid-containing receptor molecules (Sia) on the cell surface. HPIV-1 and HPIV-3 interact with α2,3-linked Sia [6-8] or α2,6-linked Sia [9], but the importance of more detailed modifications/branches of these Sias has only recently been examined for HPIVs [6, 8-11]. After interacting with its receptor, the HN molecule activates the viral fusion protein (F), and the viral envelope then fuses directly with the plasma membrane of the cell, releasing the viral nucleocapsid into the cytoplasm to initiate infection.
HN-receptor interaction is the critical prelude to F-triggering and viral entry, and an attractive step for blocking viral infection [12]. We have shown that HN-receptor interaction can be inhibited by small molecules that interact with the HN receptor binding site [12]. We also showed that HPIV infection in cultured monolayer cells is prevented by treating the culture with either viral or bacterial sialidase to remove cell surface Sia receptors [13]. Depending on the degree of removal of Sia receptors, varying effects were obtained, ranging from reduced spread of virus to complete inhibition of viral entry [13-16]. More recently, using human airway epithelial (HAE) cell cultures that more closely represent the natural host tissue, treatment with a sialidase has been shown to inhibit HPIV3 infection [9, 17]. The HAE model replicates the pathogenesis of HPIV3 in cotton rats, and the HAE culture can be used to assess inhibitory strategies that would be effective in vivo [17]. These data suggested that removal of Sia moieties by neuraminidase could be an effective antiviral approach for HPIV.
DAS181 is a recombinant sialidase protein containing the catalytic domain of A. viscosus sialidase and the heparin-binding domain from human amphiregulin (AR) [18], which prolongs DAS181 retention on the epithelial surface. This compound has been studied as a possible treatment for influenza infection. We have previously shown that DAS181 can effectively remove both α2,3- and α2,6-linked Sia from MDCK cells and thereby inhibit infection by all IFV strains tested, including both human and avian IFV strains and novel H1N1 [18]. We proposed that DAS181 would inhibit HPIV infection, similarly to the action of the A. ureafaciens sialidase previously tested in cultured cell monolayers[9]. The current study describes DAS181-mediated desialylation and antiviral activity in several commonly used cell lines infected with all three major serotypes of HPIV. In agreement with the predictions based on the effect of neuraminidase treatment on HPIV3 infection in HAE [17], we also demonstrated DAS181 anti-HPIV activity in HAE, and in the HPIV-3 cotton rat infection model. These data suggest that this anti-HPIV strategy warrants further study to assess its potential clinical utility for HPIV infection, and raise the possibility of a broad-spectrum antiviral agent against both IFV and PIV.
Materials and Methods
Cells and viruses
LLC-MK and CV-1 cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in MEM/EBSS media supplemented with 10% FBS, 1x Glutamax (Invitrogen, Carlsbad, CA), and 1x Antibiotic/Antimycotic solution (Sigma, St Louis, MO) at 37°C in a humidified atmosphere of 5% CO2. HAE cultures (EpiAirway™, Mattek Corporation, Ashland, MA) and maintained in manufacturer-supplied media (Air-100 media). HAE cultures were acclimated at 37°C and 5% CO2 for 18-24 hours prior to all experiments.
Virus stocks of HPIV1 (C-35), HPIV2 (Greer), HPIV3 (C243; “HPIV3C”), and Sendai (murine PIV1, strain 52) were obtained from ATCC and propagated on LLC-MK cells. HPIV3W (Wash/47885/57) was obtained from the National Institutes of Health (HA-1, NIH # 47885, cat # V323-002-020) and propagated and titered on CV-1 cells as previously described[19]. IFV A/PortChalmers/1/73 (H3N2) was obtained from ATCC, propagated on MDCK cells, and tested for drug sensitivity in HAE as described in [20].
Desialylation assay
LLC-MK cells were treated with DAS181 (100 μL/well) for 2 hours at 37°C followed by washing the cells 2x with PBS, fixing with 0.05% glutaraldehyde for 10-15 min in PBS, and blocking with 3% BSA in PBS overnight at 4°C. Sia levels were detected with a cell-based lectin ELLA as described [18]. All samples were normalized such that 100% Sia was defined as the absorbance at 450nm of untreated tissues and 0% Sia was defined as the absorbance at 450nm of untreated tissues, without the lectin incubation step. The % Sia remaining was calculated using 100% × [(Abs of DAS181 treated cells - background)/(Abs of untreated cells - background)].
Infection and treatment protocols
LLC-MK cells were infected with HPIV at indicated MOIs for 2 hours at 37°C, followed by washing and treating with DAS181. Virus production at 72 hours was quantified by cell-based ELISA. CV-1 cells were infected with HPIV and simultaneously treated with DAS181 for 90 minutes. DAS181 was reapplied for 30 minutes, followed by washing, on days 1 and 2. On day 3, viral titer was quantified by plaque assay [21].
For assessing DAS181 domains, LLC-MK cells were treated with DAS180, DAS181, or DAS185 just before infection for 2 hours at 37°C followed by washing, HPIV infection for 2 hours at 37°C, then washing again, and replenishing with either fresh media alone or media/DAS variants.
For the experiment in Figure 2 and Table 3, the wdHAE cultures were pretreated with DAS181 for 2 hours at 37°C, followed by washing and infection with IFV A/PortChalmers/1/73 (10,000 PFU/well), HPIV-2 (10 PFU/well) or HPIV3 (300,000 PFU/well) for 4 hours at 37°C. After washing away unbound virus (1x with 200 μL PBS), fresh DAS181 was added back to the cultures. Apical washes (100 mL PBS) were collected daily and stored at -80°C. After each collection fresh DAS181 was added back to the cultures. qRT-PCR analysis of all samples was carried out and dose-response data is shown for the day with peak viral titer (from wells lacking drug) (day 3 for IFV A/PortChalmers/1/73, day 4 for PIV-2, day 6 for PIV-3). Days chosen represent day of peak viral titer, for each virus, in the absence of drug.
Figure 2. DAS181 efficiently inhibits HPIV genome replication in HAE culture.
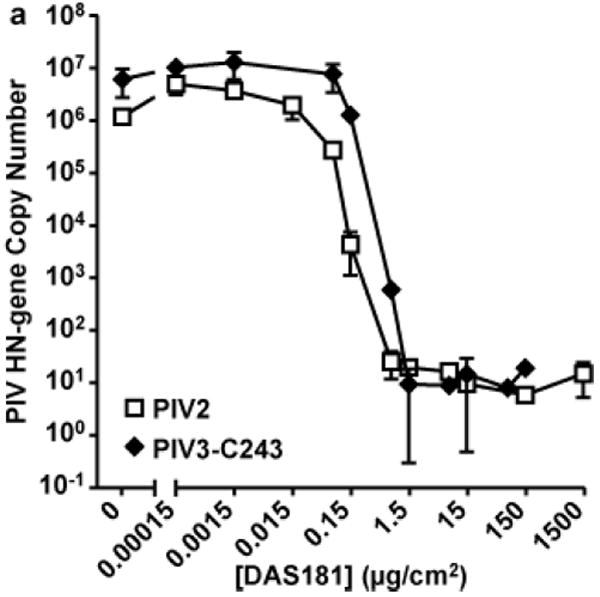
HAE were treated with DAS181 for 2 hours at 37°C followed by washing and infection with virus for 4 hours. After washing, DAS181 was added back the HAE topical surface. Fresh DAS181 was added to the HAE surface once daily. On day 4 (HPIV2) or day 6 (HPIV3) post-infection viral titer in apical wash was quantified by qRT-PCR for the PIV HN gene copy number. 1.0 ug/cm2 DAS181 = 178.6 nM DAS181. Values represent mean ± SEM of 3 replicate wells.
Table 3.
Comparative Potencies of DAS181 for IFV and PIV in HAE Cultures
| Virus | EC50 (μg/cm2) | EC90 (μg/cm2) | EC99 (μg/cm2) |
|---|---|---|---|
| IFV A/Port Chalmers/1/73 | 0.14 | 0.31 | 1.1 |
| HPIV2 | 0.03 | 0.08 | 0.12 |
| HPIV3 (C243) | 0.1 | 0.19 | 0.48 |
HAE in 96-well tissue culture plates was treated with DAS181 at various dose levels for 2 hours at 37°C, followed by washing with PBS and infection with IFV A or HPIVs. On the day of peak titer post-infection, viral titer in the apical wash was quantified by qRT-PCR assays.
To assess production of infectious virus in wdHAE (figure 3) the cultures were infected with PIV at 400 PFU/well or 4000 PFU/well for 90 minutes in the presence of DAS181. Cultures were washed, then supernatants were aspirated and the cultures were incubated at 37°C overnight. DAS181 was added apically for 30 minutes, followed by washing, on day 1 and 2. On day 3 viral titer was quantified by plaque assay on CV-1 cells [21].
Figure 3. DAS181 efficiently inhibits HPIV production of infectious progeny virions in HAE culture (row a) or CV-1 cells (row b).
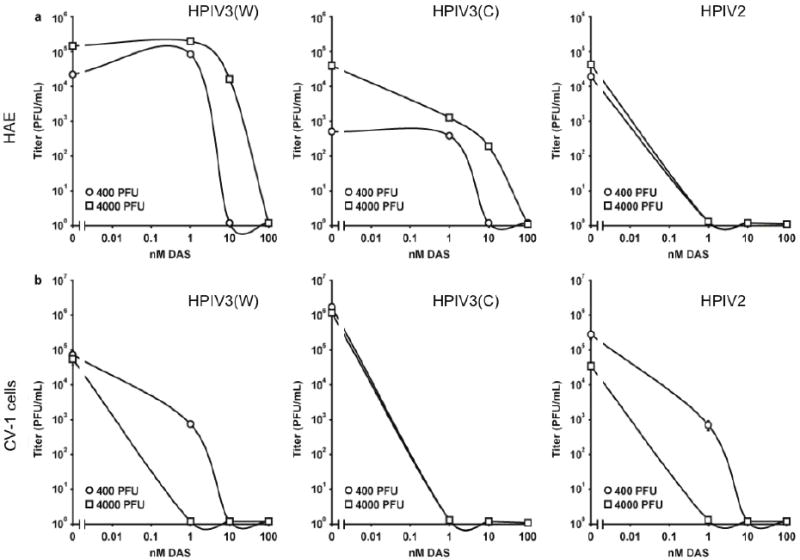
HAE were infected with either 400 (circles) or 4000 (squares) PFU of (i) HPIV3(W) (ii) HPIV3(C) or (iii) HPIV2 and treated with various concentrations of DAS181 during infection (90 mins) then daily for 30 mins until day 3. Supernatant fluids were collected and titered. Titers 3 days post infection are shown.
Cell-based ELISA
Cells were fixed in 0.05% glutaraldehyde in PBS for 10-15 minutes, blocked in 3% BSA in PBS for 15 minutes at 37°C (or 4°C overnight), then incubated with 50μL of either goat anti-PIV-1 polyclonal antibody (10μg/mL, cat # 20-PG89, Fitzgerald Industries, Concord, MA), goat anti-PIV-3 polyclonal antibody (10μg/mL, cat # 20-PG90, Fitzgerald) or mouse anti-PIV-2 monoclonal antibody (0.5 μg/mL, AbD Serotec, cat # 7140-2017) for 1 hour at 37°C. Cells were then incubated with 50μL of 1:2500 dilution of HRP-conjugated donkey anti-goat IgG (Promega, Madison, WI, cat # V805A) or 1:5000 dilution of HRP-conjugated goat anti-mouse IgG (Pierce, Rockford, IL, cat # S372B) for 1 hour at 37°C. The wells were developed with 50μL/well of TMB (Sigma), stopped with 50μL/well of 1 M H2SO4, and absorbance was quantified at 450nm. Uninfected, untreated cells were the background control. Infected, untreated cells were the 100% infection control. EC values were extrapolated from dose response curves. Unless otherwise indicated, EC values are the mean of at least 3 independent experiments.
Plaque assays
PFU was determined by counting viral plaques on CV-1 cell monolayers as previously described [17, 21, 22]. HPIV stocks used for LLC-MK cell experiments, and for the HAE experiment shown in figure 2, were titered by counting viral plaques on LLC-MK cell monolayers.
qRT-PCR
Daily apical washes were collected and stored at -80°C until viral RNA was purified from 50 μl of wash sample using Applied Biosystem’s MagMAX-96™ Viral RNA isolation kit. cDNA was synthesized with 10 μL of the purified viral RNA using Applied Biosystem’s cDNA Synthesis kit. PCR reactions were run under the conditions of one cycle of 95°C for 20 seconds, followed by 45 cycles of 95°C for 3 seconds and 60°C for 30 seconds on an ABI 7500 Fast Real-time PCR system.
Cotton rat experiments
Twenty female cotton rats aged 3 to 5 weeks (Harlan, Indianapolis) were kept under constant environmental conditions (temperature 21±1.5°C; humidity 30 to 45%). At the age of 6 - 8 weeks the rats were divided into 4 groups with five animals/group: group 1 was treated with PBS, group 2, 3, and 4 were daily intranasally administrated for three days with DAS181 at dose levels of 0.1, 0.3 or 1 mg/kg/day in 100μL volume. The first treatment was initiated one hour prior to viral challenge on day 0. All groups of cotton rats were challenged by 106 PFU of HPIV3W virus (Wash/47885/57) intranasally in 100 μL volume. Animals were euthanized by CO2 inhalation on day 3 post-infection, and lungs were homogenized with glass douncers. Viral titers in lung homogenates were quantified by plaque assay in CV-1 cells as above. The homogenates were greatly diluted before titering, so that the treatments performed in the rats would not affect titering results. Statistical analysis was performed with Prism 4.0 software. Significance determined by ANOVA with Bonferroni post-test.
Results
Desialylation of LLC-MK cells
DAS181 removes Sia from MDCK cells [18] and human tracheal tissue[23]. Since MDCK cells do not support efficient PIV infection, we tested desialylation efficacy of DAS181 in LLC-MK cells. Figure 1 shows that DAS181 efficiently removes both α(2,3)- and α(2,6)-linked Sia from LLC-MK cells in a dose-dependent manner. The desialylation efficacy of DAS181 is superior to that of DAS180, a DAS181 analogue without the AR-domain, by up to 100x, consistent with our previous observations in MDCK cells[18]. As expected, DAS185, a DAS181 analogue with one point mutation in the sialidase functional domain (Y348F) that reduces the sialidase activity by >200x (data not shown), is ineffective at desialylating LLC-MK cells, especially α(2,6)-linked Sia, shown in figure 1.
Figure 1. Desialylation of LLC-MK cells.
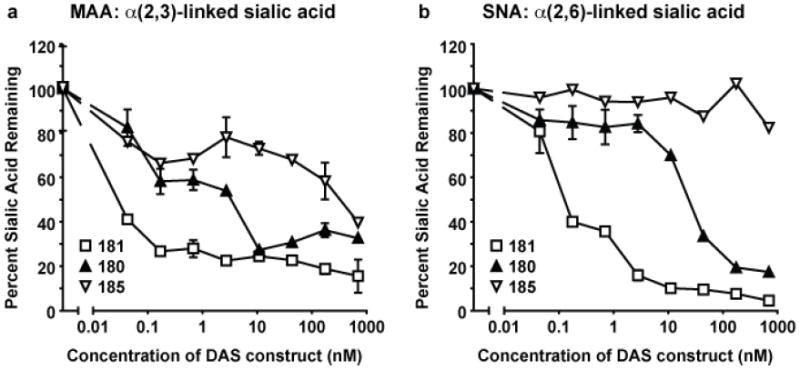
LLC-MK cells were treated with DAS181, DAS180, or DAS185 for 2 hours at 37°C followed by washing and detection of remaining sialic acid with a cell-based lectin ELLA.MAA lectin recognizes α2,3-sialic acids (A) and SNA lectin recognizes α2,6-sialic acids (B). Error bars represent standard error of the mean of 3 experiments
Anti-PIV activity of DAS181 in cultured cell lines
The anti-PIV activity of DAS181 was examined in HPIV strains in LLC-MK and CV-1 cell lines. First, we treated LLC-MK cells with DAS181 immediately following infection with HPIV-1, 2, 3 or Sendai virus (murine PIV-1). Three days later, the levels of viral replication were determined by cell-based ELISA. All tested viruses were inhibited by DAS181. The concentrations of DAS181 yielding 50%, 90%, and 99% PIV inhibition (EC50, 90, 99) were determined (table 1). The HPIV strains are more sensitive to DAS181 treatment than the murine PIV, Sendai. HPIV3, the most prevalent HPIV strain associated with severe infections, is sensitive to DAS181 even at high challenge doses (MOI up to 1). The observed broad-spectrum and efficient inhibition of PIV is consistent with previous experiments using bacterial neuraminidases to prevent infection with HPIV3 [13-16] and comparable to the anti-IFV activity previously described [18]. The antiviral potency of DAS181 is only somewhat dependent on the virus challenge dose; despite 10-fold increases in infectious dose, the EC50, EC90, and EC99 values increased to a lesser degree.
Table 1.
Inhibition of PIV Replication in LLC-MK Cells by DAS181
| MOI | EC50 (nM) | EC90 (nM) | EC99 (nM) |
|---|---|---|---|
| HPIV1 | |||
| 0.1 | 136 ± 38 | 1089 ± 332 | 1922 ± 1754 |
| 0.01 | 54 ± 46 | 240 ± 102 | 673 ± 446 |
| 0.001 | 31 ± 33 | 87 ± 53 | 155 ± 74 |
| HPIV2 (Greer) | |||
| 0.1 | 14.7 ± 5.6 | n/a | n/a |
| 0.01 | 2.5 ± 2.3 | 36.1 ± 18.9 | 58.6 ± 31.0 |
| 0.001 | 1.8 ± 0.9 | 5.1 ± 1.0 | 10.0 ± 7.3 |
| HPIV3 (C243) | |||
| 1 | 43.3 ± 11.9 | 113.6 ± 40.6 | 307.7 ± 242.1 |
| 0.1 | 15.5 ± 5.3 | 53.0 ± 20.3 | 96.1 ± 9.8 |
| 0.01 | 2.8 ± 2.7 | 16.5 ± 5.7 | 39.4 ± 15.3 |
| Sendai virus | |||
| 0.1 | 3363 ± 73 | 6908 ± 6234 | 22646 |
| 0.01 | 1283 ± 422 | 3194 ± 2835 | 12313 ± 8387 |
| 0.001 | 328 ± 50 | 1081 ± 1437 | 5988 ± 6227 |
LLC-MK cells were infected with virus at indicated MOIs for 2 hrs. After washing, culture media containing DAS181 at different concentrations were added to the cells. Viral titer was quantified by cell-based ELISA at 3 days post-infection. Titer in infected/PBS-treated cells was taken as 100% infection, and in uninfected cells as 0% infection. EC values were extrapolated from dose response curves. Values represent mean ± SEM of 3 experiments for each virus, except 2 experiments for PIV2.
To confirm anti-PIV activity of DAS181, CV-1 cells were infected with HPIV2 or HPIV3 in the presence or absence of DAS181, and viral titers determined by plaque assay. DAS181 at 1 nM completely inhibited replication of two HPIV3 strains and significantly inhibited HPIV2 (table 2).
Table 2.
Inhibition of HPIV Replication in CV-1 Cells by DAS181
| Treatment | Virus Titer (PFU) | ||
|---|---|---|---|
| HPIV2 | HPIV3 (C243) | HPIV3W | |
| PBS | 268,750±70,986 | 1,175,000±75,777 | 54,687±24,054 |
| DAS181 1 nM | 687±217 | 0 | 0 |
| DAS181 10 nM | 0 | 0 | 0 |
| DAS181 100 nM | 0 | 0 | 0 |
CV-1 cells were simultaneously infected with virus (400 PFU/well, 6-well format) and treated with DAS181 for 90 minutes. Drug was reapplied to the cells daily for 30 min on days 1 and 2. On day 3, viral titer was determined via plaque assay (PFU). Values represent mean ± SEM of 3 experiments.
As expected, the inactive DAS181 analogue, DAS185, does not exhibit anti-HPIV activity. DAS180 is effective at inhibiting HPIV3 replication in LLC-MK cells although at a lower potency than DAS181 (data not shown). These observations confirm the critical role of sialidase function in the anti-HPIV activity of DAS181.
DAS181-mediated anti-PIV activity in HAE culture
The pseudostratified well-differentiated human airway epithelium culture (wdHAE) contains all the differentiated cell types found in normal human airway epithelium, and thus mimics the human respiratory epithelium morphologically and functionally [24]. HAE replicates natural host infection with HPIV [9], and we showed that HAE faithfully reflects HPIV3 pathogenesis in the cotton rat, suggesting that the HAE culture can be used to assess inhibitory strategies that would be effective in vivo [17]. We have previously demonstrated that DAS181 removes Sia from the HAE apical surface and prevents IFV infection of HAE [20]. We tested whether DAS181 inhibits HPIV infection of HAE. HAE were pre-treated with DAS181 for 2 hours, then infected with HPIV2 or HPIV3. Several days later, apical wash samples were collected and analyzed by qRT-PCR to monitor viral replication levels. In this experiment, treatment doses are expressed as μg/cm2 of apical surface area, instead of concentration (nM). Figure 2 shows that at dose levels >0.1 μg/cm2 apical area, replication of both HPIV strains was effectively inhibited.
To assess for the ability of DAS181 to inhibit production of progeny virus and thus prevent ongoing infection, we tested the effect of treatment on the production of infectious viruses (figure 3). We determined the effect of DAS181 on viral titer of HPIV2 and two different strains of HPIV3, HPIV3C and HPIV3W. HAE were infected with either 400 or 4000 PFU of HPIV2, HPIV3C or HPIV3W for 90 minutes in the presence of various concentrations of DAS181. The supernatant fluids were then aspirated to maintain the integrity of the air/liquid interface. HAE were incubated at 37°C, and each day for 3 days, medium containing the appropriate concentration of DAS181 was added apically for 30 minutes to the cultures. This treatment served to expose the cells to DAS181, and also to collect viral particles that had budded from the HAE during the previous 24 hours. In parallel, comparison sets of cultured monolayer cells (CV-1) were infected in the presence of the same concentrations of DAS181. Figure 3 shows the viral titers from day 3 post-infection, at various DAS181 concentrations, in HAE cultures (a) or CV-1 cells (b). Identical experiments were performed for HPIV3W (left), HPIV3C (center) or HPIV2 (right), using either 400 or 4000 PFU. Concentrations as low as 0.1nM DAS181 inhibited infectious virus production for HPIV2, while somewhat higher concentrations were needed to inhibit infectious virus production for HPIV3, depending on the PFU in the initial inoculum. After infection with 400PFU, concentrations of DAS181 above 1nM reduced infectious virus production for HPIV3C and HPIV3W. After infection with a 10x larger inoculum (PFU of 4000), a higher concentration of DAS181 (10nM) is needed to inhibit viral growth.
We further compared anti-HPIV activity of DAS181 in the HAE cultures against the previously observed activity of DAS181 against IFV in the same model system [20]. As shown in Table 3, in the HAE model system DAS181 exhibits potent and comparable inhibition against HPIV3, HPIV2 and an IFV strain A/PortChalmers/1/73.
Anti-HPIV3 activity in a cotton rat model
With demonstrated anti-HPIV activity against all four tested HPIVs in vitro, we next tested anti-HPIV activity in vivo using a well established HPIV3 cotton rat infection model [25, 26]. Twenty female cotton rats were divided into 4 groups, and all groups were infected with 106 PFU HPIV3W per rat via intranasal administration (i.n.). All groups were treated daily (100μL i.n.) for three days starting one hour pre-infection, with group 1 receiving PBS (untreated control), and groups 2, 3, and 4 receiving DAS181 at 0.1, 0.3, or 1mg/kg/day, respectively. All the animals were euthanized on day 3 post-infection for determination of viral replication in lung by plaque assay. The resulting viral titer data demonstrate the effective suppression of HPIV3W infection by DAS181 in vivo, as reflected by significant and dose dependent inhibition of HPIV3W titer in the rat lungs (figure 4). Note that these cotton rat data reflect the result of pre-treatment plus continued treatment; the effect of treatment only after infection remains to be evaluated.
Figure 4. Viral titer in the lungs of HPIV3W-infected cotton rats treated with DAS181.
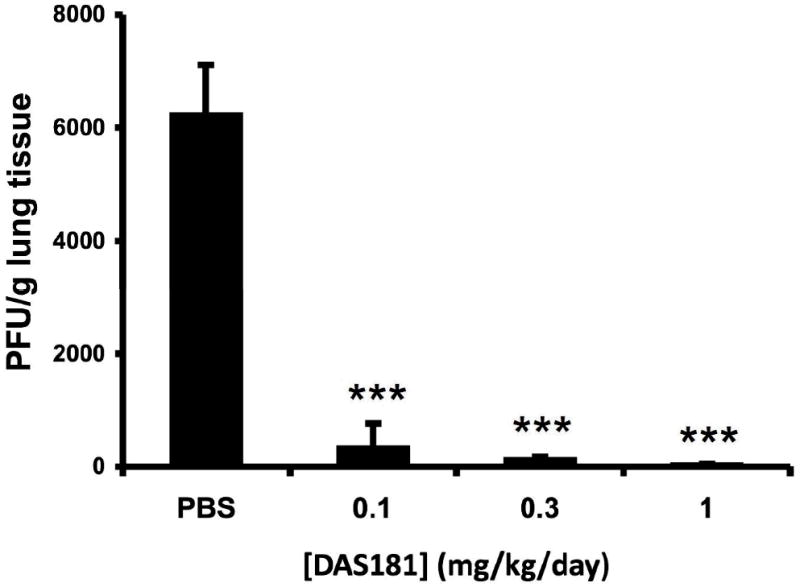
20 female cotton rats were infected with 106 PFU of HPIV3W via intranasal administration. Animals were divided into 4 groups and treated with either PBS or DAS181 at three different dose levels (0.1, 0.3, 1 mg/kg) daily via intranasal administration (100μl, q.d.x3, starting 1h before infection). On day 3 post-infection, the lungs were harvested, homogenized, viral titer was determined by plaque assay, and expressed as plaques / gram lung tissue. Values represent the mean ± SEM of 5 animals per group. *** = p<0.001 as determined by ANOVA with Bonferroni post-test.
Discussion
Therapies for parainfluenza and other respiratory viruses are urgently needed [12, 27]. Respiratory viruses in the paramyxovirus family, including HPIV, RSV and metapneumovirus, have lagged far behind influenza virus in terms of the development of effective antiviral drugs and vaccines, despite the recognized impact of these diseases in children [1], and the more recently recognized importance of these pathogens in the adult population, particularly older adults[28]. And while for respiratory syncytial virus, effective strategies of prophylaxis are available to protect the groups at most risk [1, 29], there are no similar strategies available for the parainfluenza viruses. In recent years, fundamental mechanisms of HPIV entry and infection have been identified, opening the way for development of antiviral strategies that subvert these mechanisms [12]. To directly prevent or debilitate the infection process, the most straightforward strategy described here directly interferes with viral entry by removing the portion of Sia receptors used for binding by the parainfluenza HN protein and thereby preventing the first step in infection.
While human IFV strains predominantly recognize (2,6)-linked Sia as the receptor, HPIV strains have different receptor specificities[7, 30]. Sendai virus (SV) (murine parainfluenza virus type 1) binds (2,3)-linked Sia as the receptor [31, 32]. HPIV-1 recognizes (2,3)-linked Sia as the receptor, while HPIV-3 recognizes (2,3)- and (2,6)-linked Sia equally well [7]. In human respiratory epithelium, (2,6)-linked Sia is more abundant and is present on both the ciliated and nonciliated cells, while (2,3)-linked Sia is less abundant and is restricted to ciliated cells [33]. Avian influenza H5 and H7 strains have high affinity to α2,3-linked Sia with various subterminal sugar structures [8, 34], while some viruses especially prefer subterminal structures which are sulfated at the 6 position of Gal or GlcNAc, or fucosylated at the 3 position of GlcNAc. Human parainfluenza viruses, HPIV1 and HPIV3, also recognize sulfated or fucosylated α2,3-linked Sia as receptors.
DAS181 carries the sialidase functional domain derived from sialidase of Actinomyces viscosus. The A. viscosus sialidase has broad substrate specificity and potent enzymatic activity [20]. DAS181 inhibits cell binding and infection by a wide range of IFV strains [18, 35]. Data in this report show potent inhibition of DAS181 against several different PIV strains. Collectively, these results indicate that DAS181 is effective at inactivating a broad range of Sia receptors regardless of their subterminal modifications, including the sulfated or fucosylated Sia receptors recognized by HPIVs as well as by avian IFVs.
The neuraminidase inhibitor zanamivir is a sialic acid analog that competitively inhibits the influenza neuraminidase by engaging the active site, and is a clinically effective drug for prophylaxis and treatment of influenza [36, 37]. For HPIV3, zanamivir inhibits HN-receptor interaction but does not inhibit HPIV3 infection at clinically relevant concentrations [12, 38]. In contrast, DAS181 is at least as efficient at inhibiting HPIV as IFV. Our data suggest that DAS181 doses effective for IFV will be effective for PIV as well (Table 3). The different sensitivity of different HPIVs to DAS181 is reflected in different EC50 values. For instance, the weaker activity against Sendai virus (mouse PIV-1), may relate to the lower sensitivity of Sendai virus Sia receptor to DAS181.
DAS181 was previously shown to inhibit a recombinant strain of HPIV3 in a high-throughput antiviral screening assay[39]. Among 23 compounds tested in the assay, DAS181 exhibited the highest potency in EC50, and is at least 60-fold more potent than the other HPIV inhibitory compounds emerging from the screen [39]. The demonstration of anti-PIV activity for DAS181 in both HAE, a culture model that reflects the human airway, and in the cotton rat animal model, suggests that further study is warranted to evaluate the anti-HPIV effect of DAS181 in the clinical setting. DAS181 has been well tolerated in phase 1 clinical trials (unpublished data), and a phase 2 clinical trial to evaluate efficacy of DAS181 against influenza will be initiated shortly. This approach, if effective, could thus be used even in cases where respiratory viral diagnosis is delayed or absent. This ability would be useful given the urgent need for therapy for these viruses, especially for the vulnerable populations of children, elderly individuals, the immunocompromised and patients with underlying airway disease.
Acknowledgments
We thank Shaklee Corporation for support of the development of our human airway epithelial tissue model (A.M. and M.P.), and we (A.M. and M.P.) are grateful to Laura Palermo for helpful discussions and experimental support of the human airway epithelial tissue model and to Christine C. Yokoyama for helpful discussions and assistance with preparing the figures.
Financial support: The work at NexBio was supported by NIH grant U01AI070281 and NIH NIAID contract HHSN266200600015C. The work at Weill Cornell was supported by Public Health Service grant AI31971 from the National Institutes of Health (NIAID) and a March of Dimes Research Grant to AM, and by an American Lung Association Research grant to MP. AM, MP and SN received funds from NexBio to cover a portion of the costs of experimental reagents used for this project.
Footnotes
Potential conflicts of interest: Q.-X.L, G.T.B., C.T, D.W., M.Y., and F.F. are/were employees of NexBio, a developer of DAS181. A.M. serves on advisory boards or as a consultant for Medimmune, GlaxoSmithKline, and Roche. A.M., M.P., and S.N. received funds from NexBio to cover a portion of the costs of experimental reagents used for this project. S.P., no conflicts to report.
References
- 1.Loughlin GM, Moscona A. The cell biology of acute childhood respiratory disease: therapeutic implications. Pediatr Clin North Am. 2006;53:929–59. doi: 10.1016/j.pcl.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134:1141–8. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhi SA, Cutland C, Zhu Y, et al. Transmissibility, infectivity and immunogenicity of a live human parainfluenza type 3 virus vaccine (HPIV3cp45) among susceptible infants and toddlers. Vaccine. 2006;24:2432–9. doi: 10.1016/j.vaccine.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Wright PF. Current status of vaccines for parainfluenza virus infections. Pediatr Infect Dis J. 2008;27:S123–5. doi: 10.1097/INF.0b013e318168b76f. [DOI] [PubMed] [Google Scholar]
- 6.Amonsen M, Smith DF, Cummings RD, Air GM. Human parainfluenza viruses hPIV1 and hPIV3 bind oligosaccharides with alpha2-3-linked sialic acids that are distinct from those bound by H5 avian influenza virus hemagglutinin. J Virol. 2007;81:8341–5. doi: 10.1128/JVI.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T, Portner A, Scroggs RA, et al. Receptor specificities of human respiroviruses. J Virol. 2001;75:4604–13. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls JM, Chan RW, Russell RJ, Air GM, Peiris JS. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008;16:149–57. doi: 10.1016/j.tim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Bukreyev A, Thompson CI, et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79:1113–24. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar E, Barroso IM. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj J. 2006;23:5–17. doi: 10.1007/s10719-006-5433-0. [DOI] [PubMed] [Google Scholar]
- 11.Porotto M, Fornabaio M, Greengard O, Murrell MT, Kellogg GE, Moscona A. Paramyxovirus receptor-binding molecules: engagement of one site on the hemagglutinin-neuraminidase protein modulates activity at the second site. J Virol. 2006;80:1204–13. doi: 10.1128/JVI.80.3.1204-1213.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115:1688–98. doi: 10.1172/JCI25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moscona A, Peluso RW. Fusion properties of cells persistently infected with human parainfluenza virus type 3: Participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991;65:2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moscona A, Peluso RW. Fusion properties of cells infected with human parainfluenza virus type 3: Receptor requirements for viral spread and virus-mediated membrane fusion. J Virol. 1992;66:6280–6287. doi: 10.1128/jvi.66.11.6280-6287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscona A, Peluso RW. Analysis of human parainfluenza virus 3 receptor binding variants: evidence for the use of a specific sialic acid-containing receptor. Microbial Pathogenesis. 1996;20:179–184. doi: 10.1006/mpat.1996.0016. [DOI] [PubMed] [Google Scholar]
- 16.Ah-Tye C, Schwartz S, Huberman K, Carlin E, Moscona A. Virus-receptor interactions of human parainfluenza viruses types 1, 2 and 3. Microb Pathog. 1999;27:329–36. doi: 10.1006/mpat.1999.0313. [DOI] [PubMed] [Google Scholar]
- 17.Palermo L, Porotto M, Yokoyama C, et al. Human parainfluenza virus infection of the airway epithelium: the viral hemagglutinin-neuraminidase regulates fusion protein activation and modulates infectivity. J Virol. 2009;83:6900–6908. doi: 10.1128/JVI.00475-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50:1470–9. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscona A, Peluso RW. Relative affinity of the human parainfluenza virus 3 hemagglutinin-neuraminidase for sialic acid correlates with virus-induced fusion activity. J Virol. 1993;67:6463–6468. doi: 10.1128/jvi.67.11.6463-6468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triana-Baltzer GB, Babizki M, Chan MC, et al. DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J Antimicrob Chemother. 2009;65:275–84. doi: 10.1093/jac/dkp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin Perlman S, Jordan M, Brossmer R, Greengard O, Moscona A. The use of a quantitative fusion assay to evaluate HN-receptor interaction for human parainfluenza virus type 3. Virology. 1999;265:57–65. doi: 10.1006/viro.1999.0024. [DOI] [PubMed] [Google Scholar]
- 22.Murrell M, Porotto M, Weber T, Greengard O, Moscona A. Mutations in human parainfluenza virus type 3 HN causing increased receptor binding activity and resistance to the transition state sialic acid analog 4-GU-DANA (zanamivir) J Virol. 2003;77:309–317. doi: 10.1128/JVI.77.1.309-317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholls JM, Aschenbrenner LM, Paulson JC, et al. Comment on: concerns of using sialidase fusion protein as an experimental drug to combat seasonal and pandemic influenza. J Antimicrob Chemother. 2008;62:426–8. doi: 10.1093/jac/dkn167. author reply 428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–66. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter D, Prince G, Hemming V, Porter H. Pathogenesis of human parainfluenza virus 3 infection in two species of cotton rat: Sigmodon hispidus develops bronchiolitis, while Sigmodon fulviventer develops interstitial pneumonia. J Virol. 1991;65:103–111. doi: 10.1128/jvi.65.1.103-111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince GA, Ottolini MG, Moscona A. Contribution of the human parainfluenza virus type 3 HN-receptor interaction to pathogenesis in vivo. J Virol. 2001;75:12446–51. doi: 10.1128/JVI.75.24.12446-12451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLaMora P, Moscona A. A daring treatment and a successful outcome: the need for targeted therapies for pediatric respiratory viruses. Pediatr Transplant. 2007;11:121–3. doi: 10.1111/j.1399-3046.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 28.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 29.Groothuis J, Simoes E, Levin M, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants ind young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 30.Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- 31.Markwell M, Moss J, Hom B, Fishman P, Svennerholm L. Expression of gangliosides as receptors at the cell surface controls infection of NCTC 2071 cells by Sendai virus. Virology. 1986;155:356–64. doi: 10.1016/0042-6822(86)90199-6. [DOI] [PubMed] [Google Scholar]
- 32.Holmgren J, Svennerholm L, Elwing H, Fredman P, Strannegard O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc Natl Acad Sci U S A. 1980;77:1947–50. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci U S A. 2004;101:4620–4. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belser JA, Blixt O, Chen LM, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc Natl Acad Sci U S A. 2008;105:7558–63. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belser JA, Lu X, Szretter KJ, et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007;196:1493–9. doi: 10.1086/522609. [DOI] [PubMed] [Google Scholar]
- 36.Moscona A. Medical management of influenza infection. Annu Rev Med. 2008;59:397–413. doi: 10.1146/annurev.med.59.061506.213121. [DOI] [PubMed] [Google Scholar]
- 37.Moscona A. Neuraminidase inhibitors for influenza. New Engl Jl Med. 2005;353:1363–73. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 38.Greengard O, Poltoratskaia N, Leikina E, Zimmerberg J, Moscona A. The anti-influenza virus agent 4-GU-DANA (Zanamivir) inhibits cell fusion mediated by human parainfluenza virus and influenza virus HA. J Virol. 2000;74:11108–14. doi: 10.1128/jvi.74.23.11108-11114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth JP, Li JK, Smee DF, Morrey JD, Barnard DL. A recombinant, infectious human parainfluenza virus type 3 expressing the enhanced green fluorescent protein for use in high-throughput antiviral assays. Antiviral Res. 2009;82:12–21. doi: 10.1016/j.antiviral.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


