Abstract
Immunotherapies targeting the amyloid-β (Aβ) peptide in Alzheimer's disease (AD) have consistently been effective in mouse studies and shown promise in clinical trials, although some setbacks have occurred. First, encephalitis was observed in a small subset of patients. More recent autopsy data from a few subjects suggests that clearance of Aβ plaques may not halt cognitive deterioration once impairments are evident, emphasizing the need for other more effective approaches at that stage of the disease.
Another important target in AD is the neurofibrillary tangles and its precursors, composed primarily of hyperphosphorylated tau proteins, which correlate well with the degree of dementia. As Aβ and tau pathologies are likely synergistic, targeting both together may be more effective, and perhaps essential as early diagnosis prior to cognitive decline is currently unavailable. Also, Aβ immunotherapy results in a very limited indirect clearance of tau aggregates, showing the importance of developing a separate therapy that directly targets pathological tau. Our findings in two tangle mouse models indicate that active immunization targeting an AD phospho-tau epitope reduces aggregated tau in the brain and prevents/slows progression of the tangle-related behavioral phenotype, including cognitive impairment. These antibodies enter the brain and bind to pathological tau within neurons although the therapeutic effect may at least in part be due to clearance of extracellular tau that may have biological effects.
We are currently clarifying the mechanism of these promising findings, determining its epitope specificity as well as assessing the feasibility of this approach for clinical trials.
Keywords: Tau, immunotherapy, vaccine, immunization, phosphorylation
INTRODUCTION
Over 20 years ago, several mutations were discovered in the amyloid precursor protein (APP) around the amyloid-β (Aβ) cleavage site or within the peptide in familial forms of Alzheimer's disease (AD) and related congophilic amyloid angiopathies. These important findings led to the overriding focus of AD therapies on this particular peptide. Initially, most of these studies focused on developing inhibitors of its aggregation and/or its production. More recently, harnessing the immune system to clear Aβ has been particularly promising, and various immunotherapies targeting Aβ are currently in clinical trials [1,2]. While some encouraging results have been reported [3-10], recent preliminary findings from the Phase I AN1792 trial suggest that it may be too late to target Aβ for clearance once cognitive impairments are evident [11]. Specifically, several Alzheimer's patients in the trial had substantial or near complete removal of Aβ plaques although experiencing severe end-stage dementia at death. It should be emphasized, however, that biochemical analysis of Aβ remained to be performed in these individuals. Prior report on two subjects from this trial showed that clearance of Aβ deposits was associated with a sharp elevation of soluble Aβ that likely contains oligomers [12], which numerous animal studies have shown to be toxic and detrimental to cognition [13]. More prolonged vaccination regimen may be needed to clear these Aβ remnants which will hopefully be clarified in the ongoing active and passive immunotherapy trials targeting Aβ.
Although tau pathology is another major hallmark of AD and the key hallmark of most forms of frontotemporal dementia, relatively few studies have described potential therapeutic approaches [14-16]. A primary reason for this discrepancy is that most of its aggregates are found within neurons, which complicates its targeting for clearance. However, it should be kept in mind that although Aβ aggregation may initiate the pathological cascade in at least some forms of AD, Aβ and tau pathologies are likely synergistic based on experimental animals studies [17-21] as well as on post-mortem analysis of AD brains [22,23]. Furthermore, tau pathology [24,25] and synapse loss [26-28] correlate much better with dementia severity than Aβ deposition. Hence, targeting these pathologies rather than or in concert with Aβ may be more efficacious, particularly after the onset of cognitive deterioration.
The focus here will be to briefly review the concept and initial findings of a particular therapeutic approach, namely immunotherapy targeting pathological hyperphosphorylated tau proteins in AD and related tauopathies.
TAU IMMUNOTHERAPY – MOUSE STUDIES
The objective of our approach was to generate antibodies via active immunization that selectively or specifically recognize the pathological hyperphosphorylated tau protein. These antibodies should then promote clearance of tau aggregates that would restore or improve neuronal function. An immediate concern with this design and with therapies targeting tau in general is that this protein is primarily found intracellularly. Therefore, any effective treatment needs to find its way into the cell. As addressed later and detailed previously [29], several studies have actually shown that antibodies do enter the brain and neurons, particularly under pathological conditions, supporting this rather provocative idea. In addition, it is conceivable that removal of extracellular tau may have a biological effect [30], and possibly facilitate clearance and/or secretion of intracellular tau. Furthermore, while our studies were underway or being reviewed, reports from related fields strengthened the validity of our concept. Namely, vaccination with recombinant α-synuclein has been shown to lead to clearance of intraneuronal aggregates of the same sequence in transgenic Parkinson's model mice [31], and anti-Aβ antibodies are taken up into neurons in culture [32] and promote clearance of intracellular Aβ aggregates [32]. In addition, antibodies against the β-secretase cleavage site of the amyloid precursor protein (APP) can be taken up into CHO cells expressing human APP751 which also reduces intracellular as well as extracellular Aβ levels [53]. It is also noteworthy, that studies focusing on APP metabolism have employed antibodies that bind to the cell surface of APP to follow APP internalization to endosomal-lysosomal compartments [33,34].
Based on information from computer algorithms, known tau epitopes and pilot studies in wild-type mice, we immunized transgenic mice (JNPL3) expressing the P301L tau mutation [35] with a 30 amino acid tau fragment that contained two phosphorylation sites that are prominent in AD (Tau379−408[P-Ser396,404] [36]. Alum adjuvant was chosen as it promotes antibody response over a cytotoxic T cell response [37], and is still the only approved adjuvant for human use. We selected homozygous animals for the study as their tau pathology is more robust and less variant than in heterozygous JNPL3 mice. However, in mice with this aggressive progression of tau-related pathology and functional impairments, complete prevention of the disease cannot be expected. Furthermore, because of the severe phenotype at a relatively early age which starts with tau aggregation as early as at 2−3 months of age and culminates in a moribund paralysis at about 12 months of age, this model is not well suited to assess reversal of the modeled disease. The vaccination was initiated once the mice had a fully developed immune system at about 2 months of age when tau aggregates are already forming in their brains. The immunized mice did significantly better on motor tests relevant to their phenotype with better effect observed at an earlier stage of the pathology (5 months) than at a later stage (8 months). Accordingly, more significant clearance of tau aggregates was observed at the earlier time point. The tau antibodies generated in these mice recognized pathological tau on brain sections demonstrating their selectivity/specificity. Furthermore, when purified, labeled with FITC and injected into the carotid artery of JNPL3 P301L mice, these antibodies were prominently observed within the brain and specifically within neurons where they co-labeled with monoclonal antibodies that specifically target pathological tau. FITC-labeled pooled mouse IgG from wild-type controls entered the brain as well although its labeling appeared to be nonspecific. Uptake of FITC-labeled antibodies was not evident in wild-type animals suggesting an impaired blood-brain-barrier (BBB) in the JNPL3 P301L mice that is likely to be essential for the efficacy of the immunotherapy. Similar scenario can be envisioned in tauopathies as the BBB is likely to be impaired in these diseases. Then, with prophylactic therapy circulating tau antibodies would be relatively inert until progressive inflammatory changes associated with the brain pathology will lead to gradual opening of the BBB with concomitant increase in antibody access into the brain and subsequent clearance of tau aggregates.
As the homozygous JNPL3 P301L model develops severe motor impairments its cognitive status is not easily assessed as intact mobility is required for the extensive maze running that is an integral component of most cognitive tests in rodents. Additionally, a model with a slower rate of tau pathology should more accurately reflect the treatment potential of this approach in the human condition that takes years or decades to result in functional impairments. With these issues in mind, we assessed the therapeutic potential of the same tau immunogen in a novel model of tangle pathology. Importantly, our preliminary findings indicate a complete prevention of cognitive deterioration in three different tasks, and comparable clearance of tau aggregates within the brain [38].
MECHANISM OF CLEARANCE
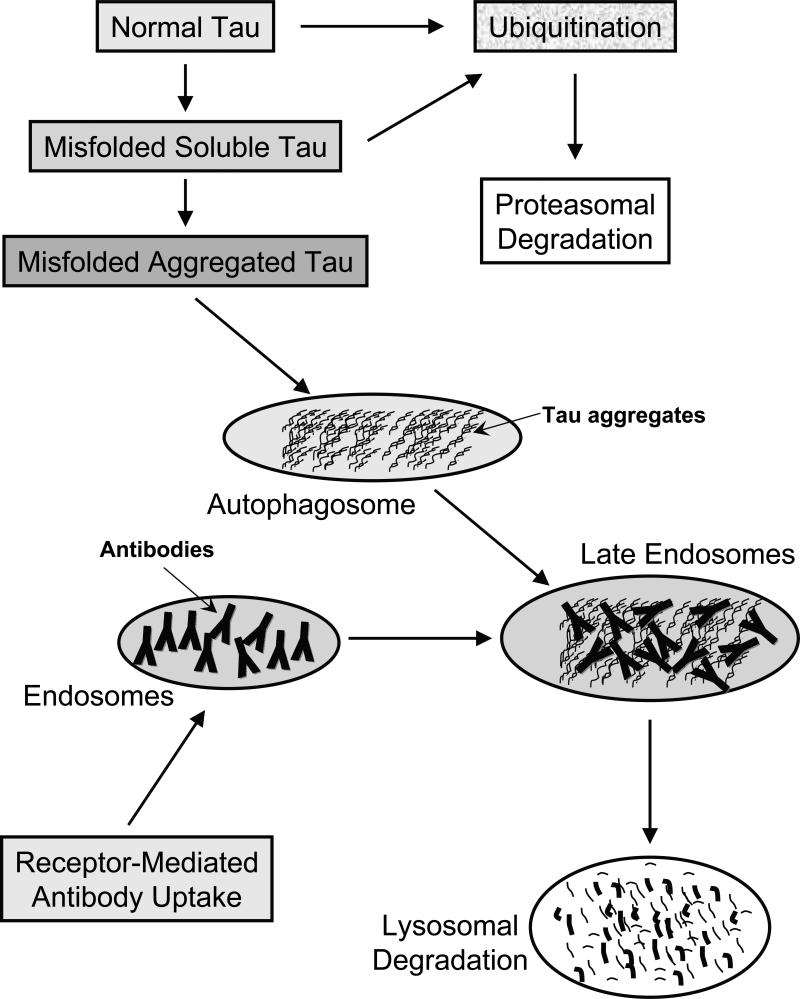
Before considering how immunotherapy targeting phospho-tau epitopes may lead to clearance of pathological tau proteins it is appropriate to outline briefly the cellular mechanisms that are likely involved in tau metabolism (Figure 1). The two major protein degradation pathways in eukaryotic cells are the proteasomal and lysosomal systems [39]. Generally, short lived proteins are thought to be mainly degraded by proteasomes and long-lived proteins mainly by autophagy via lysosomes. Furthermore, it appears that soluble misfolded proteins are preferentially degraded by the proteasome system and by autophagy when its capacity is exceeded [40]. On the other hand, small and larger aggregates should not be able to access the proteosome and would therefore be removed solely by autophagy. While there are several forms of autophagy, removal of tau aggregates is likely mediated through macroautophagy. It is characterized by the formation of a double-membrane bound structure, the autophagosome, which engulfs cytoplasmic entities, in this case tau aggregates. It can then fuse with late endosomes and/or lysosomes in which the aggregates undergo enzymatic degradation. Under pathological conditions, such as in AD this pathway is likely overwhelmed leading to large amounts of tau aggregates within the cytosol that over time form paired helical filaments, one of the major characteristic feature of the disease.
Figure 1. Clearance of normal and pathological tau within neurons.
Normal tau is likely to be marked by ubiquitination for subsequent proteasomal degradation. Misfolded soluble tau should be cleared by the same route whereas once it aggregates, those assemblies are presumably too large for the proteosome and could be encapsulated within the autophagosome.
Receptor-mediated antibody uptake will place those in endosomes that can then fuse with autophagosomes in late endosomes, in which the antibodies may bind to the tau aggregates. At that stage and/or when the endosomes fuse with lysosomes, these antibodies may promote disassembly of the aggregates that should facilitate their cleavage by lysosomal enzymes.
Also, before addressing how tau antibodies may lead to clearance of tau aggregates, one needs to envision how these antibodies can end up within the brain in the extracellular milieu and within neurons. It is well established that low levels of antibodies (0.1%) can enter the brain under normal conditions [41]. IgG has been shown to cross the BBB via adsorptive-mediated transcytosis [42], and it can be speculated that some or perhaps most of the uptake may be through the circumventricular organs that because of their function do not have a classic BBB [43,44]. As we have observed in the homozygous JNPL3 P301L mouse model, a much larger percentage of IgG enters the CNS with advanced tau pathology [36]. Interestingly, we have also observed in this model an age-related increase in tau autoantibodies [36], which can be due to diminished immunoprivilege of the CNS following a gradual opening of the BBB as tau pathology escalates. Likewise, the BBB is compromised in various neurodegenerative diseases such as AD [45], indicating that a similar scenario can play out in these human conditions. Immunologists are also well aware of the fact that antibody-secreting cells can enter the brain and secrete antibodies locally. These observations regarding antibody access to the CNS and into neurons are also supported by numerous other animal studies as we have discussed previously in more detail [29,46].
It is likely that tau antibodies can target pathological tau both extra- and intracellularly. The extracellular clearance can be envisioned to occur similar to what is thought to take place with antibodies targeting Aβ; antibody binding to the aggregates (oligomers to fibrils) may directly promote their disassembly and as well signal microglia to clear the antibody-protein complexes. This removal may prevent potential direct or indirect toxic effect of extracellular tau aggregates. It should also be noted that it is conceivable that extracellular tau is not solely derived from dead cells but that it is secreted from cells and it may have an extracellular function [30]. If that is the case, then antibody-mediated clearance of extracellular tau may promote secretion of intracellular tau through a shift in equilibrium. This equilibrium may be bidirectional, in other words extracellular tau may be taken up into not only microglia for clearance but also neurons and astrocytes. This uptake pathway may be more prominent under pathological conditions and may explain in part the anatomical spread of tangle pathology with the progression of the disease. Another way to clear intracellular tau is via antibody uptake. As we have discussed previously in more detail, neurons have several receptors that can bind IgG and intraneuronal antibodies have been detected in numerous studies, often under pathological conditions [29]. The site of antibody-tau interaction within the cell is likely to take place in the endosomal/autophagy-lysosomal system. Ultrastructural analysis of the JNPL3 P301L mouse model has shown prevalent lysosomal and autophagic vesicles associated with tau filaments [47], as well as lysosomal complexes in neuronal cultures expressing various tau mutations [48]. Separately, antibodies have been detected within lysosomes by immunoelectroscopy [49].
TOXICITY
Potential toxic effects associated with this type of therapy are mainly two-fold: 1) Antibody-binding to normal tau may impair its function and/or promote its clearance which could have detrimental effects on neuronal integrity; and 2) activation of the immune system (or the antibodies themselves in case of passive immunization) may lead to adverse reactions. Both of these issues have been addressed previously in more detail [29,46]. Briefly, regarding the first issue, by targeting phosphorylation sites that are prominent in AD, binding of antibodies to normal tau should be minimal. In support of this, Western blot levels of normal tau do not appear to be reduced in our immunized mice [36]. In addition, tau knock-out mice are remarkably normal suggesting that other microtubule associated proteins can take over its functions [50]. Additionally, crosses between tau-knockout mice and Aβ plaque mice have less Aβ induced pathology [51], suggesting that even if this type of therapy cleared normal tau to some extent it might actually be beneficial. It is also important to note that under normal conditions these antibodies do not appear to have appreciable access to the brain or into neurons [36]. Adverse reactions related to autoimmunity will always be a concern when self-antigens are being targeted although with careful design this issue can be mostly avoided as evident by the several ongoing trials focusing on Aβ.
In contrast to these observations, tau pathology and encephalitis-like functional deficits have been reported in wild-type mice immunized with recombinant tau protein [52]. However, these adverse reactions may have been caused by the very strong adjuvants used [Complete Freund's Adjuvant (CFA) and Pertussis toxin (PT)] and/or the choice of full-length unphosphorylated human tau administered to mice expressing only endogenous mouse tau. In our studies, we use tauopathy selective/specific phosphorylated fragments of the human tau protein in mice that express human tau, administered with alum adjuvant which is the only approved adjuvant for human use. Alum is a mild immunostimulant and promotes an antibody (Th2) response whereas CFA and PT elicit primarily a strong cytotoxic T-cell response which is not called for when self-antigens such as tau are being targeted.
CONCLUDING REMARKS
The new era of immunotherapy for protein conformational disorders continues to be promising, although the design of this approach is continuously evolving. As these diseases often have more than one aggregating entity whose pathologies are intertwined, like Aβ and tau in AD, combination therapies are likely to be more effective. Obviously, prophylactic treatment should be most efficacious but presymptomatic diagnostic markers are needed for that to become reality.
ACKNOWLEDGMENTS
Dr. Sigurdsson is supported by NIH grants AG02197, AG032611, DK075494, The Alzheimer's Drug Discovery Foundation, the Alzheimer's Association, and the Irma T. Hirschl/Monique Weill-Caulier Trust
References
- 1.Wisniewski T, Konietzko U. Amyloid-β immunisation for Alzheimer's disease. Lancet Neurology. 2008;7(9):805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative diseases. Annu Rev Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9(4):448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer's disease. Brain Pathol. 2004;14(1):11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64(1):129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 6.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, et al. Aβ species removal after Aβ42 immunization. J Neuropathol Exp Neurol. 2006;65(11):1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 7.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, Von Rotz RC, et al. Generation of antibodies specific for β-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8(11):1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 8.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38(4):547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 9.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005;64(1):94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 10.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 11.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 12.Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, et al. Amyloid-β peptide remnants in AN-1792-immunized Alzheimer's disease patients -A biochemical analysis. Am J Pathol. 2006;169(3):1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viola KL, Velasco PT, Klein WL. Why Alzheimer's is a disease of memory: The attack on synapses by Aβ oligomers (ADDLs). Journal of Nutrition Health & Aging. 2008;12(1):51S–57S. doi: 10.1007/BF02982587. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal K, Grundke-Iqbal I. Developing pharmacological therapies for Alzheimer disease. Cellular and Molecular Life Sciences. 2007;64(17):2234–2244. doi: 10.1007/s00018-007-7221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider A, Mandelkow E. Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics. 2008;5(3):443–457. doi: 10.1016/j.nurt.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trojanowski JQ, Duff K, Fillit H, Koroshetz W, Kuret J, Murdhy D, et al. New directions for frontotemporal dementia drug discovery. Alzheimers & Dementia. 2008;4(2):89–93. doi: 10.1016/j.jalz.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigurdsson EM, Lorens SA, Hejna MJ, Dong XW, Lee JM. Local and distant histopathological effects of unilateral amyloid-β 25−35 injections into the amygdala of young F344 rats. Neurobiol Aging. 1996;17(6):893–901. doi: 10.1016/s0197-4580(96)00169-8. [DOI] [PubMed] [Google Scholar]
- 18.Sigurdsson EM, Lee JM, Dong XW, Hejna MJ, Lorens SA. Bilateral injections of amyloid-β 25−35 into the amygdala of young Fischer rats: Behavioral, neurochemical, and time dependent histopathological effects. Neurobiol Aging. 1997;18(6):591–608. doi: 10.1016/s0197-4580(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 19.Gotz J, Chen F, Van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ 42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 21.Ribe EM, Perez M, Puig B, Gich I, Lim F, Cuadrado M, et al. Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiol Dis. 2005;20:814–822. doi: 10.1016/j.nbd.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Pearson RCA, Powell TPS. The neuroanatomy of Alzheimer's disease. Rev Neurosci. 1989;2:101–122. doi: 10.1515/REVNEURO.1989.2.2.101. [DOI] [PubMed] [Google Scholar]
- 23.Delacourte A, Sergeant N, Champain D, Wattez A, Maurage CA, Lebert F, et al. Nonoverlapping but synergetic tau and APP pathologies in sporadic Alzheimer's disease. Neurology. 2002;59(3):398–407. doi: 10.1212/wnl.59.3.398. [DOI] [PubMed] [Google Scholar]
- 24.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 25.Wilcock GK, Esiri MM. Plaques, tangles and dementia : A quantitative study. Journal of the Neurological Sciences. 1982;56(2−3):343–356. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 26.Davies CA, Mann DMA, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimers-disease. Journal of the Neurological Sciences. 1987;78(2):151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 27.DeKosky ST, Scheff SW. Synapse loss in frontal-cortex biopsies in Alzheimers-disease - Correlation with cognitive severity. Annals of Neurology. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 28.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 29.Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer's disease and related tauopathies. Journal of Alzheimer's Disease. 2008;15:157–168. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Ramos A, Diaz-Hernandez M, Rubio A, Miras-Portugal MT, Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Molecular and Cellular Neuroscience. 2008;37(4):673–681. doi: 10.1016/j.mcn.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, et al. Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Tampellini D, Magrane J, Takahashi RH, Li F, Lin MT, Almeida CG, et al. Internalized antibodies to the Aβ domain of APP reduce neuronal Aβ and protect against synaptic alterations. J Biol Chem. 2007;282(26):18895–18906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki T, Koo EH, Selkoe DJ. Trafficking of cell-surface amyloid β-protein precursor. Endocytosis, recycling, and lysosomal targeting detected by immunolocalization. Journal of Cell Science. 1996;109:999–1008. doi: 10.1242/jcs.109.5.999. [DOI] [PubMed] [Google Scholar]
- 34.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes - Alternative processing into amyloid-bearing fragments. Nature. 1992;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 35.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 36.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindblad EB. Aluminium compounds for use in vaccines. Immunology and Cell Biology. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 38.Sigurdsson EM, Quartermain D, Boutajangout A. Tau immunotherapy prevents cognitive decline and clears pathological tau in a tangle mouse model. Alzheimer's & Dementia. 2008;4(4):T191–T192. [Google Scholar]
- 39.Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4(2):141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- 40.Kruse KB, Dear A, Kaltenbrun ER, Crum BE, George PM, Brennan SO, et al. Mutant fibrinogen cleared from the endoplasmic reticulum via endoplasmic reticulum-associated protein degradation and autophagy - An explanation for liver disease. Am J Pathol. 2006;168(4):1299–1308. doi: 10.2353/ajpath.2006.051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nerenberg ST, Prasad R. Radioimmunoassays for Ig classes G, A, M, D, and E in spinal fluids: normal values of different age groups. J Lab Clin Med. 1975;86(5):887–898. [PubMed] [Google Scholar]
- 42.Zlokovic BV, Skundric DS, Segal MB, Lipovac MN, Mackic JB, Davson H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the guinea pig. Exp Neurol. 1990;107(3):263–270. doi: 10.1016/0014-4886(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 43.Balin BJ, Broadwell RD, Salcman M, el Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251(2):260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- 44.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120(2):245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 45.Zlokovic B. Can blood-brain barrier play a role in the development of cerebral amyloidosis and Alzheimer's disease pathology. Neurobiol Dis. 1997;4(1):23–26. doi: 10.1006/nbdi.1997.0134. [DOI] [PubMed] [Google Scholar]
- 46.Sigurdsson EM. Immunotherapy for conformational diseases. Current Pharmaceutical Design. 2006;12(20):2569–2585. doi: 10.2174/138161206777698837. [DOI] [PubMed] [Google Scholar]
- 47.Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. Journal of Neurocytology. 2003;32(9):1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- 48.Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and tau filaments in forebrain. Molecular and Cellular Neuroscience. 2001;18(6):702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- 49.Meeker ML, Meeker RB, Hayward JN. Accumulation of circulating endogenous and exogenous immunoglobulins by hypothalamic magnocellular neurons. Brain Res. 1987;423(1−2):45–55. doi: 10.1016/0006-8993(87)90823-7. [DOI] [PubMed] [Google Scholar]
- 50.Denk F, Wade-Martins R. Knock-out and transgenic mouse models of tauopathies. Neurobiol Aging. 2009;30(1):1–13. doi: 10.1016/j.neurobiolaging.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberson ED, Scearce-Levie K, Palop JJ, Yan FR, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 52.Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Archives of Neurology. 2006;63(10):1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- 53.Arbel M, Yacoby I, Solomon B. Inhibition of amyloid precursor protein processing by beta-secretase through site-directed antibodies. Proc. Natl. Acad. Sci. USA. 2005;102:7718–23. doi: 10.1073/pnas.0502427102. [DOI] [PMC free article] [PubMed] [Google Scholar]