Abstract
There is increasing consensus regarding the importance of operationally defining and measuring functional decline in mild cognitive impairment (MCI). However, few studies have directly examined functional abilities in MCI or its presumed subtypes and, to date, reported findings have been discrepant. Nondemented older adults (n = 120) were administered a comprehensive cognitive battery measuring multiple domains as well as a performance-based functional ability measure. Participants were characterized as either cognitively normal, amnestic MCI, or non-amnestic MCI. MCI individuals demonstrated decrements in instrumental activities of daily living (IADL) relative to their cognitively normal counterparts. Specifically, participants with amnestic MCI demonstrated significant decrements in financial management, whereas those with non-amnestic MCI showed poorer performance in abilities related to health and safety. Moreover, decreased functional abilities were associated with decrements in global cognitive functioning but not memory or executive functions in the MCI participants. Finally, logistic regression demonstrated that functional abilities accurately predicted MCI subtype. Results support the need for better delineation of functional decline in MCI. Given the implications of functional status for MCI diagnosis and treatment, the direct assessment of functional abilities is recommended. Results further suggest performance-based IADL assessment may have utility in distinguishing MCI subtypes.
Keywords: Mild cognitive impairment, Older adults, Neuropsychology, Activities of daily living, Amnestic, Nonamnestic
INTRODUCTION
Controversy surrounds the establishment of specific diagnostic criteria for mild cognitive impairment (MCI). For example, whether MCI criteria should include impairment in everyday activities has provided one source of debate (Giovanetti et al., 2008). Initial MCI guidelines required that functional abilities remain intact, as this specific criterion was thought to help distinguish MCI from dementia. However, cognitive and functional abilities clearly deteriorate over the course of MCI because such change leads to progression from MCI to dementia for many individuals (Petersen, Smith, Waring, Ivnik, Tangalos, & Kokmen, 1999). Indeed, recent evidence suggests that individuals with MCI demonstrate impaired complex, or instrumental, activities of daily living (IADL) but not more basic activities of daily living (ADL) (Perneczky, Pohl, Sorg, Hartmann, Tosic, et al., 2006). Based in part on this accumulating evidence, an international working group put forth modified criteria for MCI that includes intact ADL and either minimal or no impairment in IADL (Winblad et al., 2004).
The few studies examining functional abilities across MCI subtypes have been discrepant in terms of which specific subgroups demonstrate poorer IADL performance. Some studies have demonstrated that MCI individuals with memory impairment show more functional change relative to their MCI peers with no memory impairment and cognitively normal participants (Farias, Mungas, Jagust, 2005), whereas others have reported that individuals with multiple-domain MCI show decrements relative to single-domain amnestic MCI participants (Zanetti, Ballabio, Abbatge, Cutaia, Vergani, & Bergamaschini, 2006). By contrast, at least one study (Boeve et al., 2003) did not find differences in functional abilities between amnestic MCI individuals and cognitively normal older adults, although MCI participants included in this particular study were characterized as MCI based, in part, on intact ADL.
Similarly, although functional performance generally is thought to be cognitively mediated (Tuokko, Morris, & Ebert, 2005), and associations between functional and cognitive performance have been demonstrated in MCI individuals as well as their cognitively normal counterparts (Cahn-Weiner, Malloy, Bole, Marran, & Salloway, 2000), studies have varied in terms of which specific cognitive domains contribute to functional deterioration. Several groups have reported that functional abilities that rely on memory (Farias, Mungas, Reed, Harvey, Cahn-Weiner, & DeCarli, 2006) and executive functioning are most impaired in MCI (e.g., Mariani et al., 2008; Perneczky, Pohl, Sorg, Hartmann, Tosic, et al., 2006). Findings from a longitudinal study revealed that individuals with MCI, or cognitive impairment without dementia (CIND), showed decrements in IADL relative to older adults without cognitive impairment and that measures of memory predicted future decline in money management skills (Tuokko et al., 2005). In contrast, Rozzini and colleagues (2007) reported that, in a group of amnestic MCI individuals, poor global cognitive performance at baseline and worsening executive functioning, but not worsening memory performance, were associated with conversion to Alzheimer's disease (AD) over a 1-year follow-up period.
Inconsistencies in findings across published reports may be related to variability in methodology. Studies differ in terms of variables, including criteria used to define MCI, whether MCI is divided into different subtypes, source of participants (e.g., memory clinic, population-based, etc.), definition of ADL/IADL impairment, and instruments used to assess functional status—including whether they use informant report, self-report, or performance-based instruments. Many clinicians rely on collateral sources of information, such as a spouse or other relative, when examining functional abilities. However, such reports may be biased by emotional factors, degree of insight, or the relationship between informant and patient (Loewenstein & Mogosky, 1999). Furthermore, in terms of self-report data, evidence suggests that some individuals with MCI may overestimate their functional abilities (Tabert et al., 2002).
Given these potential biases associated with informant based and self-report instruments, performance-based measures provide an attractive alternative. Such measures assess functional capacity directly by having an individual complete a task and formally evaluating performance. Advantages of performance-based instruments include standardized administration and scoring, quantitative results that can be interpreted using normative reference standards, and minimal effects of lack of insight and bias (Griffith et al., 2003).
Given discrepancies between previous studies, the nature of functional abilities in MCI has yet to be elucidated, particularly in subtypes of MCI. Accordingly, we examined everyday functioning using a performance-based measure in MCI and cognitively normal older adults. We predicted that, although individuals with MCI would not demonstrate objective impairments on a performance-based measure of IADL, they would perform more poorly than their cognitively normal counterparts. However, we hypothesized that this result would vary by MCI subtype. Specifically, based on previously published findings (e.g., Farias et al., 2005; Tuokko et al., 2005), we predicted that individuals with amnestic MCI would perform more poorly relative to their non-amnestic counterparts. We further expected that poorer functional abilities in IADL would be associated with decreased abilities related to global cognitive functioning, memory, and executive functions across our sample (see Mariani et al., 2008; Rozzini et al., 2007; Tuokko et al., 2005). Finally, we examined whether functional abilities predict cognitive status as such a finding may argue for the diagnostic utility of formal performance-based IADL assessment.
METHODS
Participants
One hundred twenty community-dwelling older adults were selected for this study from a larger cohort of research volunteers participating in a longitudinal study of normal aging. These individuals were consecutively enrolled and selected for the present study because they (1) underwent a comprehensive neuropsychological assessment including a measure of IADL (i.e., Managing Money and Health and Safety sub-scales of the Independent Living Scales [ILS], Loeb, 1996) and (2) were determined to be non-demented by consensus diagnosis based on neuropsychological, neurological, and functional data. Given that intact functional abilities were an inclusion criterion, no individual demonstrated objective impairment in IADL (defined as ILS T-scores ≤ 40). This study was approved by the UCSD institutional review board and written informed consent was obtained from all participants.
Neuropsychological Assessment
All participants received a comprehensive neuropsychological assessment. Neuropsychological tests of interest included measures from five cognitive domains: memory, attention, language, visuospatial functioning, and executive functioning. In addition, the Managing Money and Health and Safety subscales of the ILS (Loeb, 1996), a performance-based measure of IADL that was developed for assessment of older adults, were also administered. The ILS was derived from the Community Competence Scale (CCS; Loeb, 1983). The standardization edition of the CCS was renamed the ILS and included only those items from the CCS that had the best psychometric properties. The ILS has been shown to have good reliability (internal consistency α = 0.88; interrater reliability = 0.99; average 2-week test-retest r = 0.91) and validity. Concurrent validity estimates of the ILS were robust for both the WAIS-R (Full Scale IQ: r = 0.73) and MicroCog scales (e.g., Attention: r = 0.72; Memory: r = 0.58; Spatial Processing: r = 0.60; Reaction Time: r = 0.69; and Information Processing Accuracy: r = 0.81). A review of 31 performance-based instruments of functional abilities recommended the use of the ILS given its good standardization sample, detailed administration and scoring information provided in the manual, and good psychometric properties (Moore, Palmer, Patterson, and Jeste, 2007).
The ILS consists of 68 items across 5 subscales (Memory/Orientation; Managing Money; Managing Home and Transportation; Health and Safety; and Social Adjustment) and takes approximately 45 minutes to administer. Items include questions assessing factual knowledge or demonstration of an ability as well as more complex questions requiring reasoning and problem solving. Each item is scored on either a 2-point (0, 2) or 3-point (0, 1, 2) scale. Instructions for standardized administration and scoring as well as an appendix including standard scores are included in the ILS manual. Because the ILS scales tap some processes already captured by our battery of tests (e.g., memory, orientation, affect and social adjustment, etc.), we limited our administration to two of the ILS's more complex IADL scales (Managing Money and Health and Safety); both have also shown good sensitivity to dementia samples. The Managing Money subscale assesses abilities related to counting money, performing calculations, paying bills, and taking precautions with finances. The Health and Safety subscale measures awareness of an individual's own health status, abilities related to assessing health problems and dealing with medical emergencies, and knowledge of healthy behaviors.
MCI Classification
Each participant was classified as cognitively normal (NC) or MCI on the basis of neuropsychologically based criteria for MCI (see Jak, Bondi et al., 2009, for details). According to these criteria, impairment in a particular cognitive domain required that at least two performances fell greater than 1 standard deviation (SD) below age-appropriate norms in order for that domain to contribute to the MCI classification. Participants were classified as cognitively normal if, at most, performance on one measure within one or two cognitive domains fell more than 1 SD below normative expectations. MCI individuals were classified as (1) Single Domain Amnestic MCI if only memory showed impairment; (2) Single Domain Non-Amnestic MCI if only one non-memory domain was impaired; (3) Multiple Domain Amnestic MCI if memory and at least one other domain were impaired, and (4) Multiple Domain Non-Amnestic MCI if at least two non-memory domains were impaired.
Using this approach, 82 individuals were characterized as cognitively normal, 22 as amnestic MCI (8 single and 14 multiple domain) and 16 as non-amnestic MCI (12 single and 4 multiple domain). Given the small number of individuals in some of the MCI subgroups, analyses were collapsed across single- versus multiple-domain subtypes to the following three participant groups: NC, amnestic MCI, and non-amnestic MCI. There were no significant differences among the participant groups in terms of age (F2,117 = .70, p = .50; ), education (F2,117 = 1.42; p = .25; ), men/women ratio (χ2 = 5.35; p = .07), or presence of the apolipoprotein epsilon 4 allele (APOE ε4; χ2 = .17; p = .92). However, as expected, there was a significant main effect of global cognitive functioning, as measured by the DRS Total T-score, (F2,117 = 14.49; p < .001; ). Post hoc comparisons of the three groups demonstrated that both the amnestic (M = 49.77; p < .001) and non-amnestic groups (M = 51.25; p = .006) had significantly lower DRS scores than the NC group (M = 55.44) but the two MCI groups did not significantly differ from each other (p = .62). Despite these group differences, the mean DRS total score for each group was within normal limits. See Table 1 for demographic data.
Table 1.
Demographic characteristics by group
| Normal control (n = 82) |
Amnestic MCI (n = 22) |
Non-amnestic MCI (n = 16) |
||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p value * | |
| Age | 74.26 (9.38) | 74.86 (7.05) | 77.13 (8.53) | .50 |
| Education | 16.02 (2.27) | 16.50 (2.13) | 15.19 (3.17) | .25 |
| Gender (M/F) | 30/52 | 14/8 | 6/10 | .07 |
| DRS Total T-score | 55.44 (4.49) | 49.77 (5.69) | 51.25 (5.37) | <.001a,b |
| APOE (ε4−/ε4+) | 53/23 | 13/7 | 11/5 | .92 |
Superscripts indicate which groups are signifi cantly different at p < .05, assessed using post hoc Tukey tests
= NC > amnestic MCI
= NC > non-amnestic MCI.
MCI = mild cognitive impairment; DRS = Mattis Dementia Rating Scale. APOE = apolipoprotein. Genotype data were not available for eight participants (six cognitively normal and two amnestic MCI participants).
Statistical Analyses
A series of univariate analyses of variance (ANOVAs) using α-values corrected for multiple comparisons were conducted to examine group differences in functional and neuropsychological variables of interest. For the functional ability and neuropsychological analyses, an α-value of 0.002 was considered significant. In cases where the omnibus F-test demonstrated statistical significance, Tukey's HSD post hoc tests were conducted to determine which specific groups differed. Bivariate correlational analyses were performed to determine the association among neuropsychological performance and functional abilities in each of the three diagnostic groups. To avoid criterion contamination, we chose neuropsychological variables not used in diagnosis. Finally, logistic regression was used to assess the contribution of functional abilities to diagnostic group classification. All analyses were performed using SPSS version 17.
RESULTS
Group Differences in Functional Abilities
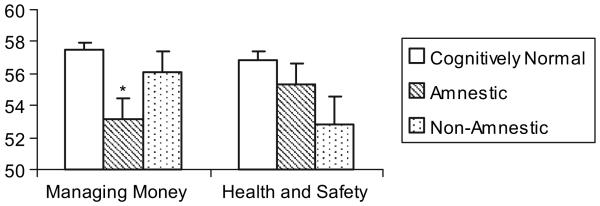
As hypothesized, there were significant group differences in functional abilities. Specifically, there was a significant group effect for the ILS Managing Money subscale (F2,1117 = 7.73 p = .001; ) and a trend for the Health and Safety subscale (F2,117 = 3.23; p = .04; ). Follow-up post hoc tests demonstrated that, on the Managing Money subscale, the amnestic MCI group (M = 53.14) performed significantly worse relative to the NC group (M = 57.45; p < .001), although there were no significant differences between the nonamnestic MCI (V = 56.13) and NC group (p = .54) or between the two MCI groups (p = .12). For the Health and Safety subscale, Tukey's post hoc tests showed a trend toward the nonamnestic MCI (M = 52.81) performing worse relative to the NC group (M = 56.82; p = .04), but no significant differences between the amnestic MCI (M = 55.36) and NC group (p = .56) or between the two MCI groups (p = .39; see Figure 1).
Fig. 1.
Instrumental activities of daily living performance by group. (* Statistically significant difference from Cognitively Normal participants, p < .001.)
Group Differences in Cognitive Abilities
Differences in cognitive performance are described in Table 2. As expected given the MCI subtypes included, memory abilities as measured by indices not included in the diagnostic characterization (i.e., DRS Memory subscale and WMS-R Visual Reproduction Percent Retention) significantly differed across the participant groups (DRS Memory: F2,117 = 20.79; p < .001; ; WMS-R Visual Reproduction Percent Retention: F2,110 = 12.29; p < .001; ). Tukey post hoc tests revealed that, on both instruments, amnestic MCI participants (DRS Memory: M = 44.55; WMS-R Visual Reproduction Percent Retention: M = 8.55) had significantly poorer memory relative to the NC (DRS Memory: M = 54.89; p < .001; WMS-R Visual Reproduction Percent Retention: M = 11.54; p < .001) and nonamnestic MCI groups (DRS Memory: M = 52.94; p = .001; WMS-R Visual Reproduction Percent Retention: M = 11.92; p = .001); however, the NC and nonamnestic MCI groups did not differ (DRS Memory: p = .54; WMS-R Visual Reproduction Percent Retention: p = .87). In contrast, executive functioning as assessed by one measure not used for diagnosis (i.e., D-KEFS Tower Test) did not differ among the groups (F2,117 = 1.67; p = .19; ). However, performance on a second executive functioning index not used for diagnosis (i.e., D-KEFS Trail Making Test Number Letter Switching) did differ among the groups (F2,116 = 8.51; p < .001; ). Tukey post hoc tests demonstrated that amnestic MCI participants (M = 10.77) performed significantly worse relative to the NC group (M = 12.90; p = .001); however, the NC and nonamnestic MCI participants (M = 11.50) did not differ (p = .07) nor did the two MCI groups (p = .61). Furthermore, there was no significant difference across groups in terms of self-reported depressive symptomatology (F 2,115 = 3.51, p = .03, ).
Table 2.
Functional abilities and neuropsychological performances by group
| Normal control |
Amnestic MCI |
Non-amnestic MCI |
Overall F |
Normative Reference | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p value * | Score | |
| MEASURES NOT USED IN THE CHARACTERIZATION OF MCI: | |||||
| ILS Managing Money | 57.45 (3.95) | 53.14 (6.19) | 56.13 (5.14) | .001a | T |
| ILS Health and Safety | 56.82 (5.66) | 55.36 (6.01) | 52.81 (7.02) | .04 | T |
| DRS Memory | 54.89 (4.63) | 44.55 (11.51) | 52.94 (6.88) | <.001a,c | T |
| WMS-R Visual Reproduction II Percent Retention | 11.54 (2.38) | 8.55 (3.58) | 11.92 (1.80) | <.001a,c | MOANS Scaled Score (SS) |
| D-KEFS Tower Test total achievement score | 12.27 (2.51) | 12.14 (2.59) | 11.00 (2.68) | .19 | SS |
| D-KEFS Trail Making Test Number-Letter Switching | 12.90 (2.16) | 10.77 (2.71) | 11.50 (2.48) | <.001a | SS |
| Geriatric Depression Scale | 4.90 (4.95) | 4.38 (3.04) | 8.33 (6.52) | .03 | raw |
| MEASURES USED IN THE CHARACTERIZATION OF MCI: | |||||
| MEMORY | |||||
| WMS-R Logical Memory I | 12.70 (2.99) | 8.00 (2.94) | 11.88 (2.94) | <.001 a,c | MOANS SS |
| WMS-R Logical Memory II | 12.76 (2.76) | 7.82 (3.23) | 12.94 (2.96) | <.001 a,c | MOANS SS |
| WMS-R Visual Reproduction I | 13.04 (2.79) | 11.00 (3.90) | 12.85 (3.36) | .03 | MOANS SS |
| WMS-R Visual Reproduction II | 12.56 (2.83) | 8.95 (4.16) | 12.85 (2.82) | <.001a,c | MOANS SS |
| CVLT 1-5 total | 55.10 (8.83) | 38.09 (7.90) | 51.69 (9.52) | <.001a,c | T |
| ATTENTION | |||||
| DRS Attention | 55.63 (4.23) | 56.14 (4.08) | 51.81 (10.55) | .03 | T |
| WAIS-R Digit span | 12.59 (3.09) | 11.55 (3.31) | 12.19 (2.74) | .37 | MOANS SS |
| Trails A | 11.89 (3.00) | 9.95 (3.32) | 10.63 (2.90) | .02 | MOANS SS |
| LANGUAGE | |||||
| BNT | 13.73 (2.53) | 11.41 (2.81) | 12.75 (2.79) | .001a | MOANS SS |
| Letter Fluency | 53.02 (10.77) | 49.27 (8.88) | 50.50 (12.06) | .29 | T |
| Category Fluency | 54.22 (11.27) | 46.05 (6.88) | 47.27 (14.43) | .003 | T |
| VISUALSPATIAL | |||||
| WISC-R Block Design | 56.01 (10.23) | 43.90 (15.55) | 42.33 (14.33) | <.001a,b | T |
| D-KEFS Visual Scanning | 11.53 (2.29) | 10.32 (3.59) | 10.31 (2.52) | .06 | SS |
| DRS Construction | 50.84 (6.10) | 49.95 (6.44) | 43.88 (11.90) | .002b | T |
| Draw-a-Clock Test | 2.88 (.40) | 2.68 (.57) | 2.63 (.62) | .06 | raw |
| EXECUTIVE FUNCTIONS | |||||
| WCST categories | 53.20 (6.40) | 49.30 (11.46) | 44.47 (9.91) | <.001b | T |
| WCST perseverative errors | 49.38 (6.52) | 46.26 (5.94) | 41.47 (11.10) | <.001b | T |
| Trails B | 12.46 (2.56) | 10.00 (2.89) | 10.63 (3.42) | <.001a | MOANS SS |
| D-KEFS Color Word Interference Inhibition | 12.23 (2.15) | 10.77 (2.35) | 10.07 (2.60) | <.001b | SS |
Signifi cance level, corrected for multiple comparisons, was set at p < .002. Superscripts indicate which groups were signifi cantly different at p < .002, assessed using post hoc Tukey tests
= NC > amnestic MCI
= NC > non-amnestic MCI
= non-amnestic MCI > amnestic MCI.
MCI = mild cognitive impairment; ILS = Independent Living Scales. DRS = Mattis Dementia Rating Scale [Mattis, 1988 ; published norms (Mattis, 1988)]. WMS-R = Wechsler Memory Scale-Revised [Wechsler, 1987 ; normative data drawn from Mayo's Older Americans Normative Studies (MOANS; Ivnik et al., 1992)]. D-KEFS = Delis-Kaplan Executive Function System [Delis, Kaplan, & Kramer, 2001 ; published norms (Delis et al., 2001)]. CVLT = California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 1987 ; normative data from Norman, Evan, Miller, & Heaton, 2000). WAIS-R = Wechsler Adult Intelligence Scale-Revised [Wechsler, 1981 ; normative data from the MOANS (Ivnik et al., 1992)]. Normative data for Trail Making Test (Reitan & Wolfson, 1985) from the MOANS (Ivnik et al., 1992). BNT = Boston Naming Test [Kaplan, Goodglass, & Weintraub, 1983 ; normative data from the MOANS (Ivnik et al., 1992)]. Normative data for letter fl uency and category fl uency from Gladsjo, Schuman, Evans, Peavy, Miller, and Heaton (1999). WISC-R = Wechsler Intelligence Scale for Children – Revised (Wechsler, 1974 ; age and education adjusted norms drawn from unpublished data derived from the UCSD Alzheimer Disease Research Center). WCST = Wisconsin Card Sorting Test (WCST-48-card version; Lineweaver, Bondi, Thomas, and Salmon, 1999).
Associations Between Cognition and Functional Abilities
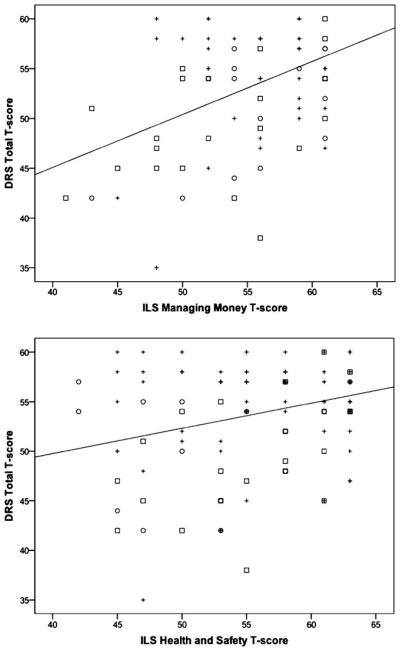
Given that discrepancies among previously published reports may be due to methodological differences, including which MCI subtypes were included and which particular functional abilities were assessed, we examined the associations among cognitive and functional performance across the entire sample and then examined the individual MCI subtypes separately. Across the entire sample, global cognitive functioning, as measured by the DRS Total T-score, was significantly associated with performance on both the ILS Managing Money (r = .48; p < .001) and Health and Safety (r = .29; p = .002) subscales (see Figure 2). In addition, memory as measured by two indices not used in diagnostic classification (i.e., DRS Memory subscale T-score and WMS Visual Reproduction Percent Retention MOANS scaled score) were significantly associated with the Managing Money (DRS Memory: r = .32; p < .001; WMS VR Percent Retention: r = .30; p = .001) but not with the Health and Safety subscale (DRS Memory: r = .11; p = .25; WMS VR Percent Retention: r = .14; p = .13). Two executive functioning measures not used in diagnosis (i.e., D-KEFS Tower Test Total Achievement scaled score and D-KEFS Trail Making Test Number-Letter Switching scaled score) were not correlated with either Managing Money (Tower Test: r = .10; p = .28; Trail Making Test: r = .11; p = .23) or Health and Safety subscale performance (Tower Test: r = .02; p = .82, Trail Making Test: r = .02; p = .84).
Fig. 2.
Scatterplots of the correlations between global cognition and Instrumental activities of daily living (IADL) performance for all participants. DRS = Mattis Dementia Rating Scale; ILS = Independent Living Scales; MCI = Mild Cognitive Impairment; + = Cognitively Normal; □ = Amnestic MCI; ○ = Non-Amnestic MCI.
When each MCI subgroup was analyzed separately, the amnestic MCI group demonstrated that both the Managing Money (r = .45; p = .04) and Health and Safety (r = .51; p = .02) subscales were significantly correlated with global cognition. However, neither the Managing Money or Health and Safety subscale were related to memory (DRS Memory: r = .14; p = .54 and r = .38; p = .08, respectively; WMS VR Percent Retention: r = .30; p = .18 and r = .27; p = .23, respectively) or executive functioning performance (Tower Test: r = .14; p = .55 and r = .05; p = .84, respectively; Trail Making Test: r = −.10; p = .66 and r = .10; p = .67, respectively) for the amnestic MCI group.
For nonamnestic MCI participants, the Managing Money (r = .58; p = .02), but not the Health and Safety subscale, was correlated with global cognition (r = .21, p = .44). Neither the Managing Money nor Health and Safety subscale were related to memory (DRS Memory: r = .18; p = .52 and r = −.04; p = .88, respectively; WMS VR Percent Retention: r = .07; p = .82 and r = .33; p = .27, respectively) or executive functioning (Tower Test: r = .21; p = .43 and r = .10; p = .72, respectively; Trail Making Test: r = −.14; p = .62 and r = −.31; p = .25, respectively) in nonamnestic MCI participants.
Diagnostic Group Classification Based on Functional Abilities
Binary logistic regression analyses showed that, when all NC participants and MCI were included in the analyses and ILS performances on the two subtests (Managing Money and Health and Safety) were the only predictor variables, it produced a significant improvement in the model relative to the null model (χ2 = 10.63; p = .005, Nagelkerke R2 = .12). The model accurately classified 72.5% of the participants as either MCI or cognitively normal. However, this model had greater specificity (95.1%) than sensitivity (23.7%). Managing Money (B = −.12; p = .02) but not Health and Safety (B = −.03; p = .51) was associated with diagnostic status.
In a second model in which only MCI participants (amnestic vs. nonamnestic) were included and both ILS subtests were the only predictor variables, a significant improvement in the model was found relative to the null model (χ2 = 10.66; p = .005, Nagelkerke R2 = .33). The model accurately classified 81.6% of the participants as either amnestic or nonamnestic MCI. More specifically, the model accurately classified 90.0% of participants as amnestic MCI and 68.8% of participants as non-amnestic MCI. Both Managing Money (B = .27; p = .02) and Health and Safety (B = −.23; p = .02) were associated with MCI subtype classification.
DISCUSSION
Our results indicate that, although their performance is still within normal limits, MCI individuals have reduced functional abilities relative to their cognitively normal counterparts. However, interestingly, the nature of this difference varies depending on MCI subtype. In addition, our findings demonstrate that decreased functional abilities are associated with decrements in global cognitive functioning but not specifically memory or executive functioning abilities in MCI. Finally, logistic regression analyses revealed that functional abilities were more effective at differentiating amnestic from nonamnestic MCI participants than in discriminating cognitively normal from MCI individuals. This finding suggests that specific patterns of functional decline may contribute to differentiation among MCI subtypes.
Importantly, our finding that individuals with MCI demonstrate decreased functional abilities adds to a growing literature indicating that IADL decline occurs in MCI (e.g., Farias et al., 2006; Giovanetti et al., 2008; Griffith et al., 2003; Peres, Chrystostome, Fabrigoule, Orgogozo, Dartigues, & Barberger-Gateau, 2006). Furthermore, this result provides additional support for the notion that intact IADL may need to be reconsidered as a diagnostic criterion for MCI and, that IADL performance may not accurately discriminate MCI from mild dementia (Perneczky, Pohl, Sorg, Hartmann, Komossa, et al., 2006).
We demonstrated group differences based on MCI sub-types such that the amnestic MCI performed more poorly relative to the NC participants on the ILS Managing Money subscale. In contrast, the non-amnestic MCI group showed a trend toward poorer performance on the ILS Health and Safety subscale. These findings suggest a possible dissociation in which amnestic MCI tends to show decline in handling finances whereas non-amnestic MCI may demonstrate decrements in other skills. Providing additional support for this notion, previously published reports have demonstrated that financial management in particular has been shown to relate to memory retrieval processes (Barberger-Gateau, Fabrigoule, Rouch, Letenneur, & Dartigues, 1999). Furthermore, Mariani and colleagues (2008) found an association between medication management and performance on executive function measures. Of note, although the two MCI groups did not differ in terms of global cognitive functioning, approximately 64% of individuals in the amnestic group demonstrated impairment in more than one cognitive domain whereas only 25% of participants in the nonamnestic group were impaired in multiple domains. Given this, it is possible that IADL differences between amnestic MCI and cognitively normal individuals may be related to the fact that many of these MCI individuals had impairments in domains in addition to memory, although our lack of associations between IADL and memory or executive functions argue against this notion. Nonetheless, given small sample sizes in some cells, we were unable to separately examine all four MCI subtypes to fully explore this issue.
Notably, very few studies examining functional abilities in MCI have compared different subtypes. However, our findings corroborate those of Kim, Lee, Cheong, Eom, Oh, and Hong (2009) who reported that, after controlling for age, gender, education, and depression, multi-domain amnestic MCI was the only subtype to perform significantly more poorly than normal participants in terms of functional abilities. Furthermore, combined with previous studies reporting decrements in financial management skills in amnestic MCI (e.g., Mariani et al., 2008; Marson et al., 2009), our findings suggest that handling finances may be a particularly useful and sensitive domain in the assessment of functional abilities in amnestic MCI. Given that financial management is an important ability in maintaining one's independence, this skill also has obvious legal implications for assessment of diminished capacity in older adults (see Wood & Moye, 2008, for discussion).
In the present sample, global cognitive functioning was more strongly associated with functional abilities than memory or executive functioning. Similarly, longitudinal studies have reported that global cognitive functioning at baseline is correlated with a faster rate of deterioration (e.g., Royall, Palmer, Chiodo, & Polk, 2004), indicating that global cognition warrants further investigation in terms of its association with everyday functioning and its ability to predict future decline. It may also be interesting to examine whether such links between functional ability and cognition differ by the type of sample recruited (e.g., clinic-based vs. volunteer). One possible explanation for this finding that performance in individual cognitive domains was not associated with functional abilities is related to the resource theory, which argues that impairment in everyday action is not deficit-based but is rather related to resource limitations. This limited capacity may be related to effort, attention, and/or brain damage and failures can occur when the individual lacks the capacity to perform a task or when capacity is directed elsewhere. Inter-individual variability in resource capacity may explain differences in performance on IADL (Schwartz et al., 1998).
Our study has limitations that must be considered. First, we used a performance-based measure. Although such measures have several advantages, they may not be as ecologically valid as they appear because individuals who are able to compensate for subtle deficits in the home environment or in daily life may be unable to do so in an unfamiliar environment such as the laboratory (Luis, Loewenstein, acevedo, Barker, & Duara, 2003). Future studies using multiple instruments including informant-based, self-report, and performance-based measures may be valuable in assessing functional impairment as well as the similarities and differences between these ADL measurement strategies. In fact, these different sources of information may complement each other when used together (Griffith et al., 2003). For instance, published reports suggest that using self-report and informant-based measures in combination and examining the discrepancy between the two may be particularly informative (e.g., Farias et al., 2005).
There is currently no gold standard for defining MCI. Most studies have adopted the requirement that one test within a cognitive domain fall 1.5 SD below the mean (e.g., Petersen & Morris, 2005), although other work by Petersen and colleagues (Jicha et al., 2006) has eschewed the use of specific neuropsychological cutoff scores and rendered diagnoses of amnestic MCI “if their memory performance was impaired out of proportion to their other cognitive domains.” Despite these varying procedures both within and across research groups, in an effort to strike a balance between reliability and sensitivity to detect mild impairment we used a comprehensive approach offered by Jak, Bondi et al. (2009) that used Heaton et al.'s lower but well-established cutoff score of 1.0 SD to identify impairment (Heaton, Grant, & Matthews, 1991; Heaton, Miller, Taylor, & Grant, 2004) and the requirement of two or more impaired scores within a cognitive domain. Taken together, the Jak, Bondi et al. criteria define impairment based on two or more test scores in a single domain to be greater than 1 SD below published norms.
Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon, and Delis (2009) noted that these “criteria were developed in consideration of the statistical maxim that multiple measures tend to provide a more reliable estimate of a cognitive construct than a single measure (Anastasi & Urbina, 1997).” Moreover, Heaton and colleagues (1991, 2004) reported that on the expanded Halstead-Retain neuropsychological battery the majority of neurologically normal adults score within the impaired range (defined as a score below 1 SD or a T-score less than 40) on at least one measure, and the median number of tests within the normative sample falling below normal limits was 10%. In addition, they found that a cutoff score of 1 SD below the normative mean on a summary score provided the best balance between sensitivity (80%) and specificity (88%). Providing further support for the importance of using a comprehensive battery with multiple measures assessing each domain, Palmer, Boone, Lesser, & Wohl (1998) found that a sizeable minority of healthy older adults (approximately 20%) obtained one impaired score in two different cognitive domains but fewer (5% or less) earned two or more impaired scores within the same domain. These findings point to the potential difficulties in the common practice of MCI diagnosis by reliance on a single measure for assessing a cognitive domain as well as with interpreting an isolated impaired score. Furthermore, recent neuroimaging and neuropsycological studies report brain-based empirical support for the comprehensive criteria in the form of hippocampal volume changes (Jak, Houston, Corey-Bloom, Nagel, & Bondi, 2007), vascular risk profiles (Jak, Urban, et al., 2009), as well as support for multiple episodic memory measures in the diagnosis of MCI (Chang et al., 2009).
Recent studies have highlighted the usefulness of incorporating instruments that examine not only whether the task can be performed but also take into account speed of task completion (Wadley, Okonkwo, Crowe, & Ross-Meadows, 2008) and quantitative and qualitative information regarding errors (e.g., Giovanetti et al., 2008). In fact, Okonkwo, Wadle, Griffith, Ball, and Marson (2006) proposed a model for functional change in MCI in which, early in the course of decline, performances becomes slower and more error prone and, later, have marked difficulty completing such tasks. Future studies could incorporate this type of qualitative information more fully. Finally, although individuals with MCI are at risk to develop dementia, MCI is a heterogeneous disorder and, given the cross-sectional design of our study, we do not know which individuals in our sample will progress to dementia. Thus, it is impossible to determine which diagnostic approach and functional abilities are truly the most valid and sensitive to progression to AD. Longitudinal studies examining an individual's trajectory of decline over time are needed to corroborate the present findings.
Clarifying the nature of ADL/IADL decline in MCI will become increasingly important and will yield many benefits related to both detection and management. For example, including information regarding functional status as a diagnostic criterion contributes to a more stable definition of MCI as defined by fewer MCI individuals reverting back to cognitively normal (Peres et al., 2006). In addition, IADL decrements are predictive of more rapid decline (Artero et al., 2008; Purser, Fillenbaum, Pieper, & Wallace, 2005) and conversion to dementia (Peres et al., 2006), indicating that early detection of functional changes may aid in identifying those at greatest risk for additional decline (Farias et al., 2006). Furthermore, information regarding functional status facilitates communication among health care providers, patient and family education (Giovanetti et al., 2008), and healthcare planning and management (Perneczky, Pohl, Sorg, Hartmann, Komossa, et al., 2006). Knowledge of functional status contributes to a better understanding of the association between cognitive impairment and quality of life (Tuokko et al., 2005) and, therefore, will help guide the development of interventions aiming to improve functional skills and allow individuals to remain independent.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health [F31 NS059193 (K.J.B.), K24 AG026431 (M.W.B.), R01 AG012674 (M.W.B.)], by Merit Review (D.C.D.) and Career Development (A.J.J.) Awards from the Department of Veterans Affairs, and by grant IIRG-07-59343 (M.W.B.) and NIRG-07-59143 (A.J.J.) from the Alzheimer's Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Alzheimer's Association or the National Institutes of Health.
REFERENCES
- Anastasi A, Urbina S. Psychological testing. Prentice-Hall, Inc; Upper Saddle River, NJ: 1997. [Google Scholar]
- Artero S, Ancelin M-L, Portet F, Dupuy A, Berr C, Dartigues J-F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:979–984. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- Barberger-Gateau P, Fabrigoule C, Rouch I, Letenneur L, Dartigues J-F. Neuropsychological correlates of self-reported performance in instrumental activities of daily living and prediction of dementia. Journals of Gerontolology. Series B. Psychological Sciences and Social Sciences. 1999;54:P293–P303. doi: 10.1093/geronb/54b.5.p293. [DOI] [PubMed] [Google Scholar]
- Boeve B, McCormick J, Smith G, Ferman T, Rummans T, Carpenter T, et al. Mild cognitive impairment in the oldest old. Neurology. 2003;60:477–480. doi: 10.1212/wnl.60.3.477. [DOI] [PubMed] [Google Scholar]
- Cahn-Winer DA, Malloy PF, Bole PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. The Clinical Neuropsychologist. 2000;14:187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ, Jacobson MW, et al. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.12.024. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Corporation; New York: 1987. [Google Scholar]
- Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: Dementia, mild cognitive impairment, and healthy elders. International Journal of Geriatric Psychiatry. 2005;20:827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, DeCarli C. MCI is associated with deficits in everyday functioning. Alzheimer Disease and Associated Disorders. 2006;20:217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti T, Bettcher BM, Brennan L, Libon DJ, Burke M, Duey K, et al. Characterization of everyday functioning in mild cognitive impairment: A direct assessment approach. Dementia and Geriatric Cognitive Disorders. 2008;25:359–365. doi: 10.1159/000121005. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Belue BS, Sicola A, Krzywanski S, Zamrini E, Harrell L, et al. Impaired financial abilities in mild cognitive impairment: A direct assessment approach. Neurology. 2003;60:449–457. doi: 10.1212/wnl.60.3.449. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Retain Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults Scoring Program. Psychological Assessment Resources, Inc; Odessa, FL: 2004. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo's older Americans normative studies: WMS-R norms for ages 56-94. The Clinical Neuropsychologist. 1992;6:49–82. [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Corey-Bloom J, Nagel BJ, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in non-demented older adults. Dementia and Geriatric Cognitive Disorders. 2007;23:382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Urban S, McCauley A, Bangen KJ, Delano-Wood L, Corey-Bloom J, et al. Profile of hippocampal volumes and stroke risk varies by neuropsychological definition of mild cognitive impairment. Journal of the International Neuropsychological Society. 2009;15:890–897. doi: 10.1017/S1355617709090638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Archives of Neurology. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Kim KR, Lee KS, Cheong H-K, Eom J-S, Oh BH, Hong CH. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2009;27:278–285. doi: 10.1159/000204765. [DOI] [PubMed] [Google Scholar]
- Lineweaver TT, Bondi MW, Thomas RG, Salmon DP. A normative study of Nelson's (1976) modified version of the Wisconsin Card Sorting Test in healthy older adults. The Clinical Neuropsychologist. 1999;13:328–347. doi: 10.1076/clin.13.3.328.1745. [DOI] [PubMed] [Google Scholar]
- Loeb PA. Validity of the community competence scale with the elderly. St. Louis University; 1983. Unpublished doctoral dissertation. [Google Scholar]
- Loeb PA. ILS: Independent living scales manual. Psychological Corp, Harcourt Brace Jovanovich; San Antonio, TX: 1996. [Google Scholar]
- Loewenstein DA, Mogosky B. Functional assessment of the older adult patient. In: Lichtenberg PA, editor. Handbook of assessment in clinical gerontology. John Wiley & Sons, Inc; New York: 1999. pp. 529–554. [Google Scholar]
- Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: Directions for future research. Neurology. 2003;61:438–444. doi: 10.1212/01.wnl.0000080366.90234.7f. [DOI] [PubMed] [Google Scholar]
- Mariani E, Monastero R, Ercolani S, Rinaldi P, Mangialasche F, Costanzi E, et al. for the ReGAI Study Group Influence of comorbidity and cognitive status on instrumental activities of daily living in amnestic mild cognitive impairment: Results from the ReGAI project. International Journal of Geriatric Psychiatry. 2008;23:523–530. doi: 10.1002/gps.1932. [DOI] [PubMed] [Google Scholar]
- Marson DC, Martin RC, Wadley V, Griffity HR, Snyder S, Goode PS, et al. Clinical interview assessment of financial capacity in older adults with mild cognitive impairment and Alzheimer's disease. Journal of the American Geriatrics Society. 2009;57:806–814. doi: 10.1111/j.1532-5415.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale: Professional manual. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- Moore DJ, Palmer BW, Patterson TL, Jeste DV. A review of performance-based measures of functional living skills. Journal of Psychiatric Research. 2007;41:97–118. doi: 10.1016/j.jpsychires.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Norman MA, Evan JD, Miller WS, Heaton RK. Demographically corrected norms for the California Verbal Learning Test. Journal of Clinical and Experimental Neuropsychology. 2000;22:80–95. doi: 10.1076/1380-3395(200002)22:1;1-8;FT080. [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Wadley VG, Griffith HR, Ball K, Marson DC. Cognitive correlates of financial abilities in mild cognitive impairment. Journal of the American Geriatrics Society. 2006;54:1745–1750. doi: 10.1111/j.1532-5415.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Boone KB, Lesser IM, Wohl MA. Base rates of “impaired” neuropsychological test performance among healthy older adults. Archives of Clinical Neuropsychology. 1998;13:503–511. [PubMed] [Google Scholar]
- Peres K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: Impact on outcome. Neurology. 2006;67:461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Sorg C, Hartmann J, Komossa K, Alexopoulos P, et al. Complex activities of daily living in mild cognitive impairment: Conceptual and diagnostic issues. Age and Ageing. 2006;35:240–245. doi: 10.1093/ageing/afj054. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Sorg C, Hartmann J, Tosic N, Grimmer T, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. International Journal of Geriatric Psychiatry. 2006;21:158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa established populations for epidemiologic studies of the elderly cohort. Journal of the American Geriatrics Society. 2005;53:1966–1972. doi: 10.1111/j.1532-5415.2005.53566.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. Journal of the American Geriatric Society. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, et al. Conversion of amnestic mild cognitive impairment to dementia of Alzheimer type is independent to memory deterioration. International Journal of Geriatric Psychiatry. 2007;22:1217–1222. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Montgomery MW, Buxbaum LJ, Lee SL, Carew TG, Coslett HB, et al. Naturalistic action impairment in closed head injury. Neuropsychology. 1998;12:13–28. doi: 10.1037//0894-4105.12.1.13. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. Functional deficits in patients with mild cognitive impairment: Prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- Tuokko H, Morris C, Ebert P. Mild cognitive impairment and everyday functioning in older adults. Neurocase. 2005;11:40–47. doi: 10.1080/13554790490896802. [DOI] [PubMed] [Google Scholar]
- Wadley VG, Okonkwo O, Crowe M, Ross-Meadows LA. Mild cognitive impairment and everyday function: Evidence of reduced speed in performing instrumental activities of daily living. American Journal of Geriatric Psychiatry. 2008;16:416–424. doi: 10.1097/JGP.0b013e31816b7303. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-revised. Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-revised manual. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- Wechsler D. Wechsler memory scale – revised. Psychological Corporation; New York: 1987. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, et al. Mild cognitive impairment – beyond controversies, towards a consensus: Report of the International Working Group on mild cognitive impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wood S, Moye J, editors. Assessment of older adults with diminished capacity: A handbook for psychologists. American Bar Association Commission on Law and Aging and the American Psychological Association; Washington DC: 2008. [Google Scholar]
- Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: A 3-year follow-up study. Journal of the American Geriatrics Society. 2006;54:580–586. doi: 10.1111/j.1532-5415.2006.00658.x. [DOI] [PubMed] [Google Scholar]