Abstract
Properties of cognitive change scores were compared in adults over age 70, for whom longitudinal changes are often negative, and in adults in two age groups under age 70, for whom the changes are often close to zero. Longitudinal assessments of three measures of memory and three measures of speed across an average interval of 2.4 years were obtained from a sample of 1,282 healthy adults between 18 and 92 years of age. Although substantial longitudinal declines were primarily apparent in adults 70 years of age and older, adults under and over age 70 were similar with respect to the variability and reliability of the cognitive changes, and in the magnitude of the correlations of the changes with each other and with variables which have been identified as risk factors for late-life cognitive decline and dementia. It is suggested that longitudinal changes in cognition can be considered to represent a paradox in that the mean values of the changes are more negative at older ages, but the change scores have similar measurement properties, and appear to be just as systematic, among adults under and over 70 years of age.
Most prior research on longitudinal changes in cognitive functioning has found significant declines primarily among adults over about 70 years of age (e.g., Ronnlund, Nyberg, Backman & Nilsson, 2005; Schaie, 2005). The dominant interpretation of these results, which is often implicit if not explicit, is that little meaningful change occurs at younger ages. Although this interpretation may be valid, it is also the case that very little is currently known about the nature and determinants of cognitive change at any age. The primary purpose of the current project was to investigate the implication that there may not be meaningful change under about 70 years of age by comparing several characteristics of longitudinal change in adults of different ages. Specifically, adults in three age groups were compared with respect to: (1) the magnitude of individual differences in change; (2) estimates of the reliability of change; (3) correlations among the changes; and (4) correlations of the changes with variables reflecting aspects of lifestyle, personality, and mood.
Cognitive changes were examined in six cognitive variables representing perceptual speed and episodic memory abilities because these abilities tend to be among the most sensitive to effects of aging (cf. Salthouse, in press). The data are derived from a project employing a measurement burst design (e.g., Nesselroade, 1991; Salthouse, 2007) in which research participants performed three different versions of each cognitive test on each of two measurement occasions. The scores are designated T11, T12, and T13 for the three versions at the first occasion, and T21, T22, and T23 for the three versions at the second occasion. The availability of three scores for each test at each occasion allowed more sensitive evaluation of change than that possible with conventional single assessment procedures.
Longitudinal data are available from a total of 1,282 adults. All of the participants had a measurement burst with three assessments at the second occasion, and 419 of them (the double burst subsample) also had three assessments at the first occasion. The other 863 participants were only administered the first version of each test on the initial occasion and all three versions on the second occasion. In order to contrast properties of cognitive change at different ages, the participants were divided into three groups, with the first group comprised of adults between 18 and 44, the second group comprised of adults between 45 and 69, and the third group consisting of adults between 70 and 92 years of age.
Several of the analyses of longitudinal change were based on the latent change model portrayed in Figure 1, in which the scores on the three test versions at each occasion serve as indicators of latent constructs. The model is based on one introduced by McArdle and Nesselroade (1994), and essentially creates a latent construct at each measurement occasion based on the scores from the three versions of each test, and then derives estimates of change from the residual of the construct on the second occasion after partialling influences from the first occasion. In addition to minimizing the impact of measurement error by focusing on the systematic variance shared among multiple variables, this approach to measuring change uses all of the available data in the analyses by relying on the full-information maximum likelihood algorithm for estimation (Enders, 2001). In other words, even the data from the participants who performed the three versions only on the second occasion contributed to the estimates in these analyses.
Figure 1.
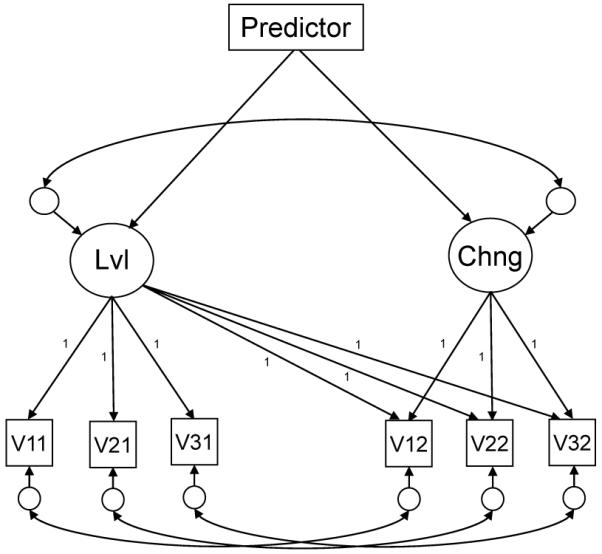
Illustration of the latent change model. Measured variables are represented by boxes and latent variables (constructs representing level or change, or residuals representing unexplained variance) are represented by circles. Lines with one arrow correspond to regression relations and lines with two arrows represent correlations (bi-directional relations).
The participants in the project also completed a set of questionnaires on the second test occasion to evaluate aspects of lifestyle, mood, and personality. The data from these questionnaires were used to examine correlations of measures of lifestyle (i.e., cognitive stimulation and physical exercise), personality (e.g., neuroticism, openness) and mood (e.g., depression, anxiety) with the longitudinal change in the measures of cognition. Variables within these categories are particularly interesting as potential predictors of cognitive change because a number of studies have reported that they are related to cognitive decline and to the onset of dementia (e.g., Fratiglioni, Pailard-Borg & Winblad, 2004; Hendrie, Albert, Butters, et al., 2006). However, it is not known whether these relations are unique to the period of late adulthood, in which case they should not be present among young and middle-aged adults, or if they reflect a phenomenon apparent across all of adulthood, in which case they may be evident to nearly the same degree at all ages.
Method
Table 1 contains descriptive characteristics of the three groups of research participants. Inspection of the entries in the table reveals that the groups are generally similar in most respects, although the older group had somewhat higher age-adjusted scaled scores for the Vocabulary, Digit Symbol, and Logical Memory variables, which suggests that they may be more cognitively select relative to their age peers than were the adults under 70 years of age.
Table 1.
Characteristics of sample
| Age Group | |||
|---|---|---|---|
| 18-44 | 45-69 | 70-92 | |
| Total Sample | |||
| Number | 352 | 665 | 265 |
| Age | 31.4 (8.6) | 56.3 (6.9) | 76.8 (5.1) |
| Proportion Females | .66 | .68 | .57 |
| Self-Rated Health | 2.1 (0.8) | 2.1 (0.9) | 2.5 (0.9) |
| Years of Education | 14.9 (2.4) | 16.1 (2.5) | 16.0 (3.0) |
| MMSE | 28.5 (1.7) | 28.7 (1.6) | 28.1 (2.0) |
| Scaled Scores | |||
| Vocabulary | 12.2 (3.3) | 13.1 (2.7) | 13.5 (3.1) |
| Digit Symbol | 11.0 (2.8) | 11.7 (2.8) | 12.0 (2.7) |
| Logical Memory | 11.4 (2.8) | 12.3 (2.8) | 12.6 (2.8) |
| Word Recall | 11.7 (3.3) | 13.1 (3.2) | 12.3 (3.0) |
| Retest Interval (years) | 2.5 (1.1) | 2.5 (1.1) | 2.4 (1.0) |
| Double Burst Sample | |||
| Number | 109 | 203 | 107 |
| Age | 29.1 (8.6) | 56.9 (6.6) | 77.3 (5.1) |
| Proportion Females | .60 | .68 | .55 |
| Self-Rated Health | 2.2 (0.9) | 2.3(0.8) | 2.6 (0.9) |
| Years of Education | 15.0 (2.3) | 15.9 (2.5) | 16.2 (3.0) |
| MMSE | 28.6 (1.6) | 28.7 (1.6) | 27.9 (1.9) |
| Scaled Scores | |||
| Vocabulary | 12.6 (3.0) | 13.0 (2.70 | 13.6 (2.9) |
| Digit Symbol | 11.0 (2.6) | 11.6 (2.9) | 12.4(2.6) |
| Logical Memory | 11.2 (2.6) | 12.0 (2.8) | 12.8 (2.5) |
| Word Recall | 11.9 (3.5) | 13.0 (3.4) | 12.5 (2.9) |
| Retest Interval (years) | 2.2 (0.6) | 2.1(0.6) | 2.2 (0.5) |
Note: Self-reported health is on a scale from 1 for excellent to 5 for poor. MMSE is the Mini-Mental Status Examination (Folstein, Folstein & McHugh, 1975). Scaled scores for tests from the WAIS III (Wechsler, 1997a) and from the WMS III (Wechsler, 1997b) are adjusted for age. The means for the scaled scores in the nationally representative normative sample are 10 with a standard deviation of 3.
The bottom portion of Table 1 reports the demographic characteristics only for the subset of participants with three assessments at each occasion (i.e., the double-burst subsample). It can be seen that these individuals are very similar to the complete samples with the exception that the retest intervals were somewhat shorter because the double measurement burst procedure was implemented after the initial occasions in the longitudinal project.
The three memory tests were the Word Recall and Logical Memory subtests from the Wechsler Memory Scale III (Wechsler, 1997a) and a locally developed paired associates test involving two lists which each contained six unrelated word pairs (for details see Salthouse, Fristoe & Rhee, 1996). The speed tests were the WAIS III (Wechsler, 1997b) Digit Symbol Substitution Test and two locally developed comparison tests requiring written same/different responses regarding pairs of letters or patterns (for details see Salthouse & Babcock, 1991).
Results1
All variables were converted into z-score units based on the mean and standard deviation of the scores from the complete sample on the first assessment. Means and standard errors for the T11 and T21 scores (i.e., the first version of the test at each occasion) for the six variables in each age group are portrayed in Figure 2. It is apparent that the means in each successive age group were lower than those in the preceding group, which reflects the cross-sectional age differences in the variables. The T21 – T11 declines among adults between 70 and 92 years of age were significant in all variables, and the effect sizes for the longitudinal changes were in the small to medium range (i.e., d values between .20 and .36). Four of the longitudinal changes were significantly negative in the 45-to-69 group, but the effect sizes were smaller than in the 70 to 92 group (i.e., d values between .09 and .11 with the exception of a d of .21 for the Pattern Comparison variable). None of the longitudinal changes were significantly negative in the 18-to-44 group, and two (Logical Memory and Digit Symbol) were significantly positive rather than negative, indicating better performance on the second occasion. The results in Figure 2 therefore replicate earlier findings of substantial longitudinal decline primarily among older adults.
Figure 2.
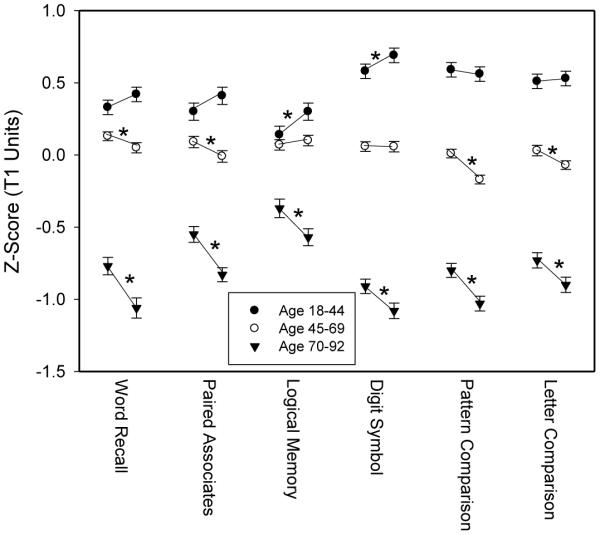
Means and standard errors in z-score units for the first (T11) and second (T21) occasion scores for adults in three age groups. Asterisks represent significant (p<.01) differences between scores at the first and second occasions.
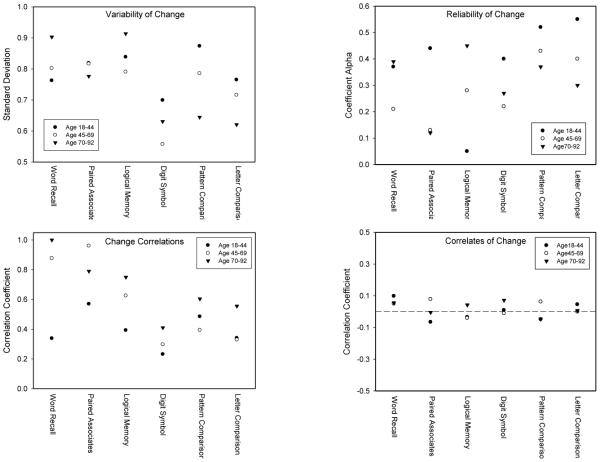
The subsequent analyses were designed to compare properties of the longitudinal changes in the three age groups. One possibility is that very few of the young and middle-aged adults exhibited any longitudinal decline, whereas in the older group there might have been a mixture of some people with large declines and others with little or no decline. A mixture of this type might be expected to result in larger individual differences in change in the older group, and this was examined by using the standard deviation of the T21-T11 change score as the measure of variability. Standard deviations of the changes for each variable in the three groups of adults are portrayed in the top left panel of Figure 3. The change variance was largest in the 70-and-over group for the Word Recall and Logical Memory variables, but that group had the smallest variance for the Paired Associates, Pattern Comparison and Letter Comparison variables. Taken in combination, these results suggest that there are few systematic age-related effects on individual differences in change.
Figure 3.
Between-person standard deviation of change (top left), estimated reliability of change (top right), average correlation with other variables representing the same cognitive ability (lower left), and median correlation of the cognitive change with 13 mood, personality, or lifestyle predictors (lower right).
Another factor that could be contributing to the age differences in longitudinal change is that the individual differences might be more systematic in the older group. That is, the total variability in change might be similar in the three groups, but the longitudinal change might be more consistent or reliable in the older group. Reliability of the longitudinal change in the double-burst subsample with three assessments at each occasion was computed with coefficient alpha by using the three separate changes as the “items” (i.e., change in the first test version T21-T11, change in the second test version T22-T12, and change in the third test version T23-T13). The estimated reliabilities computed in this manner are portrayed in the top right panel of Figure 3.
It can be seen that all of the reliability estimates were quite modest, with a range from about .1 to .5. The reliability estimate was highest in the 70-and-over group for the Word Recall and Logical Memory variables, but it was smallest in that group for the Paired Associates, Pattern Comparison, and Letter Comparison variables. At least based on these results, therefore, longitudinal change does not appear to be any more systematic at older ages than at younger ages.
Estimates of the reliabilities of the changes can also be derived from the formula that the reliability of the change is equal to reliability at each occasion minus the correlation across occasions divided by 1 minus the correlation across occasions (i.e., Cohen & Cohen, 1983, p. 414). Data from the complete sample of participants were used in these analyses because the correlation between the scores on the first and the second versions at the second occasion was used as the estimate of reliability, and the T21-T11 correlation was used as the across-occasion correlation. The estimated reliabilities computed in this fashion are presented in the top of Table 2, along with the T21-T11 correlations. It can be seen that all of the reliability estimates were less than .38, with medians of .14 in the youngest group, .08 in the middle group, and .17 for the 70-and-over group. The low values are primarily attributable to the fact that the T21-T11 correlations were nearly the same magnitude as the single occasion reliabilities. However, the important point to note in the current context is that the values are similar in each age group, and just as with the direct estimates of change reliability, there is no evidence that the changes were more consistent or systematic in the 70-and-over group.
Table 2.
Reliabilities and variances of cognitive changes
| Age Group | ||||||
|---|---|---|---|---|---|---|
| 18-44 | 45-69 | 70-92 | ||||
| Estimated reliabilities of change | ||||||
| T1-T2 | Rel. | T1-T2 | Rel. | T1-T2 | Rel. | |
| Word Recall | .64 | .18 | .60 | .17 | .65 | .18 |
| Paired Associates | .64 | .19 | .65 | −.01 | .51 | .17 |
| Logical Memory | .68 | .05 | .63 | .19 | .58 | .31 |
| Digit Symbol | .73 | −.43 | .80 | −.82 | .72 | −.32 |
| Pattern Comparison | .61 | .44 | .56 | .31 | .68 | .37 |
| Letter Comparison | .70 | .07 | .67 | −.12 | .73 | .11 |
| Variance of latent changes | ||||||
| Mean (SE) | Mean (SE) | Mean (SE) | ||||
| Word Recall | .06 (.02) | .04 (.01) | .07 (.03) | |||
| Paired Associates | .09 (.02) | .02 (.01) | .02 (.02) | |||
| Logical Memory | .03 (.02) | .03 (.01) | .11 (.03) | |||
| Digit Symbol | .13 (.03) | .10 (.02) | .14 (.03) | |||
| Pattern Comparison | .18 (.04) | .13 (.02) | .11 (.02) | |||
| Letter Comparison | .16 (.03) | .10 (.02) | .08 (.02) | |||
Note: T1-T2 refers to the (stability) correlation of the score on the first test version on each occasion across the retest interval. Rel. is the estimate of reliability computed by subtracting the T1-T2 correlation from the T21-T22 correlation, and dividing the difference by 1 minus the T1-T2 correlation. Variances of latent changes are derived from the latent change model portrayed in Figure 1.
The remaining analyses were based on the latent change model portrayed in Figure 12. Data from all participants were used in these analyses even though only a subset of the participants had complete three-assessment data at both occasions because the full-information maximum likelihood algorithm uses all of the available data in deriving the estimates. As noted earlier, an advantage of the latent change procedure is that it provides more sensitive measures of change than conventional difference scores. As an illustration of this differential sensitivity, the simple correlation of the longitudinal difference in the Digit Symbol variable with the longitudinal difference in the Word Recall variable was .12, whereas the correlation between the corresponding latent changes was .47.
An initial set of analyses examined the variance of the latent changes. These estimates, along with their standard errors, are presented in the bottom of Table 2. Inspection of the entries reveals that the change variance was largest in the oldest group for three of the variables, but it was smallest in that group for two variables. As with the measures of variability in the simple difference scores, therefore, there was no consistent relation between age and the magnitude of individual differences in cognitive change.
The next analyses consisted of computing correlations among the latent changes for the three variables representing the same cognitive ability (e.g., Recall with Paired Associates, and Recall with Logical Memory). The averages of the two correlations for each variable are portrayed in the bottom left panel of Figure 3. It can be seen that the average correlations for the Recall variable in the 70-and-over group were very high, and most of the correlations in this group were somewhat larger than those in the other two groups. However, all of the correlations among the latent changes were significantly greater than zero except for the Word Recall – Logical Memory and the Digit Symbol – Letter Comparison correlations in the 18-to-44 group.
Correlations between the change in variables from the same ability (e.g., memory) and in variables from different abilities (i.e., memory and speed) were also examined. The median correlations between variables from the same ability were .45, .48, and .50 for the young, middle, and old groups, respectively, and the median correlations between variables from different abilities were .17 for the young group, .26 for the middle group, and .38 for the older group. The higher change correlations for variables within a given ability compared to those for variables from different abilities suggests that there is a stronger coupling of change for variables representing the same ability than among variables representing different abilities. Of particular importance in the current context, however, was that this difference was evident in adults within each age group and not just among adults over 70.
Finally, correlations of the latent change parameters with various mood and personality variables (e.g., depression – Radloff, 1977; anxiety - Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983; positive and negative affect - Watson, Clark & Tellegen, 1988; Satisfaction with Life – Diener, Emmons, Larsen & Griffin, 1985; Need for Cognition - Cacioppo, Petty, Feinstein & Jarvis, 1996; and the “Big 5” personality traits - Goldberg, Johnson, Eber, Hogan, Ashton, Cloninger & Gough, 2006) were examined. Two lifestyle variables were also included; physical exercise, represented by the total number of hours per month devoted to either running or walking, and cognitive activity, represented by the number of hours per week participating in seven activities with high rated cognitive demands (i.e., reading newspapers, using a computer, driving a car, reading non-fiction books, working on crossword puzzles, handling finances, and writing).
As is apparent in the bottom right panel of Figure 3, the medians of the correlations of these variables with longitudinal changes in the cognitive variables were very close to zero in all three age groups. Similar analyses were conducted on a composite cognitive score created by averaging z-scores across the six cognitive variables. The median correlations with the composite cognitive variable were −.02 for the 18-to-44 group, .02 for the 45-to-69 group, and .03 for the 70-to-92 group. There were also little systematic trends in any of the predictor variables. To illustrate, the correlations between the composite cognitive variable and the physical activity variable were −.02, .05, and −.13, respectively, in the young, middle, and older groups, and those between the composite cognitive variable and the cognitive activity variable were .07, .02, and −.03, respectively.
Discussion
The combination of results described above can be considered to represent a paradox in that substantial longitudinal declines in measures of cognitive functioning occur primarily among adults 70 years of age and older, but adults under and over 70 are very similar in various properties of the changes, such as the magnitude of individual differences and the estimated reliability of the changes, and the size of correlations of the changes with changes in other cognitive variables and with various predictor variables.
How can this paradox of substantial decline primarily in the older group, despite nearly equivalent magnitude of individual differences, reliability, and correlations with other variables, be explained? It should be noted that the failure to detect significant decline in the young and middle-aged groups is unlikely to be a simple problem of low power because the sample sizes in the 18-to-44 and 45-to-69 groups were larger than that in the 70-to-92 group.
One possibility that might contribute to the paradox is that the factors affecting the mean value of change are not necessarily the same as the factors contributing to individual differences in change. For example, positive benefits of prior test experience could diminish with increasing age, such that the mean values of change become progressively more negative at older ages. However, if individual differences in the magnitude of retest effects are independent of individual differences in other determinants of longitudinal change, there could be age differences in mean change without age differences in various properties of change. Retest effects are only one possible factor that might operate in this fashion, and other factors could occur in addition to, or instead of, retest effects. Regardless of the number or identity of these other factors, however, the key point for the current argument is that the mean value of longitudinal change could be affected without influencing properties of the change measures.
It is noteworthy that there were no consistent correlations of the longitudinal cognitive changes with measures of personality, mood, or aspects of lifestyle. Some correlates of change might have been expected at least in the 70-and-over adults based on reports in the published literature. However, it is important to recognize that the prior research in this area has been inconsistent, as is evident by considering results with three categories of predictor variables - cognitive stimulation, physical activity, and neuroticism. Smaller longitudinal declines in cognitive performance among people with more cognitive activity have been reported several times, but this pattern was apparent in only 1 of 18 comparisons in the Hultsch, Small, Hertzog and Dixon (1999) study (i.e., in 0 of 9 variables with “active lifestyle” as the measure of cognitive activity, and in 1 of 9 variables with “novel information processing” as the measure of cognitive activity), in only 2 of 6 variables in the Bosma, van Boxtel, Ponds, Jeclicic, Houx, Metsemakers and Jolles (2002) study, and in different combinations of composite variables across two studies by Wilson and colleagues (Wilson, Mendes de Leon, Barnes, Schneider, Bienias, Evans, & Bennett, 2002; Wilson, Scherr, Schneider, Li, & Bennett, 2007). That is, in the 2002 study there were significant relations with working memory and perceptual speed but not with episodic memory, semantic memory, or visual spatial ability, and in the 2007 study there were significant relations with episodic memory, semantic memory, and perceptual speed, but not with working memory or visual-spatial ability. With respect to physical activity, smaller longitudinal declines in cognition among people with higher levels of self-reported physical activity have been found in several studies (e.g., Weuve, Kang, Manson, Breteler, Ware & Grodstein, 2004; Yaffe, Barnes, Nevitt, Lui, & Covinsky, 2001), but not in others (e.g., Barnes, Yaffe, Satariano & Tager, 2003; Hultsch, et al., 1999; Sturman, Morris, Mendes de Leon, Bienias, Wilson & Evans, 2006; Wilson, et al., 2002). Finally, larger cognitive declines among people with higher levels of neuroticism have been reported in some studies (e.g., Wilson, Evans, Bienas, Mendes de Leon, Schneider & Bennett, 2003; Wilson, Arnold, Schneider, Kelly, Tang & Bennett, 2006; Wilson, Arnold, Schneider, Li, & Bennett, 2007), but not in others (e.g., Arbuckle, Maag, Puskar, & Chaikelson, 1998; Hultsch, et al., 1999; Jelicic, Bosma, Ponds, van Boxtel, Houx, & Jolles, 2003; Wetherell, Reynolds, Gatz & Pedersen, 2002).
Some of the inconsistency in the prior results could be attributable to different studies using different measures of the predictor variables (e.g., evaluating cognitive activity as 0 or 1 based on no or any engagement in “mentally active sports [e.g., chess, puzzles]” or as the sum of the frequency of engagement in several activities rated as cognitively demanding), different measures of the cognitive outcome variables (e.g., single scores or composite scores), different types of samples (e.g., varying according to the age range, or with respect to the procedures used to exclude possible dementia cases), and different analytical methods (e.g., simple correlations or complex structural equation models).
A distinctive feature of the current project was the use of powerful latent change analyses to eliminate measurement error and maximize sensitivity to change. The existence of generally significant correlations among the change measures indicates that the measures of change had at least a moderate level of sensitivity. Furthermore, many of the predictor variables (i.e., Need for Cognition, CES-D, PANAS-Positive, PANAS-Negative, Satisfaction with Life, Openness, Cognitive Stimulation) were significantly correlated with the level parameter from the latent change model in one or more of the cognitive variables, which indicates that the potential predictors also had moderate sensitivity. Despite this evidence of sensitivity at the level of both predictor and criterion variables, relations to the change variables were very weak in all age groups. Until the reasons for the different results across projects are understood, researchers should be cautious in reaching conclusions regarding correlates of cognitive change in healthy adults.
To summarize, the combination of results reported above represents a paradox because substantial negative longitudinal change primarily occurs in the 70-and-over group despite similar magnitude of individual differences in change, reliability in change, and correlates among the changes in adults under and over age 70. This paradox must be resolved in order to fully understand the nature and determinants of cognitive change at any period in adulthood.
Acknowledgments
This research was supported by a grant from the Alzheimer's and Related Disease Research Award Fund and from a grant from the National Institute on Aging (R37 024270).
Footnotes
Because of the moderately large sample sizes, a significance level of .01 was used in all statistical analyses.
Fit statistics for the models were generally in the good to excellent range. TO illustrate, the CFI and RMSEA values for the models examining correlations among variables for the same cognitive ability were .93 and .04, respectively, for the memory variables, and .94 and .04, respectively, for the speed variables.
References
- Arbuckle TY, Maag U, Pushkar D, Chaikelson JS. Individual differences in trajectory of intellectual development over 45 years of adulthood. Psychology and Aging. 1998;13:663–675. doi: 10.1037//0882-7974.13.4.663. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatric Society. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MP, Ponds RW, Jelicic M, Houx P, Metsemakers J, Jolles J. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: A reciprocal association? Zeitschrift fur Gerontologie und Geriatrie. 2002;35:575–581. doi: 10.1007/s00391-002-0080-y. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Feinstein JA, Jarvis WBG. Dispositional differences in cognitive motivation: The life and times of individuals varying in need for cognition. Psychological Bulletin. 1996;119:197–253. [Google Scholar]
- Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Lawrence Erlbaum Publishers; Hillsdale, NJ: 1983. [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Enders CK. A primer on maximum likelihood algorithms available for use with missing data. Structural Equation Modeling. 2001;8:128–141. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, Gough HC. The International Personality Item Pool and the future of public-domain personality measures. Journal of Research in Personality. 2006;40:84–96. [Google Scholar]
- Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, Yaffe K, Cuthbert BN, Edwards E, Wagster MV. The NIH cognitive and emotional health project: Report of the critical evaluation committee. Alzheimer's & Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Small BJ, Hertzog C, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging. Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Bosma H, Ponds RWHM, van Boxtel MPJ, Houx PJ, Jolles J. Neuroticism does not affect cognitive functioning in later life. Experimental Aging Research. 2003;29:73–78. doi: 10.1080/03610730303704. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Structuring data to study development and change. In: Cohen SH, Reese HW, editors. Life-span developmental psychology: Methodological innovations. Erlbaum; Hillsdale, NJ: 1994. pp. 223–268. [Google Scholar]
- Nesselroade JR. The warp and woof of the developmental fabric. In: Downs R, Liben L, Palermo D, editors. Views of development, the environment, and aethestics: The legacy of Joachim F. Wohlwill. Erlbaum; Hillsdale, NJ: 1991. pp. 213–240. [Google Scholar]
- Radloff LS. The CES–D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ronnlund M, Nyberg L, Backman L, Nilsson L-G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Implications of within-person variability in cognitive and neuropsychological functioning for the interpretation of change. Neuropsychology. 2007;21:401–411. doi: 10.1037/0894-4105.21.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Major Issues in Cognitive Aging. Oxford University Press; New York: in press. [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Salthouse TA, Fristoe N, Rhee SH. How localized are age-related effects on neuropsychological measures? Neuropsychology. 1996;10:272–285. [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. Oxford University Press; New York: 2005. [Google Scholar]
- Spielberger CD, Gorsush RL, Lushene R, Vagg PR, Jacobs GA. Manual of the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Archives of Neurology. 2005;62:1750–1734. doi: 10.1001/archneur.62.11.1750. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of Positive and Negative Affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale: Third Edition. Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: Third Edition. The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance and cognitive decline in normal aging. Journal of Gerontology: Psychological Sciences. 2002;57B:P246–P255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical activity including walking and cognitive function in older women. Journal of the American Medical Association. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late life dementia. Psychosomatic Medicine. 2007;69:47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. Journal of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Li Y, Bennett DA. The relation of cognitive activity to risk of developing Alzheimer's> disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women. Archives of Internal Medicine. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]