Abstract
We previously reported that endothelin A (ET-A) receptor antagonism attenuates carcinoma-induced pain in a cancer pain mouse model. In this study, we investigated the mechanism of ET-A receptor-mediated antinociception and evaluated the role of endogenous opioid analgesia. Squamous cell carcinoma (SCC) cell culture treated with the ET-A receptor antagonist (BQ-123) at 10−6 M and 10−5 M significantly increased production and secretion of β-endorphin and leu-enkephalin, respectively. Behavioral studies were performed by inducing tumors in the hind paw of female nude mice with local injection of cells derived from a human oral SCC. Significant pain, as indicated by reduction in withdrawal thresholds in response to mechanical stimulation, began at four days after SCC inoculation and lasted to 18 days, the last day of measurement. Local administration of either naloxone methiodide (500 µg/kg), selective antagonists for µ-opioid receptor (CTOP, 500 µg/kg) or δ-opioid receptor (naltrindole, 11 mg/kg), but not κ-opioid receptor (nor-BNI, 2.5 mg/kg), significantly reversed antinociception observed from ET-A receptor antagonism (BQ-123, 92 mg/kg) in cancer animals. These results demonstrate that antagonism of peripheral endothelin-A receptor attenuates carcinoma pain by modulating release of endogenous opioids to act on opioid receptors in the cancer microenvironment.
Keywords: endothelin, cancer, pain, cancer mouse model, oral cancer
Introduction
Pain is a primary determinant of a poor quality of life for cancer patients, especially head and neck cancer patients.13 Seventy-five to ninety percent of terminal cancer patients must cope with opiate-resistant pain related to cancer progression.43,44,53,57 Eighty-five percent of cancer patients experience severe pain in their final days of life.60 Opiates are initially effective in cancer pain management, but they are associated with a number of adverse side effects including tolerance 9,40,41,49, immunosuppression 22, nausea, vomiting, respiratory depression and even respiratory arrest. Once morphine tolerance develops, there are no effective analgesic regimens available. The etiology of cancer pain is unknown, but may involve mediator-dependent signaling by cancer cells to primary afferent sensory neurons in the cancer micro-environment.
One candidate mediator responsible for cancer pain is endothelin (ET).51,52,59 ET is a potent vasoactive peptide first isolated from porcine aortic endothelial cells63 and initially shown to play a role in inflammatory pain.23 It is closely related to the sarafotoxins derived from the venom of burrowing asps.36 Three isopeptides of endothelin, endothelin-1, 2, and 3 (ET-1, ET-2, and ET-3, respectively) have been identified.33 ET-1 is a 21 amino acid peptide synthesized from its precursor, pre-pro ET-1, via a proteolytic cleavage facilitated by the metalloproteinase, endothelin converting enzyme (ECE).27 ET exerts its physiological effect through binding of two G-protein coupled receptors (GPCRs), endothelin A (ET-A) and endothelin B (ET-B). ET-1 is highly expressed in different cancers 1,2,4,37 and selective antagonism of ET-A receptors has been shown to attenuate pain in prostate cancer 64, bone cancer 18,20,51, adenomcarcinoma 12, and melanoma 25. Moreover we have demonstrated that injection of an ET-A receptor antagonist into the site of the tumor attenuated squamous carcinoma-induced nociception in a cancer pain mouse model.52,59 These findings indicate that ET-A receptors are implicated in cancer pain signaling and that the antinociceptive effect of ET-AR antagonists is due to antagonism of ET-A receptors in the cancer microenvironment.19,26
ET-A receptor activity on primary afferent neurons leads to downstream signal transduction that involve elevation in intracellular levels of calcium 56,65 and cAMP61, activation of phospholipases (PLC-A and PLC-D) and protein kinase C (PKC), and regulation of membrane ionic channels 34, which all ultimately result in activation of peripheral nociceptors.26 Attenuation of carcinoma pain with ET-A receptor antagonist can lead to effects on components of the signal transduction pathway. Studies in both rats and mice have demonstrated that ET-A receptor antagonist potentiates morphine analgesia and prevents development of tolerance by promoting coupling of G-proteins to opioid receptors.6–8,28 Hence we hypothesize that antagonism of ET-A receptors can potentially inhibit afferent nociceptor signal transduction or potentiate innate opioid analgesic pathways to modulate cancer pain.
Opioid analgesic pathways are activated through activation of the other isotype of endothelin receptors, endothelin-B receptors (ET-BR), found on non-neuronal cells. Activation of ET-B receptors on skin keratinocytes with selective ET-B receptor agonists stimulates the release of β-endorphin, which suppresses ET-1-induced pain behavior in mice and rats.35 Both ET-A and ET-B receptors are expressed on cell membranes of squamous cell carcinoma.5 Since activation of ET-B receptor on squamous cells modulates pain through endogenous opioids, we wanted to evaluate the role of opioid analgesia in ET-AR-mediated antinociceptive effects in carcinoma. In the present study, we investigated whether ET-A receptor antagonism affects endogenous opioid secretion, and whether attenuation of carcinoma pain with ET-A receptor antagonist is dependent on opioid receptors. These findings have been partly presented at an annual meeting of the American Pain Society.54
Materials and Methods
Cell culture
HSC-3, an oral SCC cell line (ATCC, Manassas, VA) derived from a human tongue SCC, was cultivated in Dulbecco’s Modification of Eagle’s Medium (DMEM) with 4.5 g/L glucose, L-glutamine and sodium pyruvate, supplemented with 10% fetal bovine serum, 25 µg/mL fungizone, 100 µg/mL streptomycin sulfate, and 100 units/mL penicillin G. Primary normal oral keratinocytes (NOK) were harvested from normal gingival tissues using a modified technique described by Hybbinette et al.31 Collection of oral epithelium was approved by the UCSF Committee on Human Research and consent was obtained from patients. Tissues were washed in 70% sterile ethanol, cut into 5 mm square sections, and incubated in 0.25 mg/mL dispase I at 37°C for approximately 3 hrs. The separated epidermis was minced and transferred to 0.25% trypsin for 5 min incubation at 37°C, then stopped with 0.25–0.5 mg/mL soybean trypsin inhibitor (Invitrogen, Carlsbad, CA) and centrifuged at 1300 rpm for 8 min. The sedimented cells were resuspended in Defined Keratinocyte Serum-free media (Invitrogen, Carlsbad, CA) supplemented with 50 µg/mL gentamicin and 25 µg/mL fungizone and cultivated at 37°C in 5% CO2.
Real-time quantitative RT-PCR in cell culture
Because ET-A receptors have not been previously quantified in oral SCC we used RT-PCR to quantify and compare transcript expression levels of ET-1 and ET-A receptor in oral SCC and normal oral keratinocytes. ET-1 and ET-A receptor mRNA expression levels were measured in the HSC-3 cell line relative to the normal oral keratinocyte control. 104 cells were cultivated in 300 µL of DMEM supplemented with 10% fetal bovine serum, 25 µg/mL fungizone, 100 µg/mL streptomycin sulfate, and 100 units/mL penicillin G on 96-well cell culture plates until 75%–80% confluent. Cells were then harvested and lysed for quantitative PCR analysis using the TaqMan® Gene Expression Cells-to-CT Kit (Applied Biosystems/Ambion, Austin, TX), performed at the Genome Analysis Core Facility (Helen Diller Family Comprehensive Cancer Center, UCSF). Samples were run on an ABI 7700 Prism (PE Biosystems, Foster City, CA). Relative expressions of ET-1 and ET-A receptor mRNA were calculated using the comparative Ct method as previously described.16,59 Analysis was carried out using the software supplied with the ABI 7700 Prism. Overexpression was defined as expression >2.0 relative to the reference gene β-N-acetyl-glucosaminidase (β-Gus).
ELISA measurements of endogenous opioids
To evaluate the effect of ET-A receptor antagonism on opioid production and secretion in oral SCC and NOK, we used enzyme-linked immunosorbent assay (ELISA) for opioid peptide measurement. 105 cells of either HSC-3 or first-passaged normal keratinocytes were seeded onto 6-well tissue culture plates with 3 mL of DMEM supplemented with 10% fetal bovine serum, 25 µg/mL fungizone, 100 µg/mL streptomycin sulfate, and 100 units/mL penicillin G. HSC-3 cells were cultured for 24 hrs until the wells reached 70–80% confluency. Each well was washed once with PBS then incubated for 12 hrs in 1 mL of one of the following media: 1) DMEM alone, 2) DMEM with 10 ng/mL synthetic beta-endorphin (Sigma, St. Louis, MO) or leu-enkephalin (Phoenix Pharmaceuticals, Burlingame, CA), or 3) DMEM with one of ten-fold concentrations of BQ-123 (10−5M to 10−7M, American Peptide Co., Sunnyvale, CA). Culture media were collected and treated with 1× HALT Protease Inhibitor Cocktail (Pierce, Rockford, IL) before performing ELISA to detect levels of β-endorphin (MD Biosciences, St. Paul, MN), leuenkephalin and dynorphin (Phoenix Pharmaceuticals, Burlingame, CA). Opioid concentrations were calculated based on a calibration curve, with 55% supernatant recovery for β-endorphin and 12% recovery for leu-enkephalin. Dynorphin concentrations were calculated with a 1:1 recovery ratio since no synthetic human dynorphin was readily available for recovery test.
SCC paw model
The cancer pain mouse model was produced as previously described. 52,59 Experiments were performed on 5 weeks-old adult female Foxn1nu, athymic, immunocompromised mice (Charles River, Wilmington, MA) weighing 16–20 g at the time of SCC inoculation. Mice were housed in a temperature-controlled room on a 12:12 h light cycle (0700–1900 h light), with ad libitum access to food and water; estrous cycles were not monitored. All procedures were approved by UCSF Committee on Animal Research. Researchers were trained under the Animal Welfare Assurance Program. Mice were divided into three experimental groups: those receiving an injection of squamous carcinoma cells (SCC group), those receiving an injection of normal oral keratinocytes (negative control), and those receiving an injection of DMEM (sham operated). All injections were into the hindpaw. All groups were anesthetized by inhalation with 1–3% isofluorane. Cell injections consisted of 1.0 × 106 HSC-3 cells (SCC group) or NOK cells (negative control) in a vehicle consisting of 50 µL of DMEM into the plantar surface of the right hind paw. The sham-operated group received 50 µL of DMEM. Post-operative analgesia was not necessary as all injections were subcutaneous.
Behavioral testing for the SCC paw model
Behavioral testing was performed as described previously.59 Testing was performed in the afternoons between 14:00 and 16:00 h (during the light phase). Mice were placed in a plastic cage with a wire mesh floor which allowed access to the paws. Quantitative assay guidelines were used similar to a previously described technique.14 Fifteen minutes were allowed for acclimation prior to testing. The probe was applied to the mid-plantar right hind paw, or the tumor-front on the hind paw toward the later stages of tumor development. Paw withdrawal thresholds were determined in response to pressure from an electronic von Frey anesthesiometer (2390 series, IITC Instruments, Woodland Hills, CA). The amount of pressure (g) needed to produce a paw withdrawal response was measured three times on each paw separated by 3 minute intervals to allow resolution of previous stimuli. The results of three tests were averaged for each paw for that day. The SCC, negative control and sham-injected groups were tested under this paradigm at 4, 7, 9, 11, 14, 16, and 18 days post-inoculation of SCC, NOK or vehicle.
Drug administration and pain behavioral testing
To evaluate whether ET-A receptor antagonist-induced attenuation of carcinoma pain involves endogenous opioids, opioid receptor (OR) antagonists were administered following BQ-123 injection. Drug testing was performed on days 18–25 following inoculation of oral SCC into the hindpaw. Drugs were dissolved in a final volume of 20 µL PBS and injected subcutaneously into the mid-plantar hind paw at the site of greatest tumor development with a 30-gauge beveled needle. After a 15 min pre-injection reading, BQ-123 (92 ng/kg) was injected and paw withdrawal thresholds were recorded at post-injection times 5, 10, 15, 30, 45, 60, and 90 min. Then a second drug (PBS or OR antagonist) was injected and paw withdrawal testing was performed at 5, 10, 15, 30, 45, 60, 90, 120, 180 min, and 24 hr. Mice groups were injected with one of the following drug combinations: 1) BQ-123 (92 ng/kg) followed by PBS control, 2) BQ-123 followed by nonspecific OR antagonist (500 µg/kg naloxone methiodide, Sigma, St. Louis, MO), 3) BQ-123 followed by selective µ-OR antagonist (500 µg/kg Cys2-Tyr3-Orn5-Pen7-amide [CTOP], Sigma, St. Louis, MO), or 4) BQ-123 followed by selective δ-OR antagonist (11 mg/kg naltrindole [NTI], Sigma, St. Louis, MO). κ-OR antagonist (2.5 mg/kg nor-binaltorphimine [nor-BNI], Sigma, St. Louis, MO) was injected 12 hr prior to injection with PBS control or BQ-123. The investigators performing the injections and behavioral testing were blinded to the drugs being administered.
Statistical Analysis
A one-way Analysis of Variance (ANOVA) with a Tukey Multiple Comparisons post-test was used to compare the withdrawal threshold of the cancer inoculated mice and sham over 18 days. The same test was used to compare the percent change in withdrawal threshold of the SCC inoculated mice before and after drug or control injection. ELISA protein measurements and mRNA gene expressions were also analyzed with one-way ANOVA. When an Equal Variance Test failed, one-way ANOVA with Dunn’s Method for Multiple Comparison post-test was performed. For all tests a p value of less than 0.05 was considered significant. Statistical analysis was performed using SigmaPlot for Windows (Version 11.0).
Results
ET-1 and ET-A receptor mRNA expression
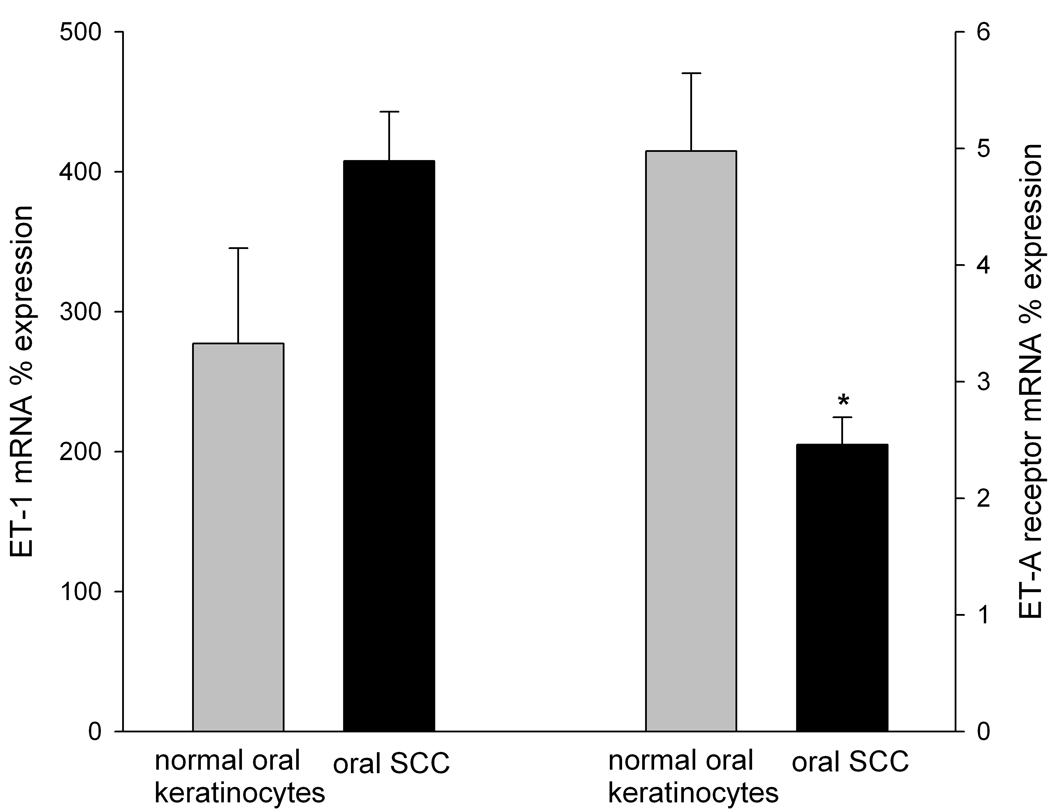
ET-1 and ET-A receptor mRNA expression levels in oral SCC and NOK controls were normalized to β-Gus and compared (Fig. 1). ET-1 mRNA expression in SCC cell culture (407.69 ±68 %) was nearly doubled that in NOK controls (277.28 ±35.09%). ET-A receptor mRNA expression in SCC (2.46 ± 0.24%) was significantly less than expression in NOK controls (4.98 ± 0.67%), p = 0.024.
Figure 1.
Mean ET-1 and ET-A receptor mRNA percent expression in NOK (n = 3) and oral SCC (n = 3) cell cultures normalized to the respective β-GUS mRNA levels. ET-AR mRNA expression was significantly lower in oral SCC relative to NOK control (p = 0.024). [* indicates significance]
ELISA measurement of endogenous opioids in cell culture
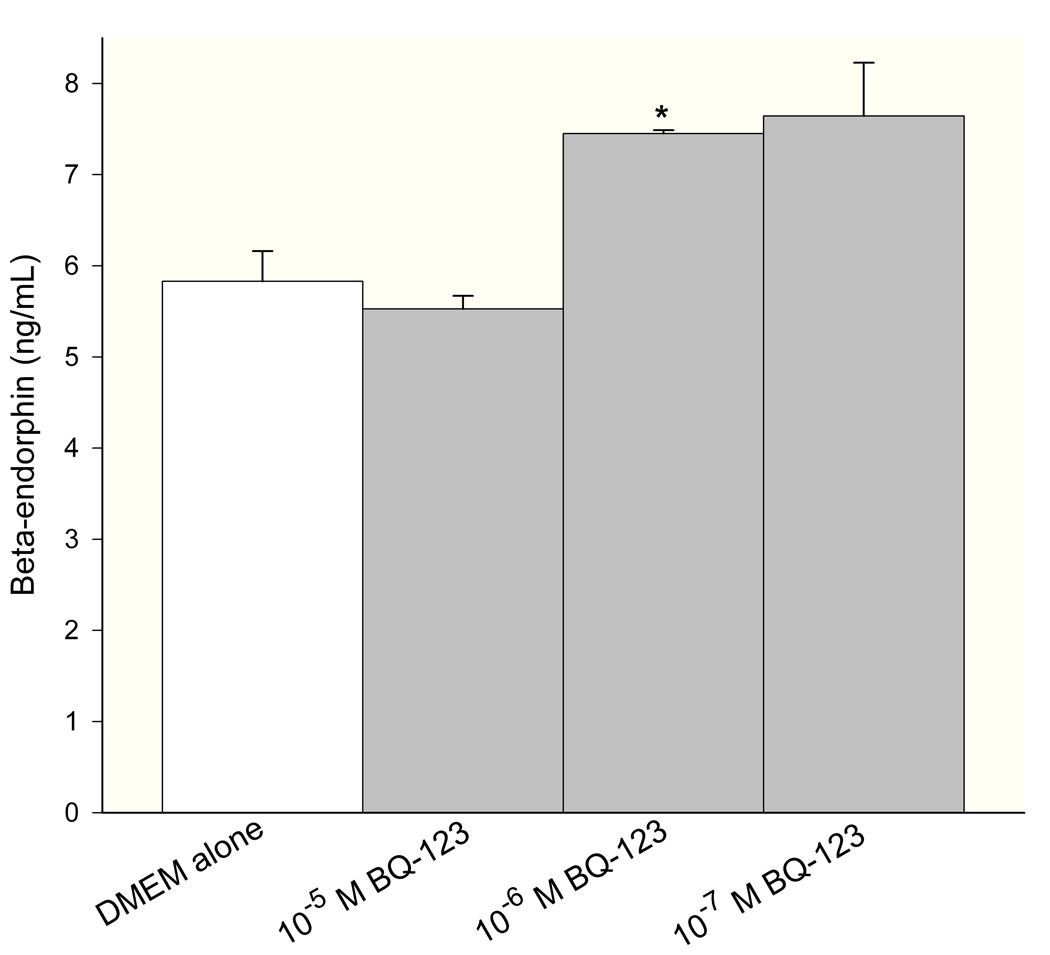
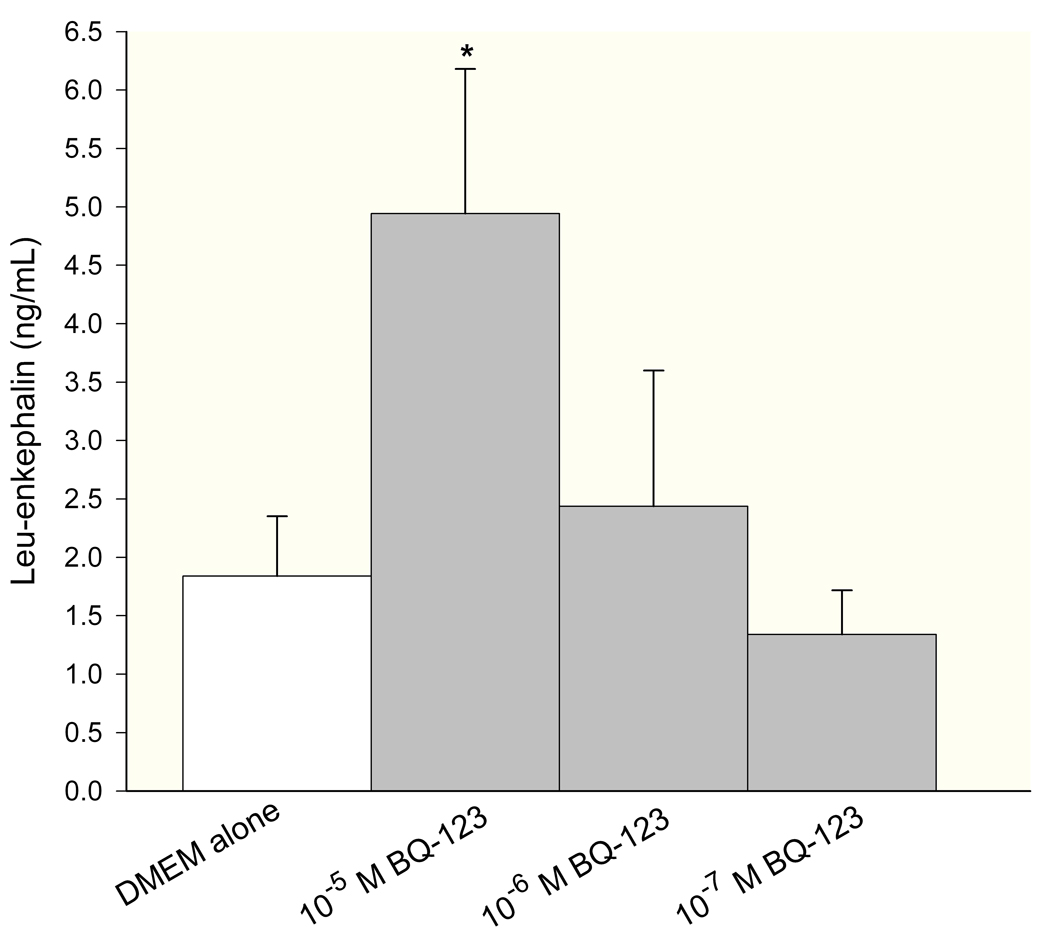
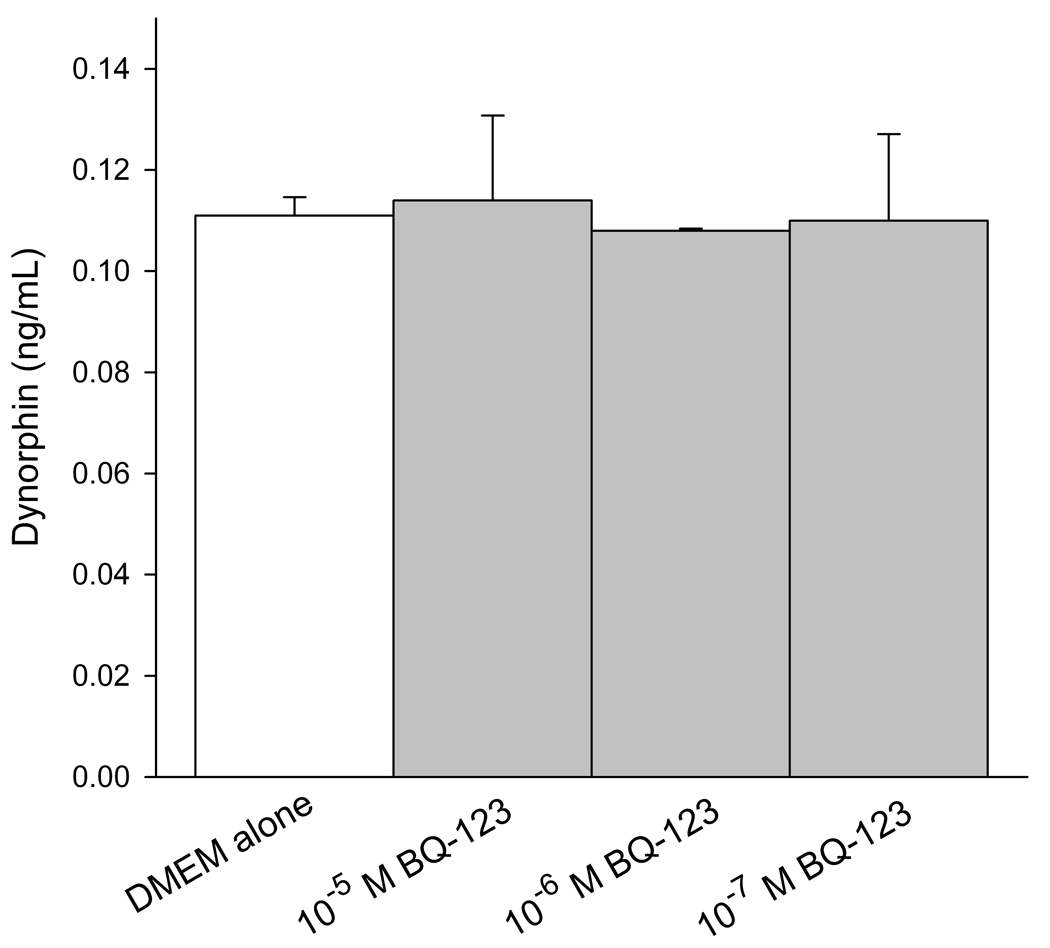
Levels of endogenous opioids (β-endorphin, leu-enkephalin, and dynorphin) were measured in conditioned media of SCC cells by ELISA. The concentration was calculated from a standard curve using a sigmoid logistics curve fitting program (Bio-Rad Laboratories, Inc., Hercules, CA) as appropriate for the opioid ELISA kits, followed by adjustments for percent recovery from synthetic peptide positive control treatments in culture. ET-A receptor antagonist treatment induced dose-responsive production of β-endorphin in SCC cells (Fig. 2a), with 10−6M BQ-123 promoting the greatest increase (7.45 ± 0.04 ng/mL, n = 3) compared to untreated control (5.83 ± 0.33 ng/mL, n = 3). Leu-enkephalin production was dose-dependent on ET-A receptor antagonist (Fig. 2b), with a significant increase at 10−5M BQ-123 (4.94 ± 1.24 ng/mL, n = 3) compared to untreated control (1.84 ± 0.51 ng/mL, n = 3). ET-A receptor antagonist had no effect on dynorphin production (Fig. 2c, n = 3).
Figure 2.
ELISA quantification of endogenous opioid concentration in SCC conditioned media under different ET-A receptor antagonist (BQ-123) treatment. (A) β-endorphin level was significantly increased when treated with 10−6 M BQ-123 compared to DMEM control (p = 0.033) (n = 3). (B) Leu-enkephalin level responded in a dose-dependent manner to BQ-123 treatment, with a significant increase at 10−5 M BQ-123 (p = 0.035) and a significant decrease at 10−8 M BQ-123 (p = 0.036) compared to DMEM control (n = 3). (C) Dynorphin level was not affected by BQ-123 incubation compared to DMEM control (n = 3). [* indicates significance]
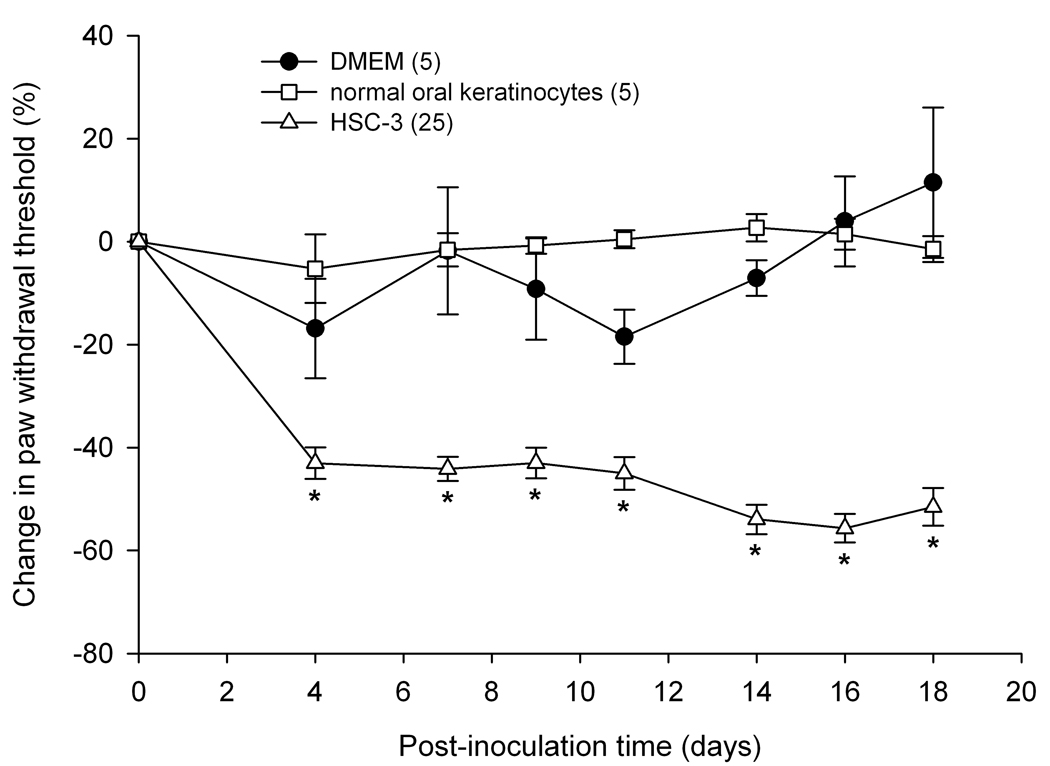
Paw withdrawal in the SCC mouse model
To determine whether SCC inoculation produced mechanical hyperalgesia in the mouse cancer model paw withdrawal thresholds for the SCC, NOK, and sham groups were compared. Mean percent change in paw withdrawal thresholds were significantly reduced in the SCC inoculated mice as compared to both NOK and sham inoculated mice on all 18 days of behavioral testing (Fig. 3). The threshold value (g) for the SCC group at baseline prior to inoculation was 4.45 ± 0.60 g and dropped at PID18 to 2.23 ± 0.97 g. Threshold values for the NOK group maintained at 5.71 ± 0.81 g on PID18 compared to its baseline at 5.46 ± 0.57 g, while the DMEM sham-injected group measured at 5.38 ± 1.08 g compared to its baseline at 4.43 ± 1.02 g.
Figure 3.
Mean percent change in paw withdrawal threshold of the right hind paws of the HSC-3 group (n = 25), NOK group (n = 5) and sham-injected group (n = 5). Paw withdrawal thresholds in the oral SCC (HSC-3 cells) group significantly decreased starting at post-inoculation day 4 and maintained throughout until day 18 (p < 0.05) compared to both NOK and sham-injected groups. The NOK group was not significantly different from the sham-injected group. [* indicates significance]
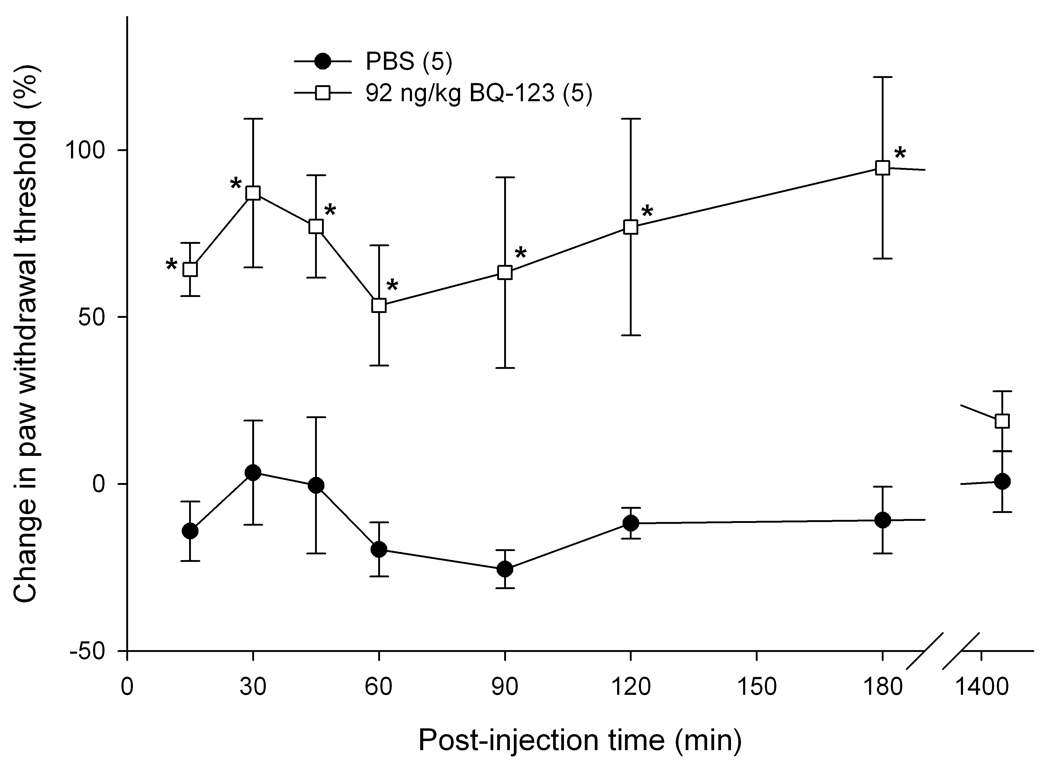
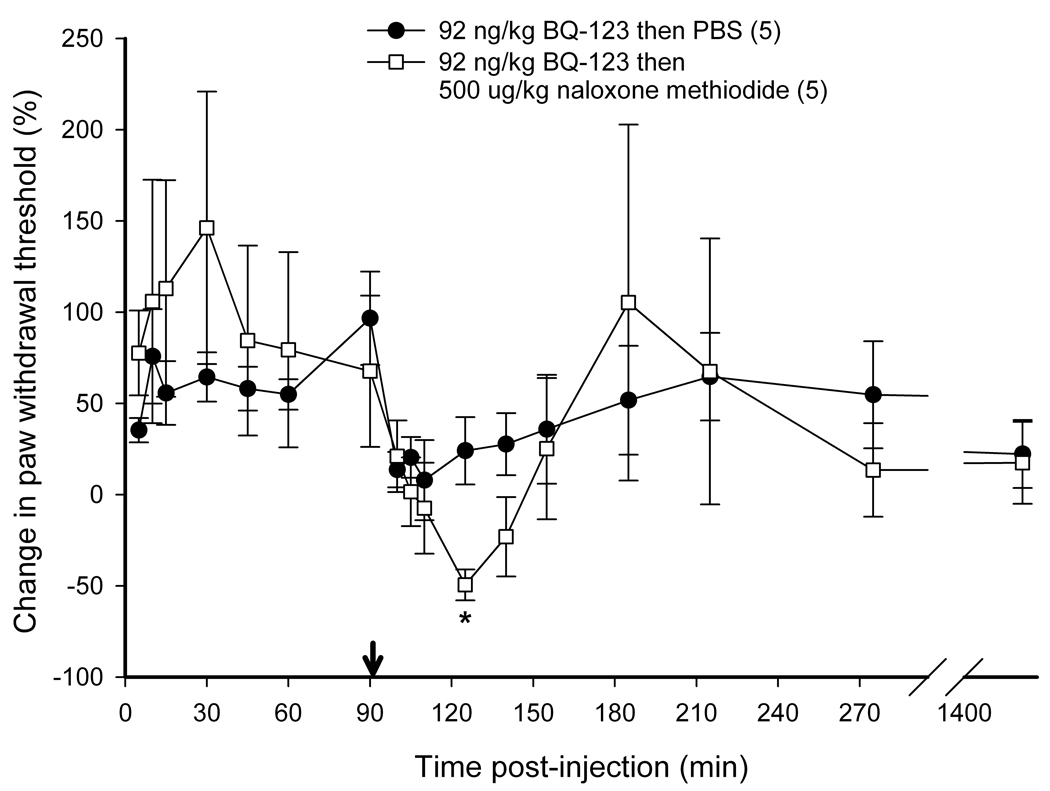
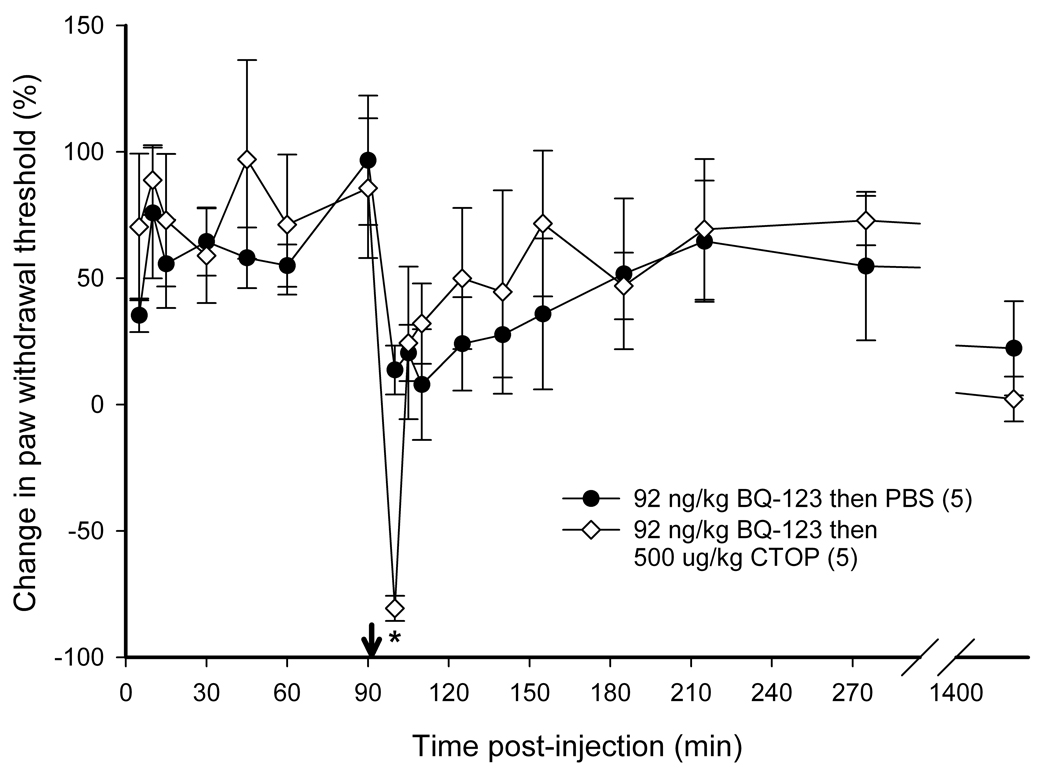
Intra-tumor antagonist administration and nociceptive behavioral testing
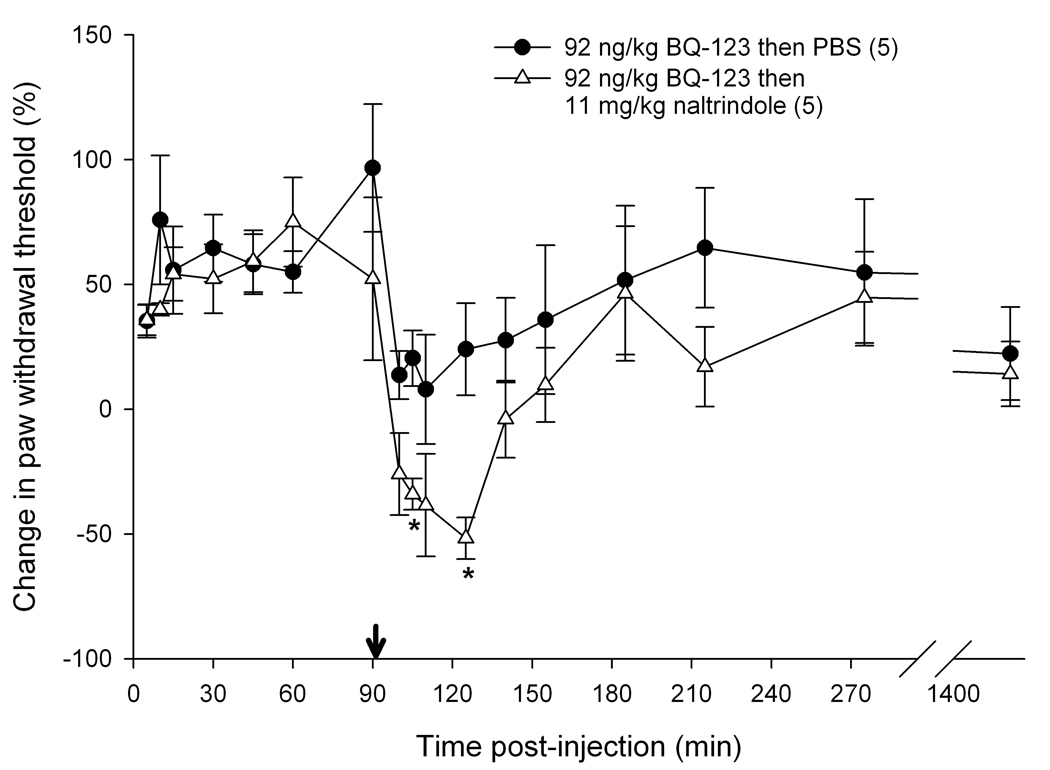
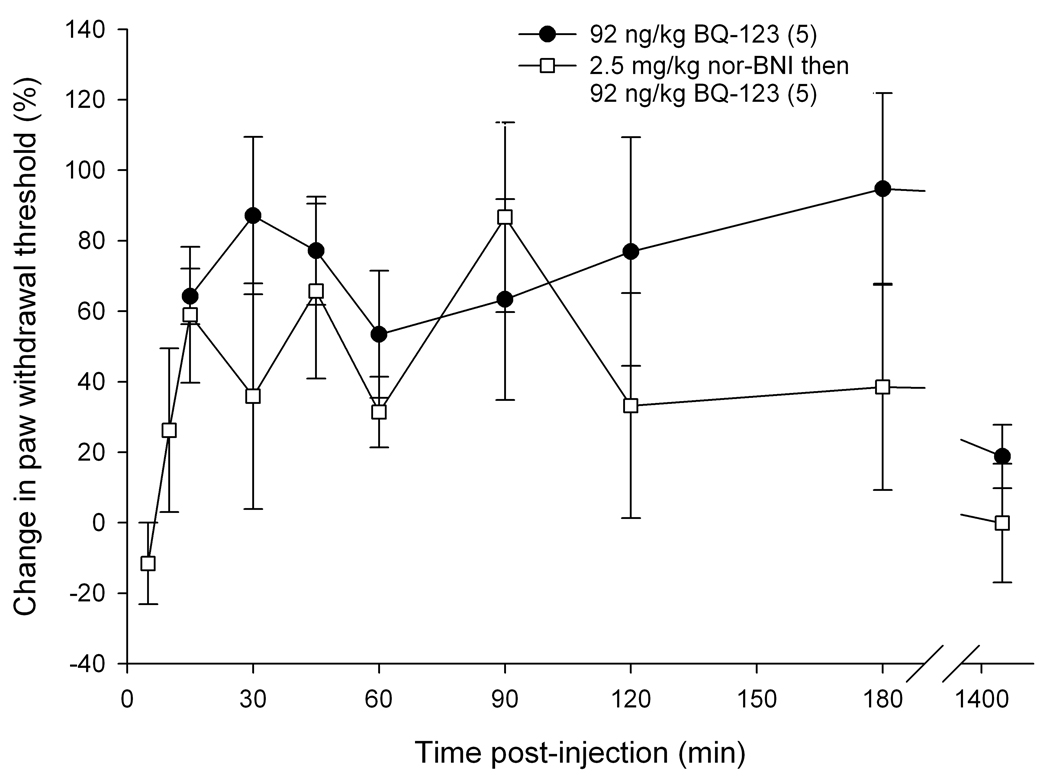
To determine if endogenous opioids were involved in the attenuation of carcinoma pain with ET-A receptor antagonist we tested whether opioid receptor (OR) antagonists could reverse the antinociceptive effect. ET-A receptor antagonism with 92 ng/kg BQ-123 significantly attenuated carcinoma-induced nociception (Fig. 4). The threshold value for the PBS control group was 2.11 ± 0.43 g at baseline, 1.74 ± 0.24 g at t = 15 min, and 1.85 ± 0.15 g at t = 3 hrs post-injection. Threshold values for the BQ-123 group were 1.18 ± 0.22 g at baseline, 1.91 ± 0.22 g at t = 15 min, and 2.24 ± 0.64 g at t = 3 hrs post-injection. Since ET-A receptor antagonism attenuated carcinoma-induced nociception for up to 3 hours post-injection (Fig. 4), OR antagonists were administered at the midpoint time, immediately after the 90 min post BQ-123 recording, to evaluate the nociceptive effect of an opioid antagonist on paw withdrawal thresholds. BQ-123 (92 ng/kg) was first injected to establish the antinociceptive behavioral response to ET-A receptor antagonist (Fig. 5, up to 90 min post-injection). Administration with a non-selective opioid receptor antagonist naloxone methiodide (500 µg/kg) decreased paw withdrawal threshold (Fig. 5a) at 30 minutes after inoculation relative to drug vehicle control (PBS), lasting no more than 45 minutes. Threshold values for the PBS control group were 1.82 ± 0.37 g at baseline before BQ-123 injection, 3.47 ± 0.70 g at t = 90 min immediately prior to PBS injection, and 2.16 ± 0.34 g at t = 125 min approximately 30 min after PBS injection, compared to the naloxone methiodide-injected group which measured 1.67 ± 0.59 g, 2.40 ± 0.83 g, and 0.84 ± 0.45 g, respectively. Selective µ-opioid receptor antagonist CTOP (500 µg/kg) immediately reversed antinociception (Fig. 5b, p < 0.001 at t = 100 min), but rapidly dissipated within 10 min. Threshold values for the PBS control group were as detailed above, while the CTOP-injected group measured at 1.70 ± 0.35 g at baseline prior to BQ-123 injection, 3.00 ± 0.56 g at t = 90 min immediately before CTOP injection, and 0.31 ± 0.17 g at t = 100 approximately 5 minutes after CTOP administration. Specific δ-opioid receptor antagonist naltrindole (11 mg/kg) also significantly reduced paw withdrawal thresholds post-injection (Fig. 5c), lasting approximately 45 minutes. Actual threshold values for the naltrindole-injected group were 1.98 ± 0.35 g at baseline before BQ-123 injection, 2.85 ± 0.87 g at t = 90 min immediately prior to naltrindole injection, 1.36 ± 0.49 g at t = 100 min about 5 minutes after naltrindole administration, and 0.93 ± 0.31 g at t = 125 min approximately 30 minutes following naltrindole. In order to achieve selective inhibition, tumor animals were pretreated with κ-opioid receptor antagonist nor-binaltorphimine (2.5 mg/kg) 12 hours before injection with BQ-123. nor- BNI had no effect on paw withdrawal thresholds compared to BQ-123 injection (Fig. 5d). Threshold values for the nor-BNI-treated group were 1.78 ±0.59 g at baseline before BQ-123 injection, 2.63 ± 0.22 g at t = 15 min after BQ-123 administration, and 2.18 ± 0.33 g at t = 3 hrs following BQ-123.
Figure 4.
Mean percent change in paw withdrawal threshold of the right hind paws of cancer-inoculated animals injected with either ET-AR antagonist (92 ng/kg BQ-123) or drug vehicle alone (PBS). BQ-123 attenuated carcinoma-induced nociception compared to PBS control in cancer-inoculated animals. Thresholds significantly increased at t = 15 min after drug injection and lasted up to 3 hrs post-injection (p < 0.05). [* indicates significance]
Figure 5.
Mean percent change in paw withdrawal threshold of the right hind paws of cancer-inoculated animals injected with ET-AR antagonist (92 ng/kg BQ-123) followed by administration of different peripheral opioid antagonist drug or PBS alone (indicated by ↓). (A) Paw withdrawal for SCC group which received naloxone methiodide at 90 min after BQ-123 injection (n = 5) compared to PBS control group (n = 5). The mean withdrawal threshold for the naloxone methiodide group had a significant negative change at about 35 min following injection of the peripheral non-specific opioid receptor antagonist (t = 125 min), compared to the PBS control group (p = 0.007). (B) Paw withdrawal for SCC group which received CTOP at 90 min after BQ-123 injection (n = 5). CTOP injection resulted in an immediate but short-lived significant reduction of the mean paw withdrawal threshold ( p < 0.001) at t = 100 min compared to the SCC group which received PBS control administration. (C) Paw withdrawal for SCC group which received naltrindole at 90 min after BQ-123 injection (n = 5). The mean paw withdrawal threshold for the naltrindole group had significant negative changes at t = 105 and 125 min compared to PBS control administration (p = 0.003 and 0.006, respectively). (D) Paw withdrawal for SCC group which received nor-binaltorphimine at 12 hrs prior to BQ-123 injection (n = 5). nor-BNI administration had no significant effect on the mean paw withdrawal threshold compared to the PBS control group. [* indicates significance]
Discussion
In the current study we demonstrated that endogenous opioids are responsible for the attenuation of carcinoma pain with ET-A receptor antagonist BQ-123. The non-selective opioid antagonist naloxone methiodide, as well as the selective µ-opioid and δ-opioid receptor antagonists blocked antinociception induced by ET-A receptor antagonist in the mouse model of cancer pain. Our in vitro experiments demonstrated that secretion of β-endorphin and leuenkephalin neuropeptides are induced upon cell culture treatment with BQ-123. These results confirm that carcinoma is capable of producing antinociception through the release of opioid peptides in the cancer microenvironment.
ET-1 is abundantly expressed by a number of carcinomas, including prostate 37, lung 48, breast 2 colorectal 4 and oral.46 We have demonstrated increased levels of both ET-1 protein and transcript in oral SCC.52 Endothelin-A receptor is one of two G-protein coupled receptor (GPCR) 3 isotypes that bind endothelin-1 (ET-1). ET-A receptors are localized to the oral SCC cell membrane 59. In the current study we found that expression levels of the receptor are reduced two-fold compared to NOK. ET-AR is a G-protein coupled receptor associated with many different downstream signal transduction cascades, including protein kinase C, phosphatidylinositol-3-kinase, and mitogen-activated protein kinase.34 Binding to ET-1 leads to ET-A receptor phosphorylation by the G-protein-coupled receptor kinase type 2 (GRK2) 24, internalization and recycling back to cell membrane surfaces.10 Increased ET-1 may activate feedback inhibition to downregulate ET-A receptor expression similar to the negative feedback regulation of glucocorticoid receptors by glucocorticoid-induced peroxisome proliferators-activated receptor alpha (PPARα).15
ET-A receptor antagonist BQ-123 treatment in HSC-3 significantly increased secretion of β-endorphin and leu-enkephalin peptides. BQ-123 concentrations were selected for expected optimal effects based on its reported IC50 of 22 nM.62 The higher BQ-123 concentration at 10−5 M had no significant effect, which may reflect a maximal effective dose for β-endorphin production. The same high concentration of BQ-123 has been shown to have no effect on maximal constrictive properties of porcine vascular smooth muscle cells.32 BQ-123 induced leu-enkephalin production in a dose-dependent manner. While endogenous opioids are commonly produced by neuronal cells, other cell types are also capable of making opioids, such as leukocytes 11, heart and skeletal muscle cells, visceral lining epithelial cells21, and skin keratinocytes.35 Furthermore a number of cancers have been demonstrated to produce opioids, including malignant melanoma, benign melanocytic naevi 45, small cell lung carcinoma 42, and ovarian tumors. 48 Epidermoid carcinoma cells and human foreskin keratinocytes are shown to produce proopiomelanocortin (POMC), the precursor for melanotropic, corticotropic and opioid peptides 58, but opioid production and secretion by an oral carcinoma have not been previously demonstrated. Opioids secreted by these non-neuronal cells have potentially similar functions as peptides with neural origin. β-endorphins derived from leukocytes can enhance inhibition of inflammatory pain in both humans and animals.39,55 Results from in vitro and our in vivo mouse model demonstrate that opioids secreted by carcinoma cells likely contribute to attenuation of cancer nociception induced by BQ-123. Intratumor injections with specific opioid receptor antagonists led to reversal of antinociception from ET-A receptor antagonist treatment. The mechanism by which ET-A receptor antagonism leads to endogenous opioid production and secretion in carcinoma cells is unclear; however, ET-A receptors are coupled to G-proteins that affect multiple downstream signaling pathways. Moreover endothelin-A and B receptors are capable of forming homo- and hetero-dimers through linking opposing ends of the ET-1 ligand.30 By antagonizing the ET-A receptors with BQ-123 synthetic peptides, ET-B receptors dissociate from the hetero-dimer and bind to ET-1 with a nine-fold increased affinity.30 ET-B receptor activation in skin keratinocytes have been shown to elevate β-endorphin production.35 Dissociated ET-B receptors in oral carcinoma cells might be more readily activated by ET-1, which is produced in abundance by carcinoma cells, leading to opioid secretion in the cancer microenvironment. Thus ET-A receptor antagonism indirectly leads to increase in endogenous opioid production through subsequent actions from the ET-B receptors.
In addition to ET-A receptor mediated opioid secretion from the carcinoma in the cancer microenvironment, ET-AR antagonism on the nerve terminal might directly modulate the opioid receptor. Previous studies have shown that ET-A receptor antagonism potentiates morphine analgesia and inhibits morphine tolerance 6–8,28. ET-A receptor antagonism is thought to improve peripheral opioid receptor function by preventing G-protein uncoupling at the cytoplasmic tail.7 Like the ET-A receptors, opioid receptors are also G-protein coupled receptors with seven transmembrane domains linked by alternating intracellular and extracellular loops.38 There are four major opioid receptors cloned, namely µ-, δ-, κ-, and nociceptin/orphanin FQ receptors (opioid receptor-like 1 [ORL1]).50 The G-proteins consist of three subunits – α, β, and γ, of which there are 17 genes encoding the α, 5 genes encoding the β, and 12 genes encoding the γ subunit.47 Opioid receptor functions through G-proteins coupled on the cytoplasmic tail of the receptor and uncoupling of the G-protein represents a state of tolerance.17 By preventing G-protein uncoupling with ET-AR antagonists, opioid receptors might maintain active signaling at the nerve terminal to attenuate carcinoma nociception.
Endothelin-A receptor antagonists clearly demonstrate potentials for modulating cancer pain.19,25,29,51,59,64,65 Given that peripheral administration of ET-A receptor antagonist produces antinociception through potentiation of endogenous opioids in the cancer model we studied, a drug targeting peripheral ET-A receptors could provide relief for cancer patients without complications related to opiate tolerance and withdrawal. These desirable effects of ET-A receptor antagonist show promise for management of cancer pain and may lead to improved analgesic therapy.
Perspective.
This article proposes a novel mechanism for endothelin-A receptor antagonist drugs in managing cancer-induced pain. An improved understanding of the role of innate opioid analgesia in ET-AR-mediated antinociception might provide novel alternatives to morphine therapy for the treatment of cancer pain.
Acknowledgments
Supported by NIH/NIDCR R21 DE018561
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22(4):422–431. doi: 10.1165/ajrcmb.22.4.3795. [DOI] [PubMed] [Google Scholar]
- 2.Alanen K, Deng DX, Chakrabarti S. Augmented expression of endothelin-1, endothelin-3 and the endothelin-B receptor in breast carcinoma. Histopathology. 2000;36(2):161–167. doi: 10.1046/j.1365-2559.2000.00795.x. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 4.Asham E, Shankar A, Loizidou M, Fredericks S, Miller K, Boulos PB, Burnstock G, Taylor I. Increased endothelin-1 in colorectal cancer and reduction of tumour growth by ET(A) receptor antagonism. Br J Cancer. 2001;85(11):1759–1763. doi: 10.1054/bjoc.2001.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awano S, Dawson LA, Hunter AR, Turner AJ, Usmani BA. Endothelin system in oral squamous carcinoma cells: specific siRNA targeting of ECE-1 blocks cell proliferation. Int J Cancer. 2006;118(7):1645–1652. doi: 10.1002/ijc.21525. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla S, Matwyshyn G, Gulati A. Endothelin receptor antagonists restore morphine analgesia in morphine tolerant rats. Peptides. 2003;24(4):553–561. doi: 10.1016/s0196-9781(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla S, Matwyshyn G, Gulati A. Morphine tolerance does not develop in mice treated with endothelin-A receptor antagonists. Brain Res. 2005;1064(1–2):126–135. doi: 10.1016/j.brainres.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 8.Bhalla S, Matwyshyn G, Gulati A. Potentiation of morphine analgesia by BQ123, an endothelin antagonist. Peptides. 2002;23(10):1837–1845. doi: 10.1016/s0196-9781(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 9.Brasseur L. Drugs. 1997;53 Suppl 2:10–17. doi: 10.2165/00003495-199700532-00005. [Review of current pharmacologic treatment of pain] [DOI] [PubMed] [Google Scholar]
- 10.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem. 2000;275(23):17596–17604. doi: 10.1074/jbc.M000142200. [DOI] [PubMed] [Google Scholar]
- 11.Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schafer M, Loeffler JP, Stein C. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100(1):142–148. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carducci MA, Nelson JB, Bowling MK, Rogers T, Eisenberger MA, Sinibaldi V, Donehower R, Leahy TL, Carr RA, Isaacson JD, Janus TJ, Andre A, Hosmane BS, Padley RJ. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol. 2002;20(8):2171–2180. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck. 1999;21(6):531–537. doi: 10.1002/(sici)1097-0347(199909)21:6<531::aid-hed6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck. 1999;21(6):531–537. doi: 10.1002/(sici)1097-0347(199909)21:6<531::aid-hed6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Li M, Sun W, Bi Y, Cai M, Liang H, Yu Q, He X, Weng J. Peroxisome proliferator-activated receptor alpha agonist-induced down-regulation of hepatic glucocorticoid receptor expression in SD rats. Biochem Biophys Res Commun. 2008;368(4):865–870. doi: 10.1016/j.bbrc.2008.01.152. [DOI] [PubMed] [Google Scholar]
- 16.Connelly ST, Macabeo-Ong M, Dekker N, Jordan RC, Schmidt BL. Increased nitric oxide levels and iNOS over-expression in oral squamous cell carcinoma. Oral Oncol. 2005;41(3):261–267. doi: 10.1016/j.oraloncology.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143(6):685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990;8(9):811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Davar G. Endothelin-1 and metastatic cancer pain. Pain Med. 2001;2(1):24–27. doi: 10.1046/j.1526-4637.2001.002001024.x. [DOI] [PubMed] [Google Scholar]
- 20.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9(10):2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 21.Denning GM, Ackermann LW, Barna TJ, Armstrong JG, Stoll LL, Weintraub NL, Dickson EW. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008;29(1):83–92. doi: 10.1016/j.peptides.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Eisenstein TK, Rogers TJ, Meissler JJ, Jr, Adler MW, Hilburger ME. Morphine depresses macrophage numbers and function in mouse spleens. Adv Exp Med Biol. 1998;437:33–41. doi: 10.1007/978-1-4615-5347-2_4. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13 Suppl 5:S220–S222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 24.Franco-Cereceda A, Rydh M, Lou YP, Dalsgaard CJ, Lundberg JM. Endothelin as a putative sensory neuropeptide in the guinea-pig: different properties in comparison with calcitonin gene-related peptide. Regul Pept. 1991;32(3):253–265. doi: 10.1016/0167-0115(91)90019-d. [DOI] [PubMed] [Google Scholar]
- 25.Fujita M, Andoh T, Saiki I, Kuraishi Y. Involvement of endothelin and ET(A) endothelin receptor in mechanical allodynia in mice given orthotopic melanoma inoculation. J Pharmacol Sci. 2008;106(2):257–263. doi: 10.1254/jphs.fp0072051. [DOI] [PubMed] [Google Scholar]
- 26.Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21(14):5358–5366. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant K, Loizidou M, Taylor I. Endothelin-1: a multifunctional molecule in cancer. Br J Cancer. 2003;88(2):163–166. doi: 10.1038/sj.bjc.6700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulati A, Bhalla S, Matwyshyn G. A novel combination of opiates and endothelin antagonists to manage pain without any tolerance development. J Cardiovasc Pharmacol. 2004;44 Suppl 1:S129–S131. doi: 10.1097/01.fjc.0000166242.43616.c2. [DOI] [PubMed] [Google Scholar]
- 29.Hamamoto DT, Khasabov SG, Cain DM, Simone DA. Tumor-evoked sensitization of C nociceptors: a role for endothelin. J Neurophysiol. 2008;100(4):2300–2311. doi: 10.1152/jn.01337.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada N, Himeno A, Shigematsu K, Sumikawa K, Niwa M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: possible formation of an ETA-ETB receptor heterodimer. Cell Mol Neurobiol. 2002;22(2):207–226. doi: 10.1023/A:1019822107048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hybbinette S, Bostrom M, Lindberg K. Enzymatic dissociation of keratinocytes from human skin biopsies for in vitro cell propagation. Exp Dermatol. 1999;8(1):30–38. doi: 10.1111/j.1600-0625.1999.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 32.Ihara M, Noguchi K, Saeki T, Fukuroda T, Tsuchida S, Kimura S, Fukami T, Ishikawa K, Nishikibe M, Yano M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50(4):247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- 33.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10(1):4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9(8):1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 36.Kloog Y, Sokolovsky M. Similarities in mode and sites of action of sarafotoxins and endothelins. Trends Pharmacol Sci. 1989;10(6):212–214. doi: 10.1016/0165-6147(89)90261-7. [DOI] [PubMed] [Google Scholar]
- 37.Kurbel S, Kurbel B, Kovacic D, Sulava D, Krajina Z, Dmitrovic B, Sokcevic M. Endothelin-secreting tumors and the idea of the pseudoectopic hormone secretion in tumors. Med Hypotheses. 1999;52(4):329–333. doi: 10.1054/mehy.1997.0637. [DOI] [PubMed] [Google Scholar]
- 38.Law PY, Loh HH. Regulation of opioid receptor activities. J Pharmacol Exp Ther. 1999;289(2):607–624. [PubMed] [Google Scholar]
- 39.Machelska H, Stein C. Pain control by immune-derived opioids. Clin Exp Pharmacol Physiol. 2000;27(7):533–536. doi: 10.1046/j.1440-1681.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 40.Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci Lett. 2000;280(1):13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- 41.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61(3):353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 42.Melzig MF, Nylander I, Vlaskovska M, Terenius L. Beta-endorphin stimulates proliferation of small cell lung carcinoma cells in vitro via nonopioid binding sites. Exp Cell Res. 1995;219(2):471–476. doi: 10.1006/excr.1995.1254. [DOI] [PubMed] [Google Scholar]
- 43.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69(1–2):1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 44.Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev. 1998;24(6):425–432. doi: 10.1016/s0305-7372(98)90005-6. [DOI] [PubMed] [Google Scholar]
- 45.Nagahama M, Funasaka Y, Fernandez-Frez ML, Ohashi A, Chakraborty AK, Ueda M, Ichihashi M. Immunoreactivity of alpha-melanocyte-stimulating hormone, adrenocorticotrophic hormone and beta-endorphin in cutaneous malignant melanoma and benign melanocytic naevi. Br J Dermatol. 1998;138(6):981–985. doi: 10.1046/j.1365-2133.1998.02263.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakamizo M, Pawankar R, Ohkubo K. Presence of endothelin-1 in human salivary glands and tumors. Nippon Ika Daigaku Zasshi. 1998;65(6):471–477. doi: 10.1272/jnms1923.65.471. [DOI] [PubMed] [Google Scholar]
- 47.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 48.Omar RA, el Tabbakh GH. Immunoreactive beta-endorphin in ovarian sex cord-stromal tumors. Arch Pathol Lab Med. 1987;111(5):436–439. [PubMed] [Google Scholar]
- 49.Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- 50.Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117(1):141–161. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, Mantyh PW. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience. 2004;126(4):1043–1052. doi: 10.1016/j.neuroscience.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 52.Pickering V, Jay Gupta R, Quang P, Jordan RC, Schmidt BL. Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur J Pain. 2008;12(3):293–300. doi: 10.1016/j.ejpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353(9165):1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 54.Quang PN, Schmidt BL. Endothelin receptor-mediated attenuation of carcinoma-induced nociception is opioid-depedent in mice. J Pain. 2009;10 4(Supplement 1):A:189 [Google Scholar]
- 55.Rittner HL, Brack A, Machelska H, Mousa SA, Bauer M, Schafer M, Stein C. Opioid peptide-expressing leukocytes: identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95(2):500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- 56.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46(3):325–415. [PubMed] [Google Scholar]
- 57.Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer. 2003;104(5):550–558. doi: 10.1002/ijc.10999. [DOI] [PubMed] [Google Scholar]
- 58.Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93(5):2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, Jordan RC. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11(4):406–414. doi: 10.1016/j.ejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Shedd DP, Carl A, Shedd C. Problems of terminal head and neck cancer patients. Head Neck Surg. 1980;2(6):476–482. doi: 10.1002/hed.2890020606. [DOI] [PubMed] [Google Scholar]
- 61.Sokolovsky M, Shraga-Levine Z, Galron R. Ligand-specific stimulation/inhibition of cAMP formation by a novel endothelin receptor subtype. Biochemistry. 1994;33(38):11417–11419. doi: 10.1021/bi00204a002. [DOI] [PubMed] [Google Scholar]
- 62.Tyndall JD, Pfeiffer B, Abbenante G, Fairlie DP. Over one hundred peptide-activated G protein-coupled receptors recognize ligands with turn structure. Chem Rev. 2005;105(3):793–826. doi: 10.1021/cr040689g. [DOI] [PubMed] [Google Scholar]
- 63.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 64.Yuyama H, Koakutsu A, Fujiyasu N, Tanahashi M, Fujimori A, Sato S, Shibasaki K, Tanaka S, Sudoh K, Sasamata M, Miyata K. Effects of selective endothelin ET(A) receptor antagonists on endothelin-1-induced potentiation of cancer pain. Eur J Pharmacol. 2004;492(2–3):177–182. doi: 10.1016/j.ejphar.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Zhou QL, Strichartz G, Davar G. Endothelin-1 activates ET(A) receptors to increase intracellular calcium in model sensory neurons. Neuroreport. 2001;12(17):3853–3857. doi: 10.1097/00001756-200112040-00050. [DOI] [PubMed] [Google Scholar]