Abstract
It has recently become clear that proteins associated with neurodegenerative disorders can be selectively incorporated into intraluminal vesicles of multivesicular bodies and subsequently released within exosomes. Multiple lines of research support a neuroprotective role for cystatin C in Alzheimer’s disease (AD). Herein we demonstrate that cystatin C, a protein targeted to the classical secretory pathway by its signal peptide sequence, is also secreted by mouse primary neurons in association with exosomes. Immunoproteomic analysis using SELDI TOF MS revealed the presence in exosomes of at least 9 different cystatin C glycoforms. Moreover, the over-expression of familial AD-associated presenilin 2 mutations (PS2 M239I and PS2 T122R) resulted in reduced levels of all cystatin C forms (native and glycosylated) and of amyloid-β precursor protein (APP) metabolites within exosomes. A better understanding of the mechanisms involved in exosomal processing and release may have important implications for the fight against AD and other neurodegenerative diseases.
Keywords: SELDI TOF MS, APP, Electron Microscopy, Glycosylation, Presenilin 2 mutations, Cystatin C, exosomes
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia in humans and is characterized neuropathologically by the extracellular deposition of insoluble amyloid fibrils as amyloid plaques, primarily composed of amyloid-β peptides (Aβ) (Selkoe, 1989). Mutations in the presenilin (PS) genes account for the majority of familial Alzheimer’s disease (FAD) cases (http://www.molgen.ua.ac.be/ADmutations). The majority of PS mutations result in increased production of Aβ peptides, which are derived from the larger amyloid-β precursor protein (APP). Although FAD-linked PS mutations cause increased generation of Aβ42, a large and increasing number of FAD-linked PS mutations have been shown to inhibit other PS activities. It has been recently reported that presenilins are essential for regulating neurotransmitter release (Zhang et al., 2009), suggesting that PS mutations have pathological effects beyond those caused by their abnormal proteolytic function.
Some proteins associated with AD lesions may have a role in the pathological processes leading to amyloidogenesis and neuronal degeneration and others may bind secondarily to amyloid deposits. Neuropathological and molecular studies suggest a functional link between Aβ and cystatin C (Kaeser et al., 2007; Levy et al., 2001; Mi et al., 2007; Sastre et al., 2004; Vinters et al., 1990). The physiological high concentration of cystatin C in the cerebrospinal fluid and its proliferative effect on neural rat stem cells (Taupin et al., 2000) strongly suggest that cystatin C could exert a trophic function in the brain. While Aβ is the major amyloid forming peptide in the brains of AD patients, the cysteine protease inhibitor, cystatin C, co-localizes with Aβ predominantly in amyloid-laden vascular walls, and in senile plaque cores of amyloid in brains of patients with amyloidoses (i.e. AD, Down syndrome, cerebral amyloid angiopathy, hereditary cerebral hemorrhage with amyloidosis, Dutch type and cerebral infarction) and nondemented aged individuals ( Levy et al., 2006).
New evidence shows that neurons and astrocytes have the capacity to secrete membrane proteins by incorporating them into exosomes, which are small vesicles derived from the intralumenal membranes of multivesicular bodies (Fauré et al., 2006). From their original discovery in the removal of unwanted proteins from maturing reticulocytes through their role in immune surveillance, the scope of discovered functions continues to grow (Belting and Wittrup, 2008; Février and Raposo, 2004; Schorey and Bhatnagar, 2008; Vella et al., 2008). It has been recently demonstrated that also proteins and peptides (i.e. APP, APP C-terminal fragments, APP intracellular domain, Aβ, PS) associated with AD are released in association with exosomes (Rajendran et al., 2006; Sharples et al., 2008; Vingtdeux et al., 2007). The identification of Aβ in association with exosomes is an important finding especially since other exosomal proteins, such as alix and flotillin, have been found to accumulate in the plaques of AD brains (Rajendran et al., 2006). These findings could provide potential explanation for extracellular amyloid deposition in the brain. We recently reported that FAD-linked PS2 mutations (PS2 M239I and T122R) (Binetti et al., 2003) alter cystatin C trafficking in mouse primary neurons reducing secretion of its glycosylated form (Ghidoni et al., 2007). Here we investigated the link between cystatin C and exosomes in an in vitro model of FAD.
2. Methods
2.1 Neuronal cultures and transfections
Mouse primary cortical neuronal preparation and transfections were performed as previously described (Benussi et al., 2005): after 4 days in culture, neurons were transfected using pcDNA3 void vector or pcDNA3 constructs containing human PS2 wt, PS2 M239I or T122R mutation cDNAs. Forty-eight hours after transfection, cells were lysed and conditioned media collected.
2.2 Isolation of exosomes from cell culture media
Exosomes from 3-4 × 107 neurons were prepared as described elsewhere (Théry et al., 2006). Briefly, conditioned media were collected and spun at 300 × g for 10 min. The supernatants were then sequentially centrifuged at 2000 × g for 10 min and 10000 × g for 30 min to remove cellular debris, followed by ultracentrifugation at 100000 × g for 70 min (T-8100 rotor) (Sorvall). The 100000 × g pellet was then resuspended in PBS and centrifuged for 70 min at 100000 × g. Exosomes were loaded into a continuous 0.25 to 2 M sucrose gradient and after 16 h of centrifugation at 200000 × g (TH-641 rotor) (Sorvall), fractions were collected from the top of the gradient, diluted with PBS and spun at 100000 × g for 70 min. All centrifugations were performed at 4°C. The pelleted fractions were then analyzed. For the enzymatic deglycosylation of cystatin C, 1 μl of endo-O-glycosidase (Roche) was added to each sample and incubated overnight at 37°C. Sample aliquots incubated without the deglycosylation enzyme were used as controls.
2.3 Western blot analysis
Western blot analyses were performed using the PS2 polyclonal antibody Ab-2 PC235 (Calbiochem) that recognizes the PS2 C-terminal fragment, the cystatin C polyclonal antibody (Upstate Biotechnology), the TSG101 monoclonal antibody (4A10) (Abcam), the Flotillin-2/ESA antibody (BD Biosciences), and the APP monoclonal antibody (22C11) that detects APP N-terminus (Chemicon). Means of normalized densitometric measurements were compared by Student’s t-test for independent samples.
2.4 Immunoelectron microscopy
Five μl of resuspended 2% PFA (Merk) fixed exosomes were put on glow discharged formvar-carbon coated nickel grids. After washing with PBS, the grids were incubated with 50 mM glycine/PBS for 10 min. The grids were blocked with 1% coldwater fish skin gelatin (SIGMA-ALDRICH Inc.) for 10 min, and then incubated with primary antibodies in blocking solution for 1 h at RT. After washing with PBS, Nanogold-labeled Fab’ anti-rabbit or anti-mouse (Nanoprobes) were applied in the blocking buffer for 1 h. The grids were washed with PBS, fixed in 1% Glutaraldehyde for 5 min, thoroughly washed with distilled water, and then washed with 0.02 M sodium citrate (pH 7.0) in order to reduce the background. Silver enhancement (HQ Silver enhancement kit) (Nanoprobes) was performed for 10 min at room temperature in the dark. After washing with distilled water, the grids were contrasted and embedded in a mixture of 3% uranyl acetate and 2% methylcellulose in a ratio of 1 to 9. Stained grids were examined under Philips CM-12 electron microscope and photographed with a Gatan (1k ×1k) digital camera.
2.5 Surface enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI TOF MS)
Two l of the specific monoclonal antibody (1 mg/ml) (4G8) (SIGNET) against Aβ epitope 17-24 or the cystatin C polyclonal antibody (1 mg/ml) (Upstate Biotechnology) were incubated in a humidity chamber for 2 h at RT to allow covalent binding to the PS20 ProteinChip Array (Bio-Rad Laboratories. Inc.). Unreacted sites were blocked with Tris-HCl 0.5 M, pH 8 in a humid chamber at RT for 30 min. Each spot was washed first three times with PBS containing 0.5% (v/v) TritonX-100 and then twice with PBS. The spots were coated with 5 μl of sample and incubated in a humid chamber at 4°C for 3 h (spots coated with cystatin C antibody) or overnight (spots coated with 4G8). Each spot was washed first three times with PBS containing 0.1% (v/v) TritonX-100, twice with PBS, and finally with deionized water. One μl of α-Cyano-4-hydroxy cinnamic acid (CHCA) (Bio-Rad Laboratories Inc.) was added to spots coated with 4G8 (SIGNET) and 1 μl of sinapinic acid (SPA) (Bio-Rad Laboratories Inc.) was added to each spot coated with cystatin C antibody (Upstate Biotechnology). Mass identification was made using the ProteinChip SELDI System, Enterprise Edition (Bio-Rad Laboratories Inc.).
3. Results
3.1 Cystatin C is released in association with exosomes
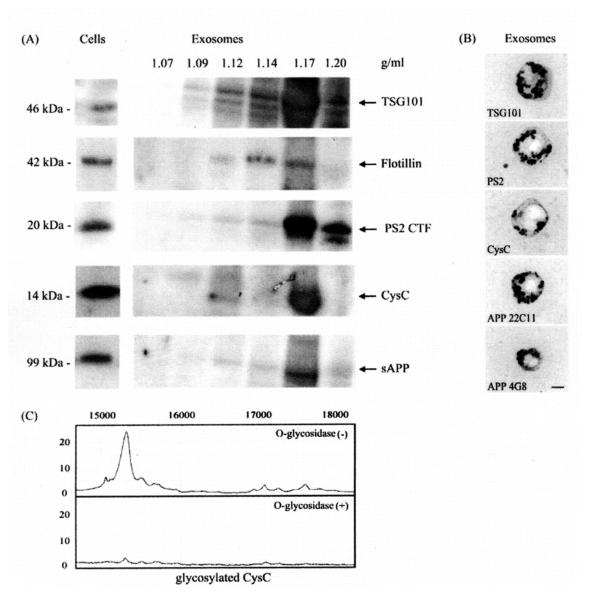
To test whether cystatin C is released in association with exosomes, we isolated these vesicles from conditioned media of mouse primary neurons through serial centrifugations and density gradient centrifugation. TSG101 and flotillin were used as marker proteins to identify exosome-containing fractions (Fig. 1A). Western blot analyses with antibodies directed against AD-associated proteins showed the presence, in these vesicles, of PS2 and APP along with cystatin C (Fig. 1A). Electron microscopy and nanogold immunolabeling of exosome-enriched fractions confirmed the Western blotting data showing labeling with antibodies to TSG101, PS2 C-terminal fragment (CTF), APP, Aβ (4G8) and cystatin C (Fig. 1B). These data highlight a non-classical secretory pathway employed by neurons to export cystatin C, a protein targeted to the classical secretory pathway by its signal peptide sequence (Abrahamson et al., 1987).
Fig. 1.
Exosomes released by mouse primary neurons contain cystatin C along with AD-associated proteins. (A) Sucrose gradient fractions of exosomal preparations from conditioned media of mouse primary neurons were immunoblotted with antibodies directed against TSG101, flotillin, PS2 CTF, cystatin C (CysC), and APP N-terminus (22C11). TSG101 and flotillin marked the exosomes-positive fractions and cystatin C, PS2 CTF, and sAPP were enriched in these fractions. (B) Exosomes-positive fractions (corresponding to 1.14 and 1.17 g/ml sucrose) were pooled and analyzed by immunoelectron microscopy using antibodies against TSG101, PS2 CTF, cystatin C (CysC), APP (22C11 and 4G8) (Scale bar, 100 nm). (C) Exosomes were subjected to SELDI-TOF MS using cystatin C antibody. Cystatin C antibody on PS20 chip array captured native cystatin C as well as at least 9 different glycoforms (glycosylated CysC) (m/z = 15060; 15315; 15510; 16965; 17100; 17280; 17490; 17630; 17810). Enzymatic deglycosylation of cystatin C glycoforms demonstrated that cystatin C in exosomes is O-glycosylated.
3.2 SELDI TOF MS reveals the presence of several different glycoforms of cystatin C in exosomes
We further studied cystatin C in exosomes by an immunoproteomic approach, using SELDI TOF mass spectrometry. Anti-cystatin C antibody on PS20 chip array captured not only native cystatin C, but also at least 9 glycosylated forms (Fig. 1C). Enzymatic deglycosylation by using the endo-O-glycosidase (Fig. 1C) showed that exosomes from mouse primary neurons transport O-glycoforms of cystatin C.
3.3 Over-expression of PS2 mutations (M239I and T122R) alters cystatin C and APP levels and their metabolites in exosomes
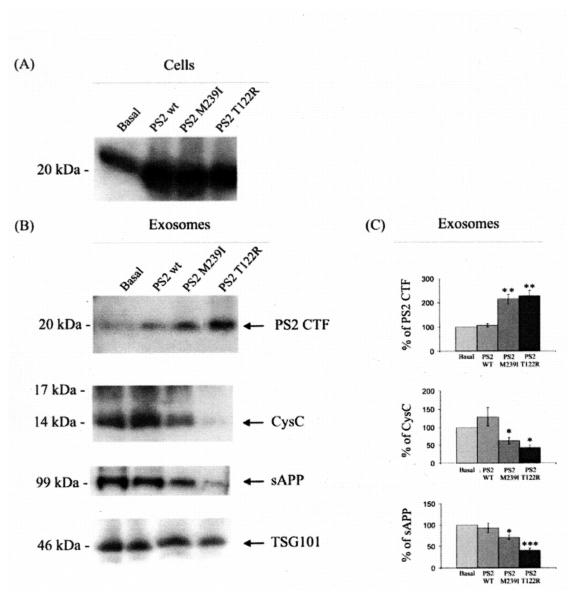
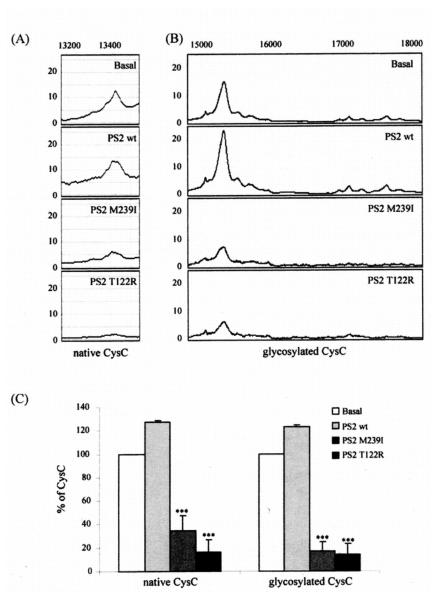
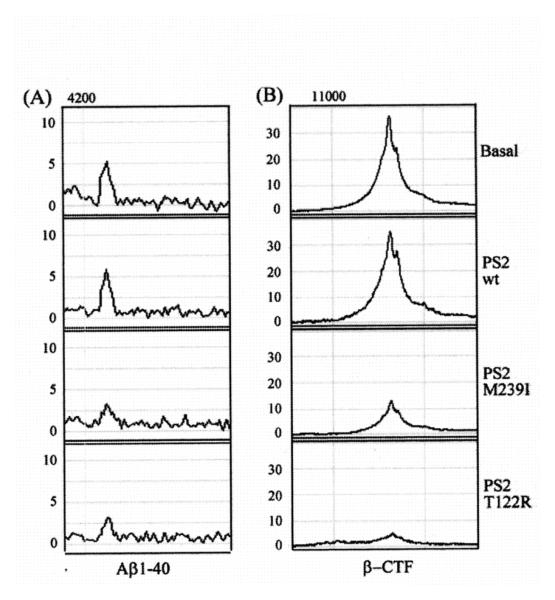
We analyzed exosomes released by mouse primary cortical neurons transfected with cDNA plasmids encoding for human wild type PS2 (PS2 wt), PS2 M239I or T122R mutations. The over-expression of PS2 proteins in neurons was verified by Western blot analysis (Fig. 2A). As shown in Fig. 2B, the over-expression of PS2 mutated proteins resulted in an enrichment of the PS2 CTF in exosomes paralleled by a decrease in cystatin C as well as a decrease in soluble APP (sAPP) (Fig. 2C). We further studied cystatin C and APP and their metabolites in exosomes by an immunoproteomic approach. As shown in Fig. 3, expression of the PS2 FAD mutations reduced the levels of both native and glycosylated forms of cystatin C in exosomes. In addition, SELDI TOF MS experiments using 4G8 revealed that exosomes released by cells over-expressing mutated PS2 contain lower levels of the APP metabolites, Aβ1-40 and β-CTF (Fig. 4A and Fig. 4B) The concentration of the less abundant Aβ1-42 in the exosomes was below the detecting sensitivity of the assay.
Fig. 2.
Over-expression of PS2 mutations (M239I and T122R) alters cystatin C and APP metabolites levels in exosomes. (A) Western blot analysis of mouse cortical primary neurons, transiently transfected with human PS2 wt, PS2 M239I, PS2 T122R or the void vector (basal), confirmed the over-expression of PS2 proteins. (B) Western blot analysis of exosomes released by mouse primary neurons over-expressing mutated PS2 protein revealed a modulation of cystatin C (CysC) and APP metabolism along with an enrichment of PS2 CTF. Analyses were done using antibodies to PS2, cystatin C, APP (22C11) and TSG101. (C) Densitometric analyses of exosomal levels of PS2 CTF, cystatin C (CysC), and sAPP. Mean values, presented as % of protein levels ± SEM, were normalized relative to the void vector (basal) and to the TSG101 protein levels. (n = 4; Student’s t-test *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Fig. 3.
SELDI TOF MS revealed a reduction of exosomal cystatin C levels mediated by PS2 mutations. Exosomes were isolated from media conditioned by mouse primary neurons transfected with human PS2 wt, PS2 M239I, PS2 T122R or the void vector (basal). (A-B) Exosomes were subjected to SELDI-TOF MS using cystatin C antibody. Cystatin C antibody on PS20 chip array captured native cystatin C (native CysC) (A) (m/z = 13.420 Da) as well as at least 9 different glycoforms (glycosylated CysC) (m/z = 15060; 15315; 15510; 16965; 17100; 17280; 17490; 17630; 17810) (B). The PS2 mutations resulted in a reduction of the whole panel of cystatin C glycoforms along with the native form of the protein. (C) Relative percentage of native and glycosylated cystatin C (CysC) forms in exosomes. The 9 different cystatin C glycoforms were represented as a single column in each experimental condition. Data presented as % of protein levels ± SEM. Mean values were normalized relative to the void vector (basal). (n = 8; Student’s t-test *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Fig. 4.
SELDI TOF MS revealed a decrease in the levels of exosomal Aβ and β-CTF mediated by expression of PS2 mutations. (A-B) SELDI TOF MS using 4G8 antibody revealed the presence of Aβ1-40 peptide (m/z = 4235) (A) and β-CTF (m/z = 11320) in exosomes (B). PS2 mutations reduced the levels of both Aβ1-40 and β-CTF. (PS2 M239I/PS2 T122R vs. PS2 wt, p < 0.01, Student’s t-test).
4. Discussion
Alzheimer’s disease is characterized by a continuous loss of neurons that are not replaced and the cause of neuronal death in AD-affected brain regions is still a matter of discussion. It was recently demonstrated that proteins associated with neurodegenerative disorders (such as AD and prion disease) can be selectively incorporated into intraluminal vesicles of multivesicular bodies and subsequently released into the extracellular environment, enriched within exosomes (Vella et al., 2008). These surprising findings brought to light previously unidentified pathways in the processing of APP and provide potential explanation for extracellular amyloid deposition in the brain (Rajendran et al., 2006; Sharples et al., 2008; Vella et al., 2008; Vingtdeux et al., 2007). Herein we demonstrate that cystatin C is also secreted by mouse primary neurons in association with exosomes. Previous studies described cystatin C secretion via the classical secretory pathway (Abrahamson et al., 1987) and it was recently reported that cystatin C is actively internalized by human cell lines, possibly by a receptor-mediated uptake of the protein (Ekström et al., 2008). A previous investigation demonstrated that glycosylated rat cystatin C is an essential cofactor of FGF2 for the proliferation of rat brain derived stem cells (Taupin et al., 2000) and that 5 different cystatin C glycoforms are indeed secreted by these adult hippocampal stem cells into conditioned media (Dahl et al., 2004). Moreover, rat glycosylated cystatin C was involved in the proliferation of adult neuronal stem cells derived from postmortem human brain (Palmer et al., 2001). In the present study, immunoproteomic analysis revealed the presence of at least 9 different cystatin C O-glycoforms in exosomes released by mouse primary neurons, suggesting a key role for exosomes in the transport of trophic factors. Multiple lines of research support a neuroprotective role for cystatin C in AD. Recent findings that low serum cystatin C levels predict the development of AD in subjects free of dementia at baseline (Sundelöf et al., 2008) suggest that low serum cystatin C levels precede clinical sporadic AD. We recently reported that FAD-linked PS2 mutations (PS2 M239I and T122R) alter cystatin C trafficking in mouse primary neurons, reducing its secretion (Ghidoni et al., 2007). We now show that the over-expression of familial AD-associated PS2 mutations results in reduced levels of all cystatin C forms and in a modulation of APP metabolites in exosomes. The reduced concentration of Aβ1-40 in exosomes, secreted by mouse primary neurons overexpressing mutated PS2, reflects the reduction of all CSF Aβ species, including Aβ1-40, in FAD patients carriers of the PS2 T122R mutation (Ghidoni et al., 2009).
We hypothesize that, in a “rarefied” neuronal network, occurring during aging, exosomes could be a key player of neuronal communication (Ghidoni et al., 2008). In this context, exosomes could easily become the Trojan horses of neurodegeneration: a wide range of factors (genetic or environmental) could modify exosomes sorting and/or composition, affecting the fate of the aging neurons. The mechanism underlying cell death could involve transport of toxic agents by Trojan exosomes. Thus, neurodegeneration in AD may be triggered by proteins spread between cells throughout the brain. Furthermore, low exosomal cystatin C levels (due to the over-expression of FAD-associated PS2 mutations) results in a reduced ability to inhibit Aβ aggregation since it had been recently demonstrated, in transgenic mice, that even subtle modifications in cystatin C expression levels affect amyloid deposition and modify disease progression (Kaeser et al., 2007; Levy, 2008; Mi et al., 2007). Thus, it appears that exosomes represent a sophisticated means of interneuronal communication. As cancer phase I clinical trails have shown (Dai et al., 2008), our knowledge of exosomes can be applied therapeutically and the use of exosomes in treatment and diagnostics is also likely to grow (Cho et al., 2005; Viaud et al., 2008). It remains unclear why different cystatin C release mechanisms exist, and which of them is involved in cell pathophysiology. Cystatin C has been implicated in various pathological conditions, including in cell invasion and metastasis in cancer, and in virus replication in cell cultures (Huh et al., 1999; Korant et al., 1985). Thus, finding cystatin C in exosomes open conceptually new lines of research in a wider context, such as immunology and virology.
A better understanding of the mechanisms involved in exosomal cystatin C processing, release, and uptake is of great therapeutic interest and may have important implications for the fight against AD and other diseases.
Acknowledgments
This work was supported by the grants: ERA-Net NEURON JTC 2008 nEUROsyn; Ricerca Corrente, Italian Ministry of Health; National Institute on Aging (AG017617), and the Alzheimer’s Association (IIRG0759699).
Footnotes
Disclosure Statement The authors declare having no actual or potential conflicts of interest in regards to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson M, Grubb A, Olafsson I, Lundwall A. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human cysteine proteinase inhibitor cystatin C. FEBS Lett. 1987;216:229–233. doi: 10.1016/0014-5793(87)80695-6. [DOI] [PubMed] [Google Scholar]
- Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J. Cell Biol. 2008;183:1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi L, Ghidoni R, Paterlini A, Nicosia F, Alberici AC, Signorini S, Barbiero L, Binetti G. Interaction between tau and alpha-synuclein proteins is impaired in the presence of P301L tau mutation. Exp. Cell Res. 2005;308:78–84. doi: 10.1016/j.yexcr.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Binetti G, Signorini S, Squitti R, Alberici A, Benussi L, Cassetta E, Frisoni GB, Barbiero L, Feudatari E, Nicosia F, Testa C, Zanetti O, Gennarelli M, Perani D, Anchisi D, Ghidoni R, Rossini PM. Atypical dementia associated with a novel presenilin-2 mutation. Ann. Neurol. 2003;54:832–836. doi: 10.1002/ana.10760. [DOI] [PubMed] [Google Scholar]
- Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int. J. Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- Dahl A, Eriksson PS, Davidsson P, Persson AI, Ekman R, Westman-Brinkmalm A. Demonstration of multiple novel glycoforms of the stem cell survival factor CCg. J. Neurosci. Res. 2004;77:9–14. doi: 10.1002/jnr.20084. [DOI] [PubMed] [Google Scholar]
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström U, Wallin H, Lorenzo J, Holmqvist B, Abrahamson M, Avilés FX. Internalization of cystatin C in human cell lines. FEBS J. 2008;275:4571–4582. doi: 10.1111/j.1742-4658.2008.06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Benussi L, Paterlini A, Missale C, Usardi A, Rossi R, Barbiero L, Spano P, Binetti G. Presenilin 2 mutations alter cystatin C trafficking in mouse primary neurons. Neurobiol. Aging. 2007;28:371–376. doi: 10.1016/j.neurobiolaging.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Benussi L, Binetti G. Exosomes: the Trojan horses of neurodegeneration. Med. Hypotheses. 2008;70:1226–1227. doi: 10.1016/j.mehy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Albertini V, Squitti R, Paterlini A, Bruno A, Bernardini S, Cassetta E, Rossini PM, Squitieri F, Benussi L, Binetti G. Novel T719P AbetaPP Mutation Unbalances the Relative Proportion of Amyloid-beta Peptides. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1142. (Ahead of print) DOI 10.3233/JAD-2009-1142. [DOI] [PubMed] [Google Scholar]
- Huh CG, Håkansson K, Nathanson CM, Thorgeirsson UP, Jonsson N, Grubb A, Abrahamson M, Karlsson S. Decreased metastatic spread in mice homozygous for a null allele of the cystatin C protease inhibitor gene. Mol Pathol. 1999;52:332–340. doi: 10.1136/mp.52.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser SA, Herzig MC, Coomaraswamy J, Kilger E, Selenica ML, Winkler DT, Staufenbiel M, Levy E, Grubb A, Jucker M. Cystatin C modulates cerebral beta-amyloidosis. Nat. Genet. 2007;39:1437–1439. doi: 10.1038/ng.2007.23. [DOI] [PubMed] [Google Scholar]
- Korant BD, Brzin J, Turk V. Cystatin, a protein inhibitor of cysteine proteases alters viral protein cleavages in infected human cells. Biochem Biophys Res Commun. 1985;127:1072–1076. doi: 10.1016/s0006-291x(85)80054-1. [DOI] [PubMed] [Google Scholar]
- Levy E. Cystatin C: a potential target for Alzheimer’s treatment. Expert Rev. Neurother. 2008;8:687–689. doi: 10.1586/14737175.8.5.687. [DOI] [PubMed] [Google Scholar]
- Levy E, Jaskolski M, Grubb A. The role of cystatin C in cerebral amyloid angiopathy and stroke: cell biology and animal models. Brain Pathol. 2006;16:60–70. doi: 10.1111/j.1750-3639.2006.tb00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F. Codeposition of cystatin C with amyloid-beta protein in the brain of Alzheimer’s disease patients. J. Neuropathol. Exp. Neurol. 2001;60:94–104. doi: 10.1093/jnen/60.1.94. [DOI] [PubMed] [Google Scholar]
- Mi W, Pawlik M, Sastre M, Jung SS, Radvinsky DS, Klein AM, Sommer J, Schmidt SD, Nixon RA, Mathews PM, Levy E. Cystatin C inhibits amyloid-beta deposition in Alzheimer’s disease mouse models. Nat. Genet. 2007;39:1440–1442. doi: 10.1038/ng.2007.29. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Calero M, Pawlik M, Mathews PM, Kumar A, Danilov V, Schmidt SD, Nixon RA, Frangione B, Levy E. Binding of cystatin C to Alzheimer’s amyloid β inhibits amyloid fibril formation. Neurobiol. Aging. 2004;25:1033–1043. doi: 10.1016/j.neurobiolaging.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Amyloid beta protein precursor and the pathogenesis of Alzheimer’s disease. Cell. 1989;58:611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ, Masters CL, Hill AF. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008;22:1469–1478. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- Sundelöf J, Arnlöv J, Ingelsson E, Sundström J, Basu S, Zethelius B, Larsson A, Irizarry MC, Giedraitis V, Rönnemaa E, Degerman-Gunnarsson M, Hyman BT, Basun H, Kilander L, Lannfelt L. Serum cystatin C and the risk of Alzheimer’s disease in elderly men. Neurology. 2008;71:1072–1079. doi: 10.1212/01.wnl.0000326894.40353.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P, Ray J, Fischer WH, Suhr ST, Hakansson K, Grubb A, Gage FH. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current Protocols in Cell Biology. John Wiley and Sons Inc.; New York: 2006. Chap 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur. Biophys. J. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- Viaud S, Ullrich E, Zitvogel L, Chaput N. Exosomes for the treatment of human malignancies. Horm. Metab. Res. 2008;40:82–88. doi: 10.1055/s-2007-1022548. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Hamdane M, Loyens A, Gelé P, Drobeck H, Bégard S, Galas MC, Delacourte A, Beauvillain JC, Buée L, Sergeant N. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 2007;282:18197–18205. doi: 10.1074/jbc.M609475200. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Nishimura GS, Secor DL, Pardridge WM. Immunoreactive A4 and gamma-trace peptide colocalization in amyloidotic arteriolar lesions in brains of patients with Alzheimer’s disease. Am. J. Pathol. 1990;137:233–240. [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Südhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]