Abstract
A novel cytokine IL-33, an IL-1 family member, signals via ST2 receptor and promotes T helper type 2 (Th2) responses, through the activation of NFκB and MAP kinases. Previous studies reported that SIGIRR (single immunoglobulin IL-1R-related molecule)/TIR8 (Toll IL-1R8) acts as negative regulator for TLR-IL-1R-mediated signaling. We now found that SIGIRR formed a complex with ST2 upon IL-33 stimulation and specifically inhibited IL-33/ST2-mediated signaling in cell culture model. Furthermore, IL-33-induced Th2 response was enhanced in SIGIRR-deficient mice compared to that in wild-type control mice, suggesting a negative regulatory role of SIGIRR in IL-33/ST2 signaling in vivo. Similar to ST2, SIGIRR was highly expressed in in vitro polarized Th2 cells, but not Th1 cells. SIGIRR-deficient Th2 cells produce higher levels of “Th2 cytokines”, including IL-5, IL-4 and IL-13 than that in wild-type cells. Moreover, SIGIRR-deficient mice developed stronger Th2 immune response in OVA-challenged asthma model. Taken together, our results suggest that SIGIRR plays an important role in the regulation of Th2 response in vivo, possibly through its impact on IL-33-ST2-mediated signaling.
Keywords: Th1/Th2 Cells, Cytokine receptors, Eosinophils, Lung
Introduction
Members of IL-1R (IL-1 receptor)/TLR (Toll-like receptor) (1) superfamily are expressed on cells of the innate immune system, providing the first line of defense against invading pathogens. The IL-1R/TLRs share a common intracellular domain (TIR domain), which mediates the activation of NFκB and MAP kinases. The IL-1R/TLR superfamily can be divided into two subgroups based on the extracellular domain: the immunoglobulin (Ig) domain-containing receptors (1) and the lecuine-rich repeat motif-containing receptors (2). The Ig domain subgroup includes IL-1R, IL-18R, ST2 and SIGIRR, while the lecuine-rich repeat motif-containing subgroup consists of a least 11 human TLRs (2–7). The IL-1R/TLR-mediated inflammatory response is critical for innate immunity and host defense against infections, whereas uncontrolled inflammation is detrimental to the host, leading to chronic inflammatory diseases. Thus, IL-1R/TLR-mediated innate immunity must have regulatory mechanisms to limit the production of harmful toxins, resulting in resolution of the inflammatory diseases. SIGIRR (also referred as TIR8) has been identified as a negative regulator for IL-1R, TLR4 and TLR9 signaling (8–10). SIGIRR is involved in colonic epithelial homeostasis, inflammation and tumorigenesis through its impact on IL-1R/TLR signaling (11,12). Recent studies have also demonstrated the critical role of SIGIRR in modulation of autoimmunity and inflammatory responses associated with infections (13,14). Therefore, it is important to identify additional IL-1R/TLRs that are inhibited by SIGIRR and determine the physiological consequences of this inhibition.
IL-33, a newly identified member of the IL-1 family (15), has been implicated in the initiation and propagation of the Th2 immune response. Like IL-1 and IL-18 (members of the IL-1 family), IL-33 is produced as pro-IL-33 that is proteolytically cleaved by caspase 1 to generate mature IL-33. Recombinant mature IL-33 has been shown to induce Th2 immunity, increase Th2 cytokines IL-4, IL-5, IL-13, splenomegaly, eosinophilia and pathological changes in mucosal tissues (15). Recently IL-33 has also been described as a chemoatractant for Th2 cells (16). IL-33 mediates its function through its interaction with receptor complex consisting of ST2 molecule and IL-1R accessory protein, leading to NFκB and MAP kinases activation (15,17). Importantly, ST2, a member of the IL-1R family member (18,19), is specifically expressed in Th2 cells (20,21), but not Th1 cells and has been reported to play a critical role in Th2 immunity (22,23).
Allergic asthma, a chronic inflammatory condition of the lung, is associated with prevailing CD4+ T cell infiltrate in the airways, bronchial hyperreactivity, pulmonary eosinophilia, hyperplasia of smooth muscle and elevated serum IgE concentration (24–26). CD4+ Th cells are essential regulators in chronic allergic diseases. Upon activation Th cells undergo differentiation into functionally distinct effector subsets (27–29). Th1 cells produce IFNγ and regulate cellular immunity (30), Th17 cells produce IL-17 and are implicated in autoimmune diseases (31), whereas Th2 cells play an essential role in orchestrating the inflammatory response associated with allergy and humoral immunity (32). Th2 cells function through the production of Th2 cytokines (IL-4, IL-5, IL-9 and IL-13) to mediate antigen-induced allergic airway inflammation, especially eosinophilia, mucus production and airways remodeling (33,34).
In this manuscript, we report that SIGIRR negatively regulates IL-33/ST2 signaling in vitro (cell culture model) and in vivo (intraperitoneal injection of IL-33). Importantly, SIGIRR-deficient mice developed stronger Th2 immune response in OVA-challenged asthma model compared to wild-type mice. Consistent with this finding, SIGIRR-deficient Th2 cells (polarized in vitro) produced higher levels of Th2-associated cytokines (including IL-4, IL-5, and IL-13) than that in wild-type cells. These results demonstrate a critical role of SIGIRR in Th2 cell function and the ability of SIGIRR to suppress Th2 immune response, possibly through its impact on IL-33-ST2-mediated signaling.
Materials and Methods
Stable and transient transfection
HEK293 cells were stably transfected with human ST2-puro vector using Fugene-6 reagent (Roche) according to the manufacturer’s instruction and after selection with puromycin (1μg/ml) either stably transfected with human SIGIRR vector together with hygromycin selection vector and selected with hygromycin B (150 μg/ml) or transiently transfected with full length hSIGIRR, ΔN, ΔTIR and ΔC mutants.
Reporter assays
2 × 105 cells were transfected with the indicated expression vectors (0, 100, 250 or 500 ng) plus 100 ng of the endothelial cell leukocyte adhesion molecule (ELAM)-1 promoter-derived NF-κB luciferase reporter plasmid, and 10 ng β-galactosidase plasmid for normalization, with a 1:3 ratio of DNA:FuGENE 6. Transfection of empty vector was used to ensure all samples received equal amounts of DNA. At 48 h after transfection, cells were stimulated with cytokines for 6 h. Cells were lysed and luciferase activity was assessed using Reporter Lysis Buffer and Luciferase Assay Reagent (Promega). All results reported represent duplicate experiments with at least three independent transfections.
Western Blot
Cells stimulated as indicated were harvested, washed once with cold phosphate buffered saline, and lysed for 30 min at 4°C in 1% Triton X-100, 150 mM NaCl, 1 mM PMSF. Cellular debris were removed by centrifugation at 10,000g for 15 min. For immunoblotting, cell extracts were fractionated by sodium dodecyl sulfate-PAGE and transferred to Immobilon-P transfer membranes (Millipore), using a wet transfer apparatus (Bio-Rad Laboratories). Immunoblot analysis was performed and the bands were visualized with horseradish peroxidase–coupled goat anti–rabbit, goat anti–mouse, or donkey anti–goat immunoglobulin as appropriate (Rockland), using the ECL chemiluminescence Western blotting detection system (GE Healthcare). Protein levels were equilibrated with the Protein Assay Reagent (Bio-Rad Laboratories).
Coimmunoprecipitation
For coimmunoprecipitations, cells were harvested, washed once with cold phosphate buffered saline and lysed in a NP-40 containing buffer (0.5% NP-40, 20 mM Hepes pH 7.4, 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 2 mM EGTA, 20 μM aprotinin, 1 mM PMSF). Cell extracts were incubated with 1 μg of Ab (anti-Flag M2) or normal IgG (negative control) for 2 h, followed by incubation for 2 h with 30μl of protein G-Sepharose beads (prewashed and resuspended in lysis buffer at a 1:1 ratio). After incubations, the beads were washed 4 times with lysis buffer, separated by SDS-PAGE, and analyzed by immunoblotting.
Th1 and Th2 polarization
CD4 positive T cells were isolated from lymph nodes by negative depletion from SIGIRR WT and KO mice. Single cell suspension was incubated with FITC-conjugated anti-CD8 (53-6.7), anti-B220 (RA3-6B2), anti-NK1.1 (PK136), anti-HSA (M1/69), anti-I-Ak/I-Ab (11-5.2 or AF6-120.1), and anti-CD16 (24G2) mAb, followed by anti-FITC microbeads. All antibodies were purchased from BD PharMingen (San Diego, CA). Magnetic separation was performed using LS magnetic column (Miltenyi). Negative fraction was collected. Purity of the CD4 T cells was usually greater than 95%. A total of 106 naïve CD4 T cells were cultured in the presence of plate bound anti CD3/CD28 (3μg/ml each), and different combinations of Abs and cytokines for 4 days: for Th1 conditions (polarization): anti IL-4 (11B11, 10μg/ml) plus IL-12 (10ng/ml); for Th2 conditions (polarization): anti IFN-γ (XMG1.2, 10μg/ml), anti IL-12 (C17.8, 10μg/ml) plus IL-4 (1000U/ml) (44). After incubation with IL-2 (10U/ml) for additional 2 days (resting), 106 cells were restimulated with plate bound anti CD3/CD28 (re-stimulation) with or without IL-33 (20 ng/ml). After 48 hr, supernatants were collected and monitored for cytokines. For real time PCR analysis RNA was isolated from wild-type ex vivo polarized Th2 cells, and ST2, SIGIRR and IL-33 gene expression was checked at different time points.
ELISA assay
IL-4, IL-5, IL-13 and IFN-gamma production was measured using DuoSet ELISA Development Systems obtaied from R&D Systems, following manufacturer’s instruction.
Mice and in vivo IL-33 treatment
SIGIRR WT and KO C57BL/6 mice were given i.p. PBS or 0.4 μg of IL-33 protein daily for 7 days.
Blood analyses
Blood was collected via cardiac puncture.
Allergen sensitization protocol
C57BL/6 and BALB/c mice were immunized with 100 ul intraperitoneal injection containing 10 ug of ovalbumin (chicken OVA, grade V; Sigma, St. Louis, MO) and 20 mg aluminum hydroxide (Sigma, St. Louis, MO on day 1 (BALB/c) or on day 1 and day 8 (C57BL/6). Mice were challenged on day 14 through 21 by exposure to approximately 40 minutes of 1% ovalbumin aerosol diluted in PBS. This was accomplished by placing mice in a 30×60cm acrylic box ventilated by NOUVAG Ultrasonic 2000 nebulizer (NOUVAG USA Inc. Lake Hughes, CA). Mice were sacrificed and processed on day 22, 24 hours after last aerosol challenge.
Bronchoalveolar lavage
The animals were anesthetized with pentobarbital. After placement of a tracheostomy tube, bronchoalveolar lavage (BAL) was performed by instilling700 μl of PBS and then withdrawing the fluid with gentle suction via the syringe. The typical BALfluid return was 300–400 μl. White blood cells were counted on a hemocytometer, while cytologic examination was performed on cytospin preparations stained using Hema3 (Fisher Scientific). Differential counts were based on counts of 100 cells using standard morphologic criteria to classify the cells as eosinophils, lymphocytes, or other mononuclear leukocytes (alveolar macrophages and monocytes). Counts were performed by a single observer who was blinded tothe study group.
Histologic analyses
Microscopic examination of mouse tissues was performed on histochoice-fixed tissue section either stained with hematoxylin/eosin (H&E) or immunostained with anti Ly-G6 FITC conjugated antibody.
Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instruction. 3μg of total RNA was then used for the reverse transcription reaction using Super Script II-reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed in AB 7300 Real Time PCR System and the gene expression was examined by SYBR GREEN PCR Master Mix (Applied Biosystem). PCR amplification was performed in triplicate, and water was used to replace cDNA in each run as a negative control. The reaction protocol included preincubation at 95°C to activate FastStart DNA polymerase for 10 min, amplification of 40 cycles that was set for 15 s at 95°C, and the annealing for 60 s at 60°C. The results were normalized with the housekeeping gene mouse β-actin. The specific primer sequences used in reaction listed as follows: mouse β-actin: 5′-GGTCATCACTATTGGCAACG-3′ and 5′-ACGGATGTCAACGTCACACT-3′; mouse IL-5: 5′-CTCACCGAGCTCTGTTGACAAG-3′ and 5′-CCAATGCATAGCTGGTGATTTTTAT-3′: mouse IL-4 : 5′-CTCATGGAGCTGCAGAGACTCTT-3′ and 5′-CATTCATGGTGCAGCTTATCGA-3′; mouse IL-1 3 : 5′-TGACCAACATCTCCAATTGCA-3′ and 5′-TTGTTATAAAGTGGGCTACTTCGATTT-3′; mouse IP-10: 5′-GACGGTCCGCTGCAACTG -3′ and 5′-GCTTCCCTATGGCCCTCATT-3′; KC: 5′-TAGGGTGAGGACATGTGTGG-3′ and 5′-AAATGTCCAAGGGAAGCGT-3′; mouse IFNγ: 5′-TGATGGCCTGATTGTCTTTCAA-3′ and 5′-GGATATCTGGAGGAACTGGCAA-3′; mouse ST2L : 5′-GCCCTCATCCAGAACAACTC-3′ and 5′-TCTTCCCTCCACTTGATGGT-3′: mouse IL-33: 5′-GGGAAGAAGGTGATGGTGAA-3′ and 5′-CCGAAGGACTTTTTGTGAAGG-3′; mouse SIGIRR: 5′-CTCCGTGACTCCTTCCTCTG-3′ and 5′-TGGGATCTTTGTCATCCTGA-3′.
ELISPOT
For ELIspot analyses, single-cell suspensions were prepared from the spleens and plated (100 or 500 × 103/100 μl) in 96-well ImmunoSpot M200 plates (Cellular Technology, Cleveland, OH) previously coated capture antibodies for IL-5 (TRFK5; eBioscience, San Diego, CA) or IFNγ (R4–6A2; eBioscience, San Diego, CA)(100 μL per well in PBS overnight at 4°C), blocked with PBS–1% bovine serum albumin (Sigma), and washed three times with PBS. Syngeneic APCs, prepared by treating adherent splenocytes with mitomycin C (50 μg/ml; 20 minutes at 37°C), were usedat 2 × 105 APCs per 100 μl. Antigen-specific responseswere elicited by the addition of OVA323–339 (Research Genetics, Birmingham, AL) at a final concentration of 4 μg/ml. After incubation (1 day at 37°C), the plates were washed, and biotinylated detection antibodies for IL-5 (TRFK4; eBioscience) or IFNγ (XMG1.2; eBioscience) in PBS–Tween–1% bovine serum albumin were added to the wells for 2 hours, followed by washing and incubation 2 hours with streptavidin-AP (DAKO, Carpinteria, CA). Spots were developed with a nitroblue tetrazolium chloride (Bio-Rad Laboratories, Hercules, CA) and 5-bromo-4-chloro-3-indolyl phosphate(Sigma Chemical Co.) mixture, visualized, and counted on an ImmunoSpot Series 1 Analyzer (Cellular Technology).
Statistical analysis
The data are presented as the mean + standard deviation. The significance of differences between two groups was determined by Student t test.
Results
SIGIRR inhibits IL-33-ST2-mediated signaling
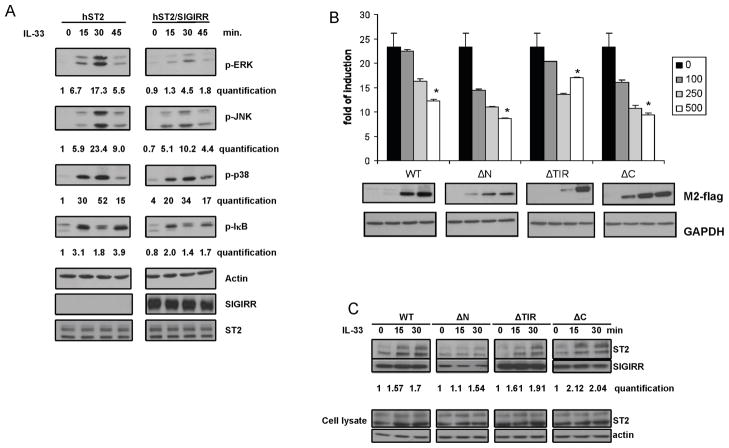
ST2, a member of IL-1R family, has recently been identified as the receptor for interleukin 33 (IL-33). IL-33 stimulation induced phosphorylation of ERK, JNK, p38 and IκBα in human 293 cells transfected with human ST2 expression construct (hST2-293 cells). SIGIRR has been shown to function as a negative regulator for signaling pathways mediated by some members of the IL-1R-TLR superfamily (9,10). We therefore examined whether SIGIRR has the ability to modulate IL-33-ST2-mediated signaling. Interestingly, expression of SIGIRR effectively inhibited IL-33-induced ERK, JNK and IκB phosphorylation in hST2-293 cells, while IL-33-induced p38 phosphorylation was moderately suppressed in the same transfected cells (Fig. 1A). Furthermore, IL-33-induced NFκB-dependent luciferase activity was much reduced in hST2-293 cells transfected with SIGIRR compared to that transfected with vector control, indicating the inhibitory effect of SIGIRR on IL-33-induced NFκB activation (Fig. 1B).
Figure 1. SIGIRR inhibits IL-33-ST2-mediated signaling.
(A) hST2-293 cells (293HEK cells stably transfected with human ST2 expression construct, hST2) and hST2/SIGIRR-293 cells (293HEK cells stably transfected with human ST2 and flag-tagged human SIGIRR, hST2/SIGIRR) were untreated or treated with IL-33 (50 ng/ml), followed by Western analysis with antibodies against p-ERK, p-JNK, p-p38, p-IκB, actin, SIGIRR and ST2. The bands were quantified with ImageJ (NIH) and normalized to actin, shown numbers are relative to time point „0 min” hST2. (B) Increasing amounts of Flag-tagged full-length hSIGIRR (WT, 1–410 amino acids), SIGIRR deletion mutants ΔN [deletion of Ig domain (amino acids 1–118)], ΔTIR [deletion of TIR domain (amino acids 161–313)], ΔC [deletion of C-terminal tail (amino acids 315–410)] or empty vector (pCDNA 3.1) was co-transfected with NFκB-luc (100ng) into hST2-293 cells. Cell lysates from the transfected cells untreated or treated with IL-33 (50 ng/ml) for 6 hrs were subjected to luciferase assay. The data were presented as fold of induction of luciferase activity in treated cells versus untreated cells. Shown are the averages and standard deviation from three independent experiments. The cell lysates were also analyzed by Western blot with anti-flag and anti-GAPDH, *P<0.05 versus 0 ng. (C) hST2-293 cells were transfected with flag-tagged full-length hSIGIRR (WT) and SIGIRR deletion mutants (ΔN, ΔTIR, and ΔC). Cell lysates from the transfected cells untreated or stimulated with IL-33 (50 ng/ml) were immunoprecipitated with anti-flag (SIGIRR) antibody, followed by Western analysis with anti-flag, anti-hST2 and anti-actin antibodies. The bands were quantified with ImageJ (NIH) and normalized to cell lysates, shown numbers are relative to time point „0 min”.
The TIR domain and Ig domain of SIGIRR are sufficient to inhibit ST2 signaling
We investigated whether SIGIRR exerts the inhibitory effect on IL-33 signaling through its interaction with ST2 molecule upon stimulation. Cell lysates from untreated or IL-33-treated hST2-293 cells transfected with flag-tagged SIGIRR were immunoprecipitated with anti-flag (M2), followed by western analyses with anti-flag and anti-hST2 antibodies. As shown in Fig. 1C, SIGIRR indeed formed a complex with ST2 upon IL-33 stimulation, suggesting that SIGIRR inhibits IL-33-mediated signaling through its interaction with the receptor complex.
SIGIRR deletion mutants were used to determine the domain(s) of SIGIRR required for its interaction with ST2. While deletion of C-terminal tail (ΔC) did not have any impact on the ability of SIGIRR to interact with ST2, ΔTIR had slightly reduced interaction with ST2 compared to full-length SIGIRR upon IL-33 stimulation (Fig. 1C). Although Ig domain deletion mutant (ΔN) was expressed at lower level (compared to ΔTIR or ΔC), it was still able to form a complex with ST2 upon IL-33 stimulation (Fig. 1C). The SIGIRR deletion mutants were then examined for their ability to inhibit IL-33-ST2 signaling. While ΔC and ΔN can inhibit IL-33-ST2 signaling as efficiently as the full-length SIGIRR, ΔTIR can partially inhibit (Fig. 1B). Taken together, these results suggest that while both the extracellular Ig and the intracellular TIR domain of SIGIRR are able to interact with ST2, the TIR domain is more critical in inhibiting IL-33 signaling than the Ig domain.
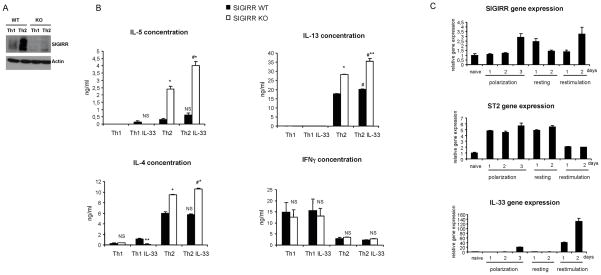
SIGIRR deficiency leads to increased production of type 2 cytokines in Th2 cells
Previous studies have showed that ST2 is strongly expressed on Th2 but not Th1 cells (20,21). Since SIGIRR exerts inhibitory effect on ST2 signaling, it is important to determine whether SIGIRR is co-expressed with ST2. To examine SIGIRR expression in T cell subsets, naïve CD4 positive T cells were isolated from wild-type and SIGIRR-deficient mice and polarized in vitro into Th1 and Th2 cells, followed by western analysis with anti-SIGIRR antibody. As shown in Fig. 2A, SIGIRR was expressed at a higher level in Th2 cells than that in Th1 cells, indicating a possible functional role of SIGIRR in this T cell subset. Importantly, Th2 cytokines (IL-4, IL-5 and IL-13) were indeed significantly increased in SIGIRR-deficient Th2 cells compared to that in wild-type Th2 cells during re-stimulation, indicating a negative regulatory role of SIGIRR in Th2 cell function (Fig. 2B). Interestingly, while ST2 expression was induced on day 1 of polarization, SIGIRR expression was induced on day 3 of polarization and during re-stimulation, suggesting that SIGIRR might serve as a negative feedback control in Th2 cell polarization and re-stimulation (Fig. 2C). It is intriguing that IL-33 expression was highly induced during re-stimulation, suggesting that the increased Th2 cytokine production in SIGIRR-deficient Th2 cells might be due to the action of endogenous IL-33 (Fig. 2C). Whereas IL-33 treatment only slightly induced IL-13, but not IL-4 or IL-5 (statistically not significant) in Th2 cells from wild-type mice, Th2 cytokines (IL-5, IL-13 and IL-4) were moderately and significantly elevated in SIGIRR-deficient Th2 cells upon IL-33 stimulation (Fig. 2B), suggesting the potential role of SIGIRR in modulating IL-33-ST2 signaling in Th2 cells. Taken together, these results implicate the potential role of SIGIRR in the regulation of Th2 immune response, possibly via inhibition of IL-33-ST2 signaling in Th2 cells. Future studies are required to understand the detailed mechanism by which SIGIRR modulates IL-33-ST2 signaling in Th2 cells.
Figure 2. Increased production of Th2 cytokines in Th2 cells from SIGIRR-deficient mice.
(A) Cell extracts prepared from Th1 and Th2 cells polarized ex vivo from wild-type and SIGIRR-deficient mice were blotted with anti-SIGIRR and anti-actin antibodies. (B) Th1 and Th2 cells polarized ex vivo from wild-type and SIGIRR-deficient mice were re-stimulated with anti-CD3/28 antibody in the presence or absence of IL-33 (20 ng/ml). After 48 hrs, the supernatants were measured for IL-4, IL-5, IL-13 and IFNγ concentration with ELISA. Shown is the representation from at least three independent experiments. NS-not significant, *P<0.005 versus SIGIRR WT, #P<0.05 versus Th2 non treated. (C) Total RNA from naïve and ex vivo polarized Th2 cells, isolated from wild-type C57BL/6 mice was subjected to real-time PCR analysis for the levels of SIGIRR, ST2 and IL-33 mRNA at different time points of polarization, resting and re-stimulation.
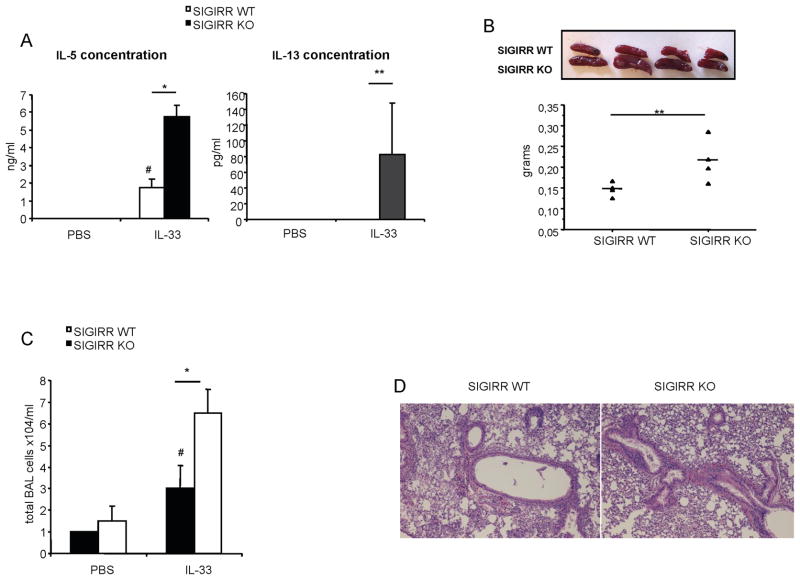
SIGIRR deficiency increases IL-33-dependent Th2 immune response
Previous studies reported that intraperitoneal injection of recombinant IL-33 was able to induce Th2 immunity, including production of Th2 cytokines (IL-4, IL-5 and IL-13), splenomegaly, eosinophilia, and inflammatory responses in the lung, indicating the critical role of IL-33 in mediating Th2-associated immune responses (15). To examine the role of SIGIRR in IL-33-ST2 signaling in vivo, wild-type and SIGIRR-deficient mice were treated with recombinant IL-33 through intraperitoneal injections. As shown in Fig. 3A, IL-33 stimulation induced significantly higher levels of IL-5 and IL-13 in the serum of SIGIRR-deficient mice compared to that in wild-type mice. Furthermore, SIGIRR-deficient mice displayed more severe splenomegaly in response to IL-33 stimulation than the wild-type control mice (Fig. 3B). Consistent with this, IL-33 treatment resulted in greater numbers of Bronchalveolar lavage (BAL) cells in SIGIRR-deficient mice compared to that in wild-type mice (Fig. 3C). More severe inflammation was observed in lungs of SIGIRR-deficient mice injected with IL-33 than that in wild-type mice (Fig. 3D). Taken together, these results demonstrated that SIGIRR-deficient mice were hyper responsive to IL-33, indicating a negative regulatory role of SIGIRR for IL-33-ST2-mediated Th2 immunity.
Figure 3. SIGIRR deficiency increases IL-33-dependent Th2 immune response.
(A–B) Wild-type and SIGIRR-deficient mice (n=7) were intraperitonealy injected daily either with 0.4 μg of IL-33 protein or with PBS for 7 days and blood and spleens were collected. IL-5 and IL-13 serum concentration was measured by ELISA (A) and the spleens from mice injected with IL-33 or PBS were weighted (B). (C–D) Wild-type and SIGIRR-deficient mice (n=6) were intraperitonealy injected daily either with 0.4 μg of IL-33 protein or with PBS for 7 days and BAL and lung tissues were collected. Cells in BAL fluid (C) were counted and lung sections (D) were stained with H&E. *P<0.001, **P<0.05 versus SIGIRR WT, #P<0.05 versus SIGIRR WT PBS.
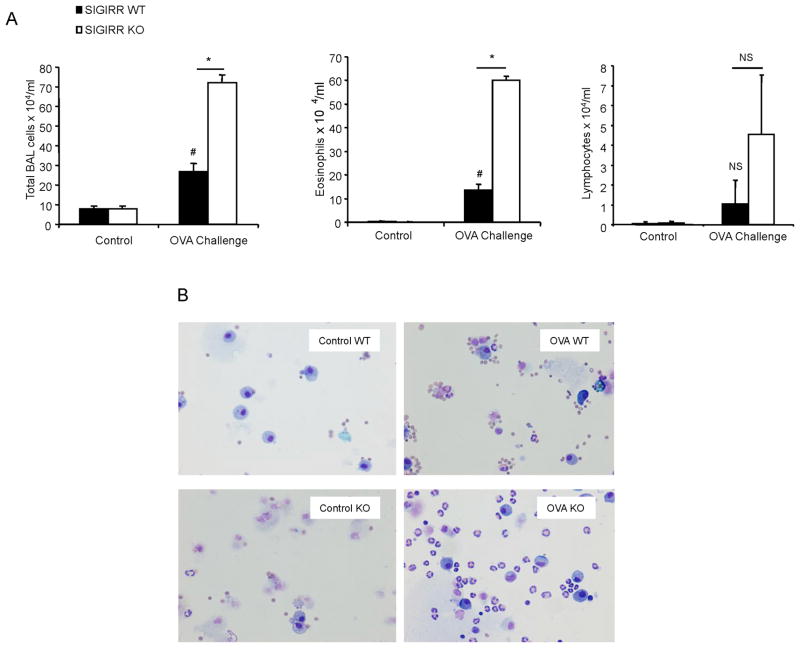
SIGIRR deficiency leads to hyper allergic pulmonary inflammation
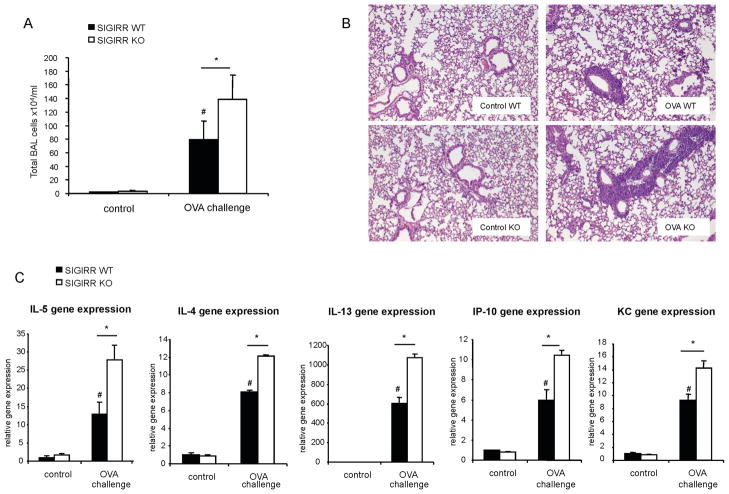
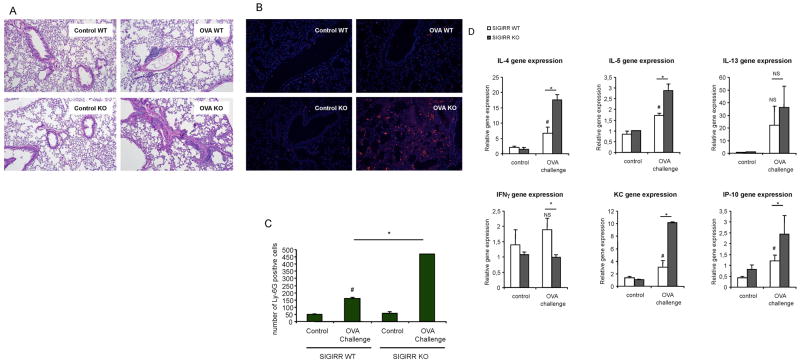
It is well known that antigen-induced allergic pulmonary inflammation is mediated by Th2 cells and their cytokines (IL-4, IL-5 and IL-13). We then examined the role of SIGIRR in allergic pulmonary inflammation. Female C57BL/6 or BALB/c SIGIRR-deficient mice (n = 9 for C57BL/6; n=6 for BALB/c) and their wild-type littermates (n = 6 for each background) were subjected to an OVA-induced model of asthma. OVA/alum-sensitized wild-type and SIGIRR-deficient mice (on C57BL/6 or BALB/c background) were then challenged seven times with aerosolized OVA. Twenty four hours after last challenge, the mice were analyzed for Bronchoalveolar lavage (BAL) cells and lung inflammation. Eosinophils and lymphocyte counts were increased in both C57BL/6 and BALB/c SIGIRR-deficient mice compared to that in their wild-type control mice (Fig. 4A-B and Fig. 6A). Histological analysis of lung tissue showed that SIGIRR deficiency leads to enhanced lung inflammation (Fig. 5A and Fig. 6B). Lung and airway recruitment of granulocytes, predominantly eosinophils and neutrophils, were increased in both C57BL/6 and BALB/c SIGIRR-deficient mice compared with their wild-type mice (Fig. 5A-C and Fig. 6B). The increased asthma phenotype in the C57BL/6 or BALB/c SIGIRR-deficient mice correlated with increased expression of Th2 cytokines (IL-4 and IL-5) and chemokines (including KC and IP-10) in the lung tissues (Fig. 5D and Fig. 6C). Th2 cytokine IL-13 was only significantly increased in BALB/c, but not in C57BL/6 SIGIRR-deficient lung tissues compared to wild-type control mice (Fig. 5D and Fig. 6C).
Figure 4. SIGIRR deficiency leads to hyper allergic pulmonary inflammation in C57BL/6 mice.
(A) Total BAL and differential cell count were analyzed in samples from control or OVA-sensitized and challenged (daily aerosol challenge with OVA for 7 days) wild-type (n=9) and SIGIRR-deficient mice (n=6), *P<0.05 versus SIGIRR WT, #P<0.05 versus SIGIRR WT control, NS-not significant. (B) Cytospins prepared from the BAL from control or OVA-sensitized and challenged wild-type and SIGIRR-deficient mice were stained with Hema3 and differential cell counting was performed using standard morphological criteria. Magnification ×400.
Figure 6. SIGIRR deficiency leads to hyper allergic pulmonary inflammation in BALB/c mice.
(A) Total BAL count was analyzed in samples from control or OVA-sensitized and challenged (daily aerosol challenge with OVA for 7 days) wild-type and SIGIRR-deficient (n=5), *P<0.05 versus SIGIRR WT, #P<0.05 versus SIGIRR WT control (B) The lung sections from control or OVA-sensitized and challenged wild-type and SIGIRR-deficient mice were stained with H&E. Magnification ×100. (C) Total RNA from lung tissues from control or OVA-sensitized and challenged wild-type and SIGIRR-deficient mice were subjected to real-time PCR analysis for the levels of IL-4, IL-5, IL-13, KC and IP-10 mRNA. *P<0.05 versus SIGIRR WT, #P<0.05 versus SIGIRR WT control.
Figure 5. SIGIRR deficiency leads to hyper allergic pulmonary inflammation in C57BL/6 mice.
(A) The lung sections from control or OVA-sensitized and challenged wild-type and SIGIRR-deficient mice (as described in Figure 4) were stained with H&E. Magnification ×100. (B–C) Lung sections from control and OVA-sensitized and challenged wild-type and SIGIRR-deficient mice were stained with anti-Ly-6G, *P<0.005 versus SIGIRR WT, #P<0.05 versus SIGIRR WT control. Magnification ×100. (D) Total RNA from lung tissues from control or OVA-sensitized and challenged wild-type and SIGIRR-deficient mice were subjected to real-time PCR analysis for the levels of IL-4, IL-5, IL-13, IFNγ, KC and IP-10 mRNA. *P<0.05 versus SIGIRR WT, #P<0.05 versus SIGIRR WT control, NS- not significant.
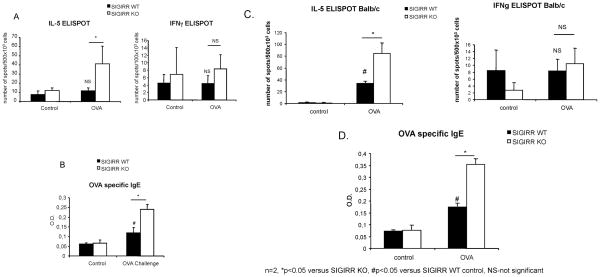
Considering the regulatory role of SIGIRR in the production of Th2 cytokines in polarized Th2 cells, we suspect SIGIRR deficiency impacts on the activation of OVA-specific T cells polarized in vivo, leading to hyper allergic pulmonary inflammation. We examined responses of SIGIRR-deficient and wild-type splenocytes 14 days after OVA-immunization (on C57BL/6 or BALB/c background). Splenocytes from SIGIRR-deficient mice showed increased frequencies of T cells secreting IL-5, but not IFNγ indicating enhanced frequencies of Th2 lineage-derived T cells, a finding consistent with enhanced Th2 immune responsiveness in SIGIRR-deficient mice (Fig. 7A and 7C). The induction of pulmonary inflammation in the mouse model of asthma requires the induction of both cellular as well as humoral immunity. Therefore, we also examined the induction of OVA-specific antibody in wild-type and SIGIRR-deficient animals after ovalbumin sensitization. SIGIRR-deficient mice demonstrated increased induction of ovalbumin specific IgE compared to wild-type mice (Fig. 7B and 7D). Collectively, SIGIRR deficiency enhanced in vivo Th2 humoral and cellular responses in a murine model of allergic asthma.
Figure 7. Hyper activation of Th2 cells and high IgE production in SIGIRR-deficient mice after OVA/Alum priming (on C57BL/6 or BALB/c background).
Wild-type and SIGIRR-deficient mice [C57BL/6, n=6 (A) or BALB/c n=3 (C)] were immunized with OVA/Alum by intraperitoneal injection. 14 days after immunization, the spleens were harvested and IL-5 and IFNγ production were measured by ELISPOT. *P<0.05 versus SIGIRR WT, NS-not significant. Serum from control or OVA-sensitized wild-type and SIGIRR-deficient mice [C57BL/6, n=6 (B) or BALB/c, n=3 (D)] were subjected to ELISA analysis for OVA-specific IgE production. *P<0.05 versus SIGIRR WT, #P<0.05 versus SIGIRR WT control.
Discussion
While the previous studies reported the role of SIGIRR/TIR8 in mucosal immunity (8,12), recent studies have indicated the critical role of SIGIRR/TIR8 in modulation of autoimmunity and inflammatory responses associated with infections (13,14). It is clear that SIGIRR/TIR8 is an important modulator for inflammatory and immune responses. In this study, we report the regulatory role of SIGIRR in adaptive immunity through its impact on Th2 cell function and antigen-induced allergic pulmonary inflammation, providing new opportunity for therapeutic strategies of asthma and other Th2-mediated allergic inflammatory responses.
Recent studies have demonstrated that IL-33 is the ligand for ST2, a member of the IL-1R family, which is specifically expressed in Th2 cells, but not Th1 cells. IL-33 has been shown to mediate its biological activities via ST2, activating NFκB and MAP kinases and driving production of Th2-associated cytokines from polarized Th2 cells. IL-33 injected i.p. resulted in splenomegaly, eosinophilia, elevated serum IgE levels, and inflammatory responses in the lung and the gut, indicating the critical role of IL-33 in mediating Th2-associated immune responses.
SIGIRR, a negative regulator of IL-1R/TLR signaling, is also specifically expressed in Th2 cells, but not Th1 cells. In this manuscript, we show that SIGIRR effectively inhibited IL-33-induced ERK, JNK, p38 and IκB phosphorylation in hST2-293 cells. Furthermore, SIGIRR formed a complex with ST2 receptor upon IL-33 stimulation, suggesting that SIGIRR probably inhibits IL-33-mediated signaling through its interaction with the receptor complex. We have previously reported that while both the extracellular Ig domain and the intracellular TIR domains are important for SIGIRR to inhibit IL-1 signaling only the TIR domain is necessary for SIGIRR to inhibit LPS signaling. We now find that both the extracellular Ig domain and the intracellular TIR are able to inhibit IL-33 signaling probably through interaction with the extracellular Ig domain and intracellular TIR of ST2, respectively.
Consistent with the high expression of SIGIRR in Th2 cells, SIGIRR deficiency leads to elevated Th2 cytokine production in Th2 cells polarized in vitro (20,21). Furthermore, antigen-induced Th2-mediated allergic pulmonary inflammation was significantly enhanced in SIGIRR-deficient mice, indicating the critical role of SIGIRR in modulating Th2 response. Several lines of evidence suggest that IL-33 signaling might play a role in SIGIRR-modulated Th2 response. First, SIGIRR interacts with ST2 and inhibits IL-33-ST2 signaling in cell culture model. Secondly, IL-33-induced Th2 response (via IL-33 i.p. injection) was significantly enhanced in SIGIRR-deficient mice, indicating the inhibitory of SIGIRR in IL-33 signaling in vivo. Thirdly, IL-33 stimulation induced higher Th2 cytokines in SIGIRR-deficient Th2 cells than that in wild-type Th2 cells. It is intriguing that IL-33 expression was highly induced during Th2 polarization and re-stimulation, suggesting that the increased Th2 cytokine production in SIGIRR-deficient Th2 cells (without exogenous IL-33 treatment) might be due to the action of endogenous IL-33. While our data showed that exogenous IL-33 induced significantly higher levels of Th2 cytokines in SIGIRR-deficient Th2 cells compared to wild-type Th2 cells, the relative low impact of exogenous IL-33 might be due to the presence of endogenous IL-33. Taken together, these results implicate the potential role of IL-33-ST2 signaling in SIGIRR-regulated Th2 cell function and Th2 immune response. Interestingly, we found that while ST2 expression was induced on day 1 of polarization, SIGIRR expression was induced on day 3 of polarization and during re-stimulation, suggesting the potential role of SIGIRR to serve as a negative feedback control. Future studies are required to understand the precise function of SIGIRR-modulated ST2 signaling in Th2 cell function.
Previous studies showed that a neutralizing antibody against T1/ST2 or a T1/ST2-Ig fusion protein inhibited Th2 cell differentiation and Th2 effectors functions in vivo, including allergic airway inflammation (20,22,23,35). While a decrease in IL-5 production and recruitment of eosinophils to the lung were observed in Nippostrongylus brasilliensis-infected ST2-deficient mice as compared to wild-type mice, absence of ST2 in mice also led to a significant defect in the OVA-induced macrophages recruitment to the lung. However, some studies utilizing ST2-deficient mice suggested that ST2 is not essential for Th2 response, suggesting a compensatory mechanism(s) in the absence of ST2 (36–38). The fact that SIGIRR deficiency led to a hyper Th2 response in the OVA-challenged asthma model indicates the essential role of SIGIRR in modulating Th2 immunity. Therefore, it is plausible that whereas IL-33-ST2-mediated Th2 immunity can be compensated by other pathway(s) in the genetically altered mice, IL-33-ST2 signaling has to be appropriately modulated through the action of regulatory molecules such as SIGIRR. Loss of such modulation (i.e. SIGIRR deficiency) leads to dysregulation of Th2 immunity, resulting in hyper Th2 immune responses and increased allergic inflammatory responses.
While ST2 is expressed in Th2 but not Th1 cells (20,21,39,40), ST2 is also expressed in other immune cells including mast cell, macrophages (41) and NKT cells (42) Recent study showed that IL-33 can promote a variety of effector responses through ST2 on human basophils, iNKT and NK cells (43). Schmitz et al. (15) reported that IL-33 is able to mediate induction of Th2 cytokines and Th2-associated inflammatory response in the absence of antigen-dependent response. It is possible that cell types other than Th2 cells are involved in IL-33-induced “Th2 response” in vivo, including mast cells, NKT and basophiles. Therefore, the impact of SIGIRR on antigen-induced allergic pulmonary inflammation might not only be due to its regulatory role in Th2 cell function. Other IL-33 responsive cell types and additional pathways (other than IL-33-ST2) might contribute to SIGIRR-modulated Th2 immune responses.
Footnotes
This work was supported by the NIH grant RO1 AI060632
Reference List
- 1.Mitcham JL, Parnet P, Bonnert TP, Garka KE, Gerhart MJ, Slack JL, Gayle MA, Dower SK, Sims JE. T1/ST2 signaling establishes it as a member of an expanding interleukin-1 receptor family. J Biol Chem. 1996;271:5777–5783. doi: 10.1074/jbc.271.10.5777. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 3.Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 4.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 5.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi O, Kawai T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Takeda K, Akira S. TLR6: A novel member of an expanding toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 8.Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor-and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233–25241. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- 10.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 11.Garlanda C, Riva F, Veliz T, Polentarutti N, Pasqualini F, Radaelli E, Sironi M, Nebuloni M, Zorini EO, Scanziani E, Mantovani A. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- 12.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, de la MC, Cua D, Vallance BA, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D’Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- 14.Lech M, Kulkarni OP, Pfeiffer S, Savarese E, Krug A, Garlanda C, Mantovani A, Anders HJ. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J Exp Med. 2008;205:1879–1888. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 17.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 18.Klemenz R, Hoffmann S, Werenskiold AK. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc Natl Acad Sci USA. 1989;86:5708–5712. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossler U, Andres AC, Reichmann E, Schmahl W, Werenskiold AK. T1, an immunoglobulin superfamily member, is expressed in H-ras-dependent epithelial tumours of mammary cells. Oncogene. 1993;8:609–617. [PubMed] [Google Scholar]
- 20.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 25.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–482. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 28.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 29.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 33.Kay AB. The role of T lymphocytes in asthma. Chem Immunol Allergy. 2006;91:59–75. doi: 10.1159/000090230. [DOI] [PubMed] [Google Scholar]
- 34.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 35.Meisel C, Bonhagen K, Lohning M, Coyle AJ, Gutierrez-Ramos JC, Radbruch A, Kamradt T. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol. 2001;166:3143–3150. doi: 10.4049/jimmunol.166.5.3143. [DOI] [PubMed] [Google Scholar]
- 36.Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, Akira S. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangan NE, Dasvarma A, McKenzie AN, Fallon PG. T1/ST2 expression on Th2 cells negatively regulates allergic pulmonary inflammation. Eur J Immunol. 2007;37:1302–1312. doi: 10.1002/eji.200636520. [DOI] [PubMed] [Google Scholar]
- 38.Senn KA, McCoy KD, Maloy KJ, Stark G, Frohli E, Rulicke T, Klemenz R. T1-deficient and T1-Fc-transgenic mice develop a normal protective Th2-type immune response following infection with Nippostrongylus brasiliensis. Eur J Immunol. 2000;30:1929–1938. doi: 10.1002/1521-4141(200007)30:7<1929::AID-IMMU1929>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Chan WL, Pejnovic N, Lee CA, Al Ali NA. Human IL-18 receptor and ST2L are stable and selective markers for the respective type 1 and type 2 circulating lymphocytes. J Immunol. 2001;167:1238–1244. doi: 10.4049/jimmunol.167.3.1238. [DOI] [PubMed] [Google Scholar]
- 40.Lecart S, Lecointe N, Subramaniam A, Alkan S, Ni D, Chen R, Boulay V, Pene J, Kuroiwa K, Tominaga S, Yssel H. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol. 2002;32:2979–2987. doi: 10.1002/1521-4141(2002010)32:10<2979::AID-IMMU2979>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Gachter T, Werenskiold AK, Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J Biol Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]
- 42.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 43.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]