Abstract
Objective
This cross-sectional study sought to confirm the presence and regional profile of previously reported changes in laminar cortical thickness in children and adolescents with Attention Deficit / Hyperactivity Disorder (ADHD) compared to typically developing healthy subjects.
Method
High-resolution MR images were obtained from 22 (19 male, 3 female; mean age: 11.7 years) children and adolescents with ADHD and 22 age and sex matched healthy control subjects (mean age: 11.7 years). Brain tissue volumes were estimated for each subject. Cortical pattern matching methods were used to sample measures of laminar thickness at high spatial frequency across homologous regions of cortex. Volume and thickness measures were compared across diagnostic groups with and without controlling for general intelligence. False discovery rate (FDR) correction confirmed regional results.
Results
Subjects with ADHD exhibited significant reductions in overall brain volume, gray matter volume and mean cortical thickness compared to healthy controls, while white matter volumes were significantly increased in ADHD. Highly significant cortical thinning (FDR-corrected p < .0006) was observed over large areas of frontal, temporal, parietal and occipital association cortices and aspects of motor cortex, but not within primary sensory regions.
Conclusions
Cortical thickness reductions present a robust neuroanatomical marker for child and adolescent ADHD. Observations of widespread cortical thinning expand upon earlier cross-sectional findings and provide further evidence to support that the neurobiological underpinnings of ADHD extend beyond prefrontal and subcortical circuits.
Keywords: gray matter thickness, structural imaging
Introduction
Attention Deficit / Hyperactivity Disorder (ADHD) represents one of the most common psychiatric disorders of youth, affecting approximately 5-12% of children and adolescents, according to recent estimates, and approximately 4-5% of adults.1-3 This high prevalence, coupled with the increased risk for co-morbid conditions including disruptive behavior disorders (∼50%)4-6 and substance abuse/dependence (∼40%),7, 8 underscores the morbidity of this disorder and the need for a better understanding of the underlying neurobiology.
Symptom clusters of inattention, impulsivity and/or hyperactivity are diagnostic of ADHD, but appear partially distinct, rendering it difficult to link these behavioral characteristics to specific neurobiological factors. Likewise, although prior brain imaging studies support that significant neuroanatomical differences exist between populations with and without ADHD, the extent, timing and regional specificity of morphometric changes remain less certain. To identify the neuroanatomical correlates of ADHD, the majority of prior structural imaging studies have assessed volumetric changes in cortical and subcortical regions. Qualitative and quantitative reviews of the literature support that children and adolescents with ADHD exhibit reductions in cerebral and cerebellar volumes, where volume/area deficits of the cerebellar vermis, the caudate and the pallidum and midsagittal callosal splenium appear amongst the most reproducible regional findings.9-12 Consistent with the hypothesis that disturbances in fronto-subcortical and frontal-striatal-cerebellar networks play an important role in the pathophysiology of ADHD, prior investigations have focused on and have reported volumetric reductions of prefrontal brain tissue.13-15 Though less frequently investigated, studies surveying multiple brain regions have also documented volumetric reductions in parietal13, 16 temporal13 and occipital cortices,14,17 although negative findings exist.14
Recently, more advanced computational image analysis strategies that allow simultaneous assessment of both global and local changes in anatomy have been applied to reveal imaging markers potentially more closely associated with ADHD-related pathophysiological processes than volume changes in arbitrarily defined regions of interest. For example, voxel-based morphometry (VBM) methods have been applied to reveal hemisphere-specific changes in gray matter density across several brain regions including frontal, parietal, temporal and cerebellar cortices in children and adolescents with ADHD.17 A recent meta-analysis of six VBM studies of ADHD, however, could only confirm regional gray matter reduction within the right putamen/globus pallidus region, though no brain region showed significant increases in gray matter density.18 Using a 3D surface-based approach to align gyral anatomy, brain surface deformations indexing local brain size reductions have been reported across both lateral prefrontal and temporal areas, although increased gray matter density was observed in posterior temporal/inferior parietal cortices bilaterally in children and adolescents with ADHD compared to controls.19 A study using deformation based morphometry methods further suggests local volume reductions across most lobar regions and the basal ganglia in ADHD, although volume enlargements were observed in the occipital lobe.20
Cortical thickness presents a measure of brain structure that may more closely reflect alterations of neural cytoarchitecture associated with neurobehavioral disturbances in ADHD. A few prior studies have assessed cortical thickness changes in children and adolescents with ADHD. Specifically, Shaw et al.21 used a fully automated measurement approach to show global cortical thinning in a large sample of children with ADHD, where significant regional findings were observed in superior prefrontal and precentral and anterior temporal cortices. Using a combined cross-sectional and longitudinal study design, these authors further demonstrated that cortical thinning remains constant over time in ADHD in all cortical regions with the exception of the right parietal cortex. A larger follow-up study, however, also demonstrated that the normal developmental trajectories for obtaining peak cortical thickness are delayed in ADHD across widespread areas of cortex including prefrontal regions.22 Furthermore, observations of reduced cortical thickness in adults with childhood onset ADHD within prefrontal, lateral inferior parietal, and cingulate cortices suggest that cortical thickness deficits persist, at least in individuals that remain symptomatic.23 One independent study of child and adolescent ADHD by Wolosin et al.24, however, failed to detect ADHD-associated changes in cortical thickness in any of the 34 gyral regions of interest examined.
To confirm and clarify the existing literature concerning cross-sectional changes of cortical thickness in childhood and adolescent ADHD, we used a sophisticated computational image analysis approach to map highly localized changes of laminar thickness between diagnostic groups. To increase statistical power, subjects with ADHD were compared with a well-matched sample of typically developing controls. Potentially confounding effects of overall brain size and general intellectual ability were additionally assessed.
Method
Subjects
Twenty-two children and adolescents with ADHD (19 male, 3 female) and 22 control subjects matched for sex and age (ADHD mean age ± SD: 11.7 ± 2.5 years, range: 7.2 - 16.0, controls: 11.7 ± 2.5 years; range: 7.7 - 16.0) participated in this study. Parents of all subjects signed consents and all subjects signed assents approved by the University of California, Los Angeles (UCLA) Institutional Review Board. Participants were recruited from local schools, pediatricians and clinics, and from ongoing studies of normal development at UCLA. Affected subjects met criteria for ADHD both according to a clinical interview with parents and the NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV);25 and scored over 1.5 SD on the SNAP-IV Rating Scale for Inattention or Hyperactive-Impulsive Scales.26 The mean SNAP scale inattention and hyperactive-inattentive scores in subjects with ADHD were 1.79 ± 0.91 and 1.30 ± 0.95 respectively for teacher ratings and 1.89 ± 0.71 and 1.21 ± 0.82 for parent ratings (data are missing for one participant). Subjects with ADHD were diagnosed as the inattentive (n = 8) or the combined type (n = 13); subtype was unspecified for one subject. Twelve subjects were comorbid for oppositional defiant disorder (ODD). Other comorbid conditions included conduct disorder (n=1), enuresis (n=1), social phobia (n=1), separation anxiety (n=1) and motor tic (n=1). Subjects with fragile X, tuberous sclerosis, generalized resistance to thyroid hormone and those taking nonstimulant psychotropic medication were excluded. Seven subjects were receiving medication (methylphenidate (n=4), dextroamphetamine (n=2), and atomoxetine (n=1)). Medicated participants were required to abstain from medication for at least 24 hours prior to scanning.
Control subjects were free from any current or lifetime history of major Axis I mental disorder by DISC interview,25 any serious medical or neurological illness and any history of closed head trauma. Additional exclusionary criteria for controls included having a first-degree relative with a history of any disruptive behavior disorder (including ADHD), antisocial personality disorder, schizophrenia or bipolar disorder. Subjects were excluded from either group if weight or height was <5th or >95th percentile for age. All children were assessed using the Block Design and Vocabulary subtests of the Wechsler Intelligence Scale for Children, Third Edition (WISC-III).27 An overlapping sample of study participants have been included in investigations of callosal thickness28 and white matter connectivity29 in ADHD.
Image Acquisition and Preprocessing
High-resolution 3D T1-weighted images were obtained contemporaneously from subjects with ADHD and controls over approximately a one year time period on the same Siemens 1.5 Tesla scanner using a sagittal acquisition protocol (TR = 24 ms, TE = 12.6 ms, flip angle = 22°, acquisition matrix 256×196, slice thickness = 1.2 mm and FOV = 240×240 mm2, voxel size = 1.3×0.9×1.2 mm3, 2 averages). Image data passed through a number of preprocessing steps including (1) correction for magnetic field inhomogeneities;30 (2) the automated removal of extra-cortical tissue31 where errors were manually corrected in each brain volume; and (3) correction for head tilt and alignment using a three-translation and three-rotation rigid-body transformation and placement into a common stereotaxic coordinate system without scaling,32, 33 and (4) automatic classification of image voxels into tissue types including gray matter, white matter and CSF using a partial volume correction method.34 Total brain volumes and volumes of each tissue compartment were measured for subsequent analysis.
Cortical Pattern Matching
Cortical pattern matching methods were employed to spatially relate homologous sulcal and gyral regions across individuals. Cortical pattern matching methods have been detailed previously.35-38 Briefly, parametric models of each hemisphere, comprising of 65 536 surface points, were extracted from each scalp-edited image volume.39 Thirty-six sulcal/gyral landmarks were manually identified on the lateral and medial hemispheric surfaces by using previously validated anatomic protocols and by following established intra- and inter-rater reliability procedures.36, 37 The sulcal landmarks were then used as anchors to drive the surrounding cortical surface anatomy of each individual into correspondence. During this process the surface-warping algorithms compute a 3D vector deformation field that records the amount of x, y, and z coordinate shift (or deformation) associating homologous cortical surface locations in each subject with reference to the average anatomical pattern of the entire study group. With the unscaled tissue classified brain volumes and cortical surface models now in spatial correspondence,34 cortical thickness was measured as the 3D distance from the cortical white-gray matter boundary to the cortical surface (gray-CSF boundary), without crossing CSF voxels, using the 3D Eikonal equation.40 A spatial filter with a radius 8 mm was applied to thickness values to circumvent misattributing the thickness of adjacent folded cortical surfaces not separated by CSF.
Statistical Analysis
Global brain volumes, cortical thickness averaged across the cortical mantle and intracranial gray matter, white matter and CSF volumes were compared between subjects with ADHD and controls using the General Linear Model (GLM). Although individuals in each group were matched for age, both Age and Sex were included as covariates in these analyses to ensure that the age spread of the study groups did not influence results. Comparisons of brain tissue compartments were performed while correcting for brain volume.
To reveal regionally specific cortical thickness changes between diagnostic groups the same statistical model was employed using the statistical package R (http://www.r-project.org/), where comparisons were performed at 65 536 spatially matched hemispheric surface locations across subjects. Sex and Age were again included in the statistical model and the cube root of overall brain volume. Interactions with Sex were not examined given the small number of female study participants.
Since statistical comparisons of cortical thickness were performed at thousands of cortical locations, False Discovery Rate (FDR) methods were used to control for multiple spatial comparisons. FDR estimates the proportion of false positive statistical tests (rejections of the null hypothesis when the null hypothesis is actually true) among all positive statistical tests. Under conditions generally accepted to hold true for imaging data, the methods used here for FDR estimates are valid even when the statistical tests are not independent due to spatial correlations in the data.41, 42 FDR was controlled for each statistical hypothesis in both hemispheres simultaneously. Since the FDR methods originally described by Benjamini and Hochberg43 are known to be increasingly conservatively biased as the proportion of true positive tests increases, we used a newer FDR method that reduces this bias. This newer methodology is the approach described by Storey44, but modified by incorporating the bootstrap estimation of the tuning parameter as described by Storey, Taylor and Siegmund.42 Using this newer methodology, we estimated the expected FDR in regions where the uncorrected p-value was less than or equal to .05.
The results of regional cortical thickness analyses were mapped onto the 3D group averaged hemispheric surface models. Uncorrected two-tailed probability maps were thresholded at a value of .05 with more significant regions encoded by corresponding colorbars. Beta maps showing the magnitude of cortical thickness changes in subjects with ADHD relative to controls were additionally generated. Finally, post-hoc analyses were performed to examine relationships between SNAP symptom ratings and cortical thickness variations within subjects with ADHD. Analyses were also performed after excluding medicated patients from the sample.
Results
Diagnostic groups had equal ratios of males and females and subjects with ADHD were matched with typically developing controls for age, (F(1,43) = .00, p = .99). In spite of recruitment efforts, mean IQ scores remained approximately 10 points lower in the group with ADHD (F(1,43) = 8.13 p = .01). Although lower IQ scores in ADHD may relate to difficulties with sustained attention during test taking and may thus not be dissociable from the diagnosis itself,45 statistical analyses were performed including IQ as an additional covariate to ensure that variations in brain morphology were not driven by differences in general intellectual ability between groups.
Subjects with ADHD exhibited significantly smaller overall brain volumes compared to healthy subjects, F(1,43) = 6.08, p < .02, although results were below the threshold of significance after including IQ as an additional covariate, F(1,43) = 2.65, p = .11. Significantly reduced overall gray matter volumes were observed in ADHD both with and without correction for IQ (F(1,43) = 10.65, p < .002 and F(1,43) = 12.83, p < .001, respectively). Individuals with ADHD, however, showed significantly increased white matter volumes both with and without correction for IQ (F(1,43) = 11.72, p < .002 and F(1,43) = 11.84, p < .001, respectively). Diagnostic groups did not differ in CSF volumes, F(1,43) = 41, p = .84 and F(1,43) = 0.78, p = .38, irrespective of correction for IQ. When cortical thickness was averaged across the cortex, subjects with ADHD showed significant global cortical thinning in comparison to controls, both with, F(1,43) = 5.80, p < .02, and without, F(1,43) = 6.45, p < .01, correction for IQ. Means and standard deviations for IQ, global brain volume, average cortical thickness and brain tissue volumes are provided in Table 1.
TABLE 1.
Mean and SDs for Morphometric Measures
| Control Group (n = 22) | ADHD Group (n = 22) | |
|---|---|---|
| Sex | 19 Male/3 female | 19 Male/3 female |
| Age (mean ± SD), y | 11.7 ± 2.5 | 11.7 ± 2.5 |
| Full estimated IQa | 103.23 ± 10.36 | 92.91 ± 13.43 |
| Overall brain volume (mean ± SD)a, cm3 | 1502.2 ± 130.5 | 1413.5 ± 124.9 |
| Average cortical thickness (mean ± SD)a, mm | 5.08 ± 0.32 | 4.76 ± 0.59 |
| Gray matter (mean ± SD)a, cm3 | 802.48 ± 75.37 | 698.83 ± 92.98 |
| Brain size-adjusted gray matter (mean ± SD)a, cm3 | 789.28 ± 55.70 | 712.04 ± 102.34 |
| White matter (mean ± SD), cm3 | 551.59 ± 80.21 | 577.67 ± 156.31 |
| Brain size-adjusted white matter (mean ± SD)a, cm3 | 525.39 ± 50.15 | 603.87 ± 112.54 |
| CSF (mean ± SD), cm3 | 148.21 ± 25.34 | 137.01 ± 29.43 |
| Brain size-adjusted CSF (mean ± SD), cm3 | 143.23 ± 21.16 | 141.99 ± 25.73 |
Note: Brain size–adjusted tissue volumes were computed by residualizing for overall brain volume and adding the unstandardized residuals to the respective group average (effectively adjusting brain volumes so that they are identical in all of the subjects).
Significant group difference.
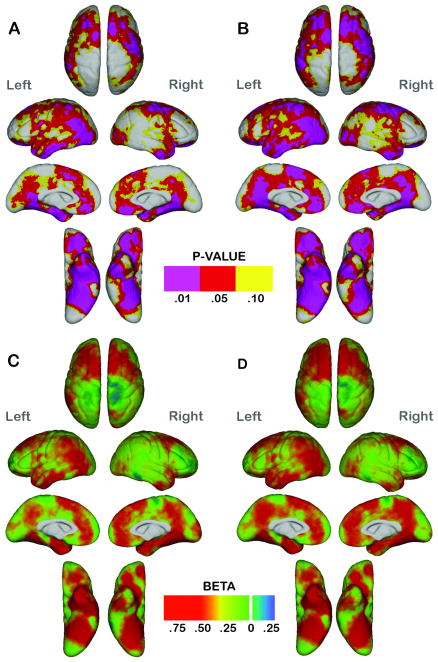
Results from statistical comparisons of cortical thickness performed at high spatial resolution are shown in Figure 1 both with (A and C) and without (B and D) general intellectual ability included as an additional covariate. Uncorrected probability maps are provided in the top panels (A and B), where the colorbar is unidirectional since significant increases of cortical thickness were not observed in subjects with ADHD compared to controls at any cortical location. The bottom panels (C and D) show the beta values associated with group differences in mm in both directions as indicated by the colorbar. Probability maps show widespread cortical thinning in individuals with ADHD compared to typically controls where the estimated FDR42 for regions with uncorrected p < .05 is .0006. Patients with ADHD exhibited significant cortical thinning prominently in bilateral dorsolateral (and aspects of precentral cortex) and orbitofrontal cortices, anterior and ventral temporal (including entorhinal cortex) and occipito-temporal cortices. Cortical thinning spanned a large portion of lateral parietal association cortex predominantly in the left hemisphere. Medially, subjects with ADHD showed significantly decreased gray matter thickness in medial prefrontal, cingulate and parieto-occipittal cortices in both hemispheres. Regional effects were similar, although slightly less pronounced after including IQ in the statistical model [Figure 1, panels B and D].
Fig. 1.
Statistical maps showing regional differences in cortical thickness between the subjects with attention-deficit/hyperactivity disorder (ADHD) compared with the controls after controlling for age, sex, and the cube root of intracranial volume (A and C) and including general intelligence scores as an additional covariate (B and D). Top: Probability maps (A and B) show thresholded uncorrected p values in color for regional cortical thinning in ADHD compared with the controls. No brain region showed significant cortical thickness increases in the subjects with ADHD. Bottom: Beta maps (C and D) show cortical thickness changes between diagnostic groups in millimeters. Positive β values index ADHD-related reductions in thickness.
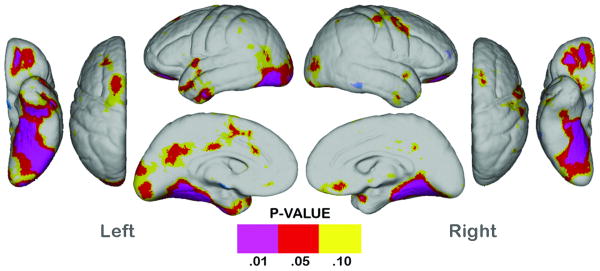
ADHD SNAP symptom ratings did not show significant associations with regional cortical thickness variations within subjects with ADHD (results not shown). Post-hoc analyses performed after removing subjects receiving medication treatment from the sample are presented in Figure 2. ADHD effects were less pronounced in this smaller subsample, particularly in dorsolateral prefrontal and anterior temporal cortices, and effects were no longer significant in parietal association areas on the lateral aspects of the hemispheres, although medially and ventrally cortical thinning was observed in a similar, though less spatially diffuse pattern.
Fig. 2.
Statistical maps showing regional differences in cortical thickness between unmedicated subjects with ADHD (n = 15) compared with the controls (n = 22). Probability maps show thresholded uncorrected p values in color for regional cortical thickness reductions. No brain region showed significant cortical thickness increases in the subjects with ADHD.
Discussion
To confirm prior cross-sectional findings and clarify discrepancies in the literature, sophisticated computational image analysis methods were employed to examine highly localized changes in cortical thickness in a well-matched sample of child and adolescent subjects with ADHD compared to typically developing controls. Mean cortical gray matter thickness was significantly reduced in ADHD (> 6%). Examination of cortical thickness performed at high spatial resolution showed that subjects with ADHD exhibit robust (up to 20%) and widespread cortical thinning over large areas of anterior and posterior association cortices, including aspects of primary motor cortex and with predominant sparing of primary sensory regions. No brain region showed significantly increased thickness in subjects with ADHD compared to age-matched controls. Standard volumetric analyses of brain tissue compartments were consistent with the majority of earlier reports where subjects with ADHD exhibited smaller brain and gray matter volumes with respect to healthy controls.9-12 White matter volumes, however, were significantly larger in ADHD as consistent with some prior observations,46 but conflicting with others.13
Cortical thickness represents the number, size, density, and arrangement of cells (neurons and neuroglia and nerve fibers) within the cortical mantle.47, 48 Age-inappropriate cortical thinning may thus reflect changes in the integrity of the neuropil stemming from disturbances in neural/synaptic development and pruning. White matter changes may also influence cortical thickness since myelinated fibers extend into the cortex. However, cortical thickness may be over or underestimated in imaging data since it is not certain to what extent gray/white and gray/CSF boundaries match actual tissue boundaries in the living brain. Signal intensity thresholds used for tissue segmentation may also vary across studies. Notwithstanding, if image parameters and pre-processing are the same for all participants, differences between diagnostic groups should remain relative since thickness or brain tissue voxel counts are biased in the same direction for all subjects.
Prominent ADHD-related cortical thinning was observed over much of dorso-lateral, orbito and mesial frontal cortices encompassing the cingulate and aspects of premotor and motor cortex, antero-lateral and mesial temporal cortices, lateral occipital cortices, medial parietal cortices, and left lateral parietal cortex. These findings are largely consistent, although more pervasive, than results from a landmark study investigating both cross-sectional and longitudinal changes of cortical thickness in child and adolescent ADHD.21 Specifically, Shaw et al.21 using completely automated procedures observed significant global cross-sectional reductions of laminar thickness in subjects with ADHD (n=163) compared to healthy controls (n=166) that were localized bilaterally to dorsal and mesial prefrontal cortices including the cingulate and antero- and mesial temporal cortices. Notably, without correction for IQ or mean cortical thickness, this prior study observed widespread sub-threshold cortical thinning and no regions of increased cortical thickness over much of the remaining cortex. Furthermore, when averaged within 56 probabilistic gyral boundaries, subjects with ADHD exhibited significant cortical thinning in almost all regions implicated in the current investigation including parietal and occipital cortex.
After controlling for differences in general intellectual ability, significant observations of cortical thinning were slightly less pronounced in ADHD [Figure 1, panels B and D], although effects still spanned the same areas of cortex. These findings may be explained in part by a loss of degrees of freedom, indicate that localized cortical thinning more closely relates to intellectual ability, or suggest that the neurobehavioral disturbances in ADHD are not dissociable from ADHD-related test taking difficulties.49 These results are again consistent with the findings of Shaw and colleagues,21 although the investigators additionally included mean cortical thickness in their statistical model, which may serve to remove ADHD-related variance for the regional effects of interest. Moreover, their study participants were of higher general intellectual ability (> 10 points) on average than those included in this study. Thus, our findings of somewhat more pronounced cortical thinning in ADHD may relate to the severity of the disorder or could be attributable to methodological differences, where in spite of smaller sample sizes, our study may have benefited from using both manual and automated methods to match gyral anatomy across subjects with less spatial smoothing.
Our findings and those of Shaw et al.21 contrast with another earlier study of cortical thickness that failed to show significant regional cortical thinning in child and adolescent ADHD, although significant reductions of intracranial volume, surface area and cortical folding were observed.24 Since sample sizes were similar, differences in results are most likely accounted for by methodological differences and/or differences in the clinical characteristics of subjects. For example, Wolosin et al.24 used automated methods to estimate and compare mean cortical thickness across the cortical mantle and within 34 gyral-based regions, where individual brain volumes appear to have been rescaled into a Talairach coordinate space. Thus, it is possible that if applied, scaling, or alternatively the lack of control for overall brain size may have influenced results. Moreover, as noted by Shaw et al.21 cortical thickness reductions in ADHD do not appear to conform well to prescribed gyral regions, thus averaging thickness across coarsely registered gyral areas may serve to decrease rather than to increase statistical power. Differences in exclusionary criteria for comorbid diagnoses may also have contributed to discrepancies in prior findings. In a post-hoc analysis, Wolosin et al.24 reported a trend for mean cortical thinning bilaterally when children comorbid for ADHD and ODD (38%) were compared with controls separately.
Our results are also partially conflicting with a previous study employing a similar approach to align cortical anatomy, but that measured cortical gray matter density to show increased density in posterior temporal/anterior parietal regions in ADHD youth with respect to controls19. However, this prior investigation did observe cortical surface deformations in prefrontal and anterior temporal regions suggestive of local brain tissue reductions that are consistent with our results. Though cortical thickness and cortical gray matter density measures may be associated, gray matter density measures reflect the number of voxels classifying as gray matter with respect to voxels classifying as other tissue types (white matter and CSF) both within and outside the cortical mantle and may thus be influenced by changes in other tissue characteristics perhaps accounting for discrepancies in findings.
ADHD-related cortical thinning was less pronounced and spatially pervasive after removing participants receiving stimulant mediation from the sample. However, we cannot be sure whether the similar, although less extensive spatial pattern of results may be attributable to more severe cortical thinning in subjects receiving medication or simply reflect the loss of statistical power by reducing the sample size by approximately one third. Some larger structural imaging studies10 suggest that morphometric abnormalities in ADHD are largely independent of comorbidities and medication exposure, although further longitudinal assessments appear warranted.
ADHD is associated with impairments in executive function including deficits in working memory, planning, set shifting and inhibitory/cognitive control suggesting the involvement of prefrontal regions and subcortical circuits.5, 49, 50 The developmentally inappropriate symptoms of inattention, impulsivity and restlessness in ADHD also implicate disturbances in prefrontal as well as parietal networks. Moreover, predominant disturbances of neural activity within fronto-striatal and fronto-parietal circuits have been reported via meta-analysis of the functional neuroimaging literature in ADHD.51 Thus, our observations of pervasive cortical thinning within dorsolateral, orbitofrontal, and medial frontal regions, and parietal cortex suggest that abnormalities in neural cytoarchitecture may constitute a biological basis for the clinical and neuropsychological profiles of ADHD.
In spite of an emphasis of the role of executive functioning and inhibitory control in ADHD, the existing literature also indicates that ADHD is associated with a heterogeneous neurocognitive profile where deficits in executive function do not present in all individuals and other functional systems are implicated.49, 52, 53 For example, some neuropsychological and electrophysiological studies suggest earlier, more generalized deficits in information processes such as in visual-spatial functioning, attention allocation and response preparation involving temporo-striatal and/or temporo-parietal networks.54-56 Prior studies also implicate altered mesiolimbic circuitry in ADHD (orbitofrontal, anterior cingulate, ventral striatum and medial temporal regions) as associated with reward, reinforcement and motivational behaviors.56 Finally, disruptions in temporo-occipital ‘ventral stream’ and parieto-occipital ‘dorsal stream’ pathways involved in object recognition/manipulation and visual-attention and analysis that underlie most cognitive processes have been implicated in ADHD, although less widely examined.57, 58 As consistent with the presentation of neurocognitve deficits, our observations of cortical thinning in anterior- and ventral temporal regions and in temporo-occipital cortex similarly suggest a complex neuroanatomical profile of ADHD extending beyond frontal and subcortical circuits.
There are several limitations associated with the current investigation. Firstly, female subjects with ADHD were underrepresented. Although prevalence is higher in males, the core features of ADHD appear similar across sex10, but developmental processes may differ by sex. Secondly, it is common for ADHD to present with other diagnoses, where ODD is the most common coexistent condition.5 Unfortunately, we were not able to address the potential confounding effects of comorbid disorders in the current study, since such analyses would have been underpowered and thus uninformative. However, an argument can be made that controlling for conditions such as ODD may remove variance that overlaps with ADHD.49 Thirdly, our cross-sectional study design did not allow us to address group differences with respect to age. Finally, the experiments performed here did not include investigation of the cerebellum and subcortical regions, also widely implicated in the disorder.
In summary, analysis strategies that enable the mapping of cortical thickness deficits with high spatial accuracy and resolution, revealed highly significant (FDR corrected p < .0006) cortical thinning over wide areas of cortex in a well-matched sample of children and adolescents with ADHD compared to typically developing controls. These results confirm and expand upon an earlier study of cortical thickness in ADHD.21 Although this study could not address whether cortical thinning is associated with a developmental delay in ADHD as previously suggested,22 our results support that when taking age into account, cortical thinning represents a robust imaging marker of the disorder. Since the observed pattern of cortical thinning is most consistent with a model of ADHD that points to deficits in multiple functional systems, future studies may focus on clarifying the nature of potential structure-function relationships.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) through grants MH073990 (Dr. Narr), the NIH Roadmap for Medical Research, U54 RR021813, and Center for Computational Biology (CCB). The NIH/National Center for Research Resources, P41 RR013642 and M01-RR00865, General Clinical Research Centers Program, provided additional support.
Footnotes
This article is the subject of an editorial by Dr. F. Xavier Castellanos in this issue.
Disclosure: The authors report no conflicts of interest.
References
- 1.Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007;20(4):386–392. doi: 10.1097/YCO.0b013e3281568d7a. [DOI] [PubMed] [Google Scholar]
- 2.Skounti M, Philalithis A, Galanakis E. Variations in prevalence of attention deficit hyperactivity disorder worldwide. Eur J Pediatr. 2007;166(2):117–123. doi: 10.1007/s00431-006-0299-5. [DOI] [PubMed] [Google Scholar]
- 3.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- 4.Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol. 2004;14(1):11–28. doi: 10.1016/s0924-977x(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Newcorn JH, Halperin JM, Jensen PS, et al. Symptom profiles in children with ADHD: effects of comorbidity and gender. J Am Acad Child Adolesc Psychiatry. 2001;40(2):137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Schubiner H. Substance abuse in patients with attention-deficit hyperactivity disorder: therapeutic implications. CNS Drugs. 2005;19(8):643–655. doi: 10.2165/00023210-200519080-00001. [DOI] [PubMed] [Google Scholar]
- 8.Wilens TE, Biederman J. Alcohol, drugs, and attention-deficit / hyperactivity disorder: a model for the study of addictions in youth. J Psychopharmacol. 2006;20(4):580–588. doi: 10.1177/0269881105058776. [DOI] [PubMed] [Google Scholar]
- 9.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- 12.Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Ment Retard Dev Disabil Res Rev. 2003;9(3):184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 14.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52(8):785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 15.Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17(3):496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 16.Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48(3):589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 17.Carmona S, Vilarroya O, Bielsa A, et al. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389(2):88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Jiang T, Cao Q, Wang Y. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR Am J Neuroradiol. 2007;28(3):543–547. [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 22.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makris N, Biederman J, Valera EM, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17(6):1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 24.Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2007;30(1):175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Swanson J. School-Based Assessments and Interventions for ADD Students. CA: K.C. Publishing; 1992. [Google Scholar]
- 27.Wechsler D. Wechsler Intelligence Scale for Children. 3rd. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 28.Luders E, Narr KL, Hamilton LS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65(1):84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton LS, Levitt JG, O'Neill J, et al. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19(17):1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEE Trans Med Imaging. 1998;17(4):653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- 31.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6(2):129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 32.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comp Assis Tomogr. 1998;22(1):139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comp Assis Tomogr. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 35.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15(6):708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 37.Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58(1):32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 38.Levitt JG, Blanton RE, Smalley S, et al. Cortical sulcal maps in autism. Cereb Cortex. 2003;13(7):728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald D, Avis D, Evans AC. Multiple Surface Identification and Matching in Magnetic Resonance Imaging. Proc SPIE. 1994;2359:160–169. [Google Scholar]
- 40.Sapiro G. Geometric partial differential equations and image analysis. Cambridge, U.K.; New York: Cambridge University Press; 2001. [Google Scholar]
- 41.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 42.Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: A unified approach. J Royal Stat Soc Series B. 2004;66:187–205. [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc, Series B. 1995;57:289–300. [Google Scholar]
- 44.Storey JD. A direct approach to false discovery rates. J Royal Stat Soc Series B. 2002;64:479–496. [Google Scholar]
- 45.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 46.Seidman LJ, Valera EM, Makris N, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 47.Parent A, Carpenter MB. Human neuroanatomy. 9th. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 48.Economo C, Parker S. The cytoarchitectonics of the human cerebral cortex. London: Humphrey Milford; Oxford University Press; 1929. [Google Scholar]
- 49.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130(1-2):3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- 51.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 52.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 53.Pennington BF. Toward a new neuropsychological model of attention-deficit/hyperactivity disorder: subtypes and multiple deficits. Biol Psychiatry. 2005;57(11):1221–1223. doi: 10.1016/j.biopsych.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Rubia K. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci U S A. 2007;104(50):19663–19664. doi: 10.1073/pnas.0710329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens MC, Pearlson GD, Kiehl KA. An FMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164(11):1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- 56.Vaidya CJ, Stollstorff M. Cognitive neuroscience of Attention Deficit Hyperactivity Disorder: current status and working hypotheses. Dev Disabil Res Rev. 2008;14(4):261–267. doi: 10.1002/ddrr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kilic BG, Sener S, Kockar AI, Karakas S. Multicomponent attention deficits in attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2007;61(2):142–148. doi: 10.1111/j.1440-1819.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 58.Vance A, Silk TJ, Casey M, et al. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry. 2007;12(9):826–832. 793. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]