Summary
Anabolic androgenic steroid (AAS) abuse is widespread. Moreover, AAS are reinforcing, as shown by self-administration in rodents. However, the receptors that transduce the reinforcing effects of AAS are unclear. AAS may bind to classical nuclear androgen receptors (AR) or membrane receptors. We used two approaches to examine the role of nuclear ARs in AAS self-administration. First, we tested androgen self-administration in rats with the testicular feminization mutation (Tfm), which interferes with androgen binding. If nuclear ARs are essential for AAS self-administration, Tfm males should not self-administer androgens. Tfm males and wild-type (WT) littermates self-administered the non-aromatizable androgen dihydrotestosterone (DHT) or vehicle intracerebroventricularly (ICV) at fixed ratio (FR) schedules up to FR5. Both Tfm and WT rats acquired a preference for the active nose-poke during DHT self-administration (66.4±9.6 responses/4h for Tfm and 79.2±11.5 for WT responses/4h), and nose-pokes increased as the FR requirement increased. Preference scores were significantly lower in rats self-administering vehicle (42.3±5.3 responses/4h for Tfm and 19.1±4.0 responses/4h for WT). We also tested self-administration of DHT conjugated to bovine serum albumin (BSA) at C3 and C17, which is limited to actions at the cell surface. Hamsters were allowed to self-administer DHT, BSA and DHT-BSA conjugates for 15 days at FR1. The hamsters showed a significant preference for DHT (18.0±4.1 responses/4h) or DHT-BSA conjugates (10.0±3.7 responses/4h and 21.0±7.2 responses/4h), but not for BSA (2.5±2.4 responses/4h). Taken together, these data demonstrate that nuclear ARs are not required for androgen self-administration. Furthermore, androgen self-administration may be mediated by plasma membrane receptors.
Keywords: Anabolic-androgenic steroids, self-administration, membrane androgen receptor, nuclear androgen receptor, testicular feminization mutation
Anabolic-androgenic steroids (AAS) are drugs of abuse. These testosterone (T) derivatives are used for athletic and aesthetic purposes (Yesalis et al., 1993). Side-effects range from hypogonadism and gynecomastia to cardiac and hepatic dysfunction (Leshner, 2000). In addition, evidence is accumulating that AAS abuse causes mood alterations (Pope and Katz, 1994), aggression (Choi and Pope, 1994, Kouri et al., 1995), and may produce dependence (Brower et al., 1991, Brower, 2002). Despite growing concerns, the underlying mechanisms of AAS abuse have not been well-understood.
In humans, it is argued that the initiation of AAS use is largely motivated by anabolic effects, but some abusers eventually develop dependence (Brower, 2002). Evidence from animal research supports this hypothesis. AAS induce conditioned place preference (CPP) in mice (Arnedo et al., 2000) and rats (Packard et al., 1997, Packard et al., 1998, Frye et al., 2002). Furthermore, hamsters voluntarily consume AAS through oral (Wood, 2002), intravenous (Wood et al., 2004), and intracerebroventricular (ICV) self-administration (DiMeo and Wood, 2004, Triemstra and Wood, 2004, Wood et al., 2004, DiMeo and Wood, 2006b).
While ICV self-administration suggests central sites of action, the specific hormones and receptors mediating AAS reinforcement are unclear. Current evidence suggests that the reinforcing effects of T are mediated by androgens, rather than through estrogens after aromatization. Male hamsters will self-administer dihydrotestosterone (DHT; DiMeo and Wood, 2006b) and other non-aromatizable androgens (Ballard and Wood, 2005). In addition, T self-administration is blocked by the anti-androgen flutamide (Peters and Wood, 2004). The question now becomes: how is the androgenic signal transduced in the brain?
The androgen receptor (AR) is a classic nuclear steroid receptor which functions as a transcription factor. ARs are sparse in structures associated with drug abuse, such as the nucleus accumbens (Acb) and the ventral tegmental area (VTA; Simerly et al., 1990, Wood and Newman, 1999). There is also evidence for gonadal steroids acting via cell surface receptors (Mermelstein et al., 1996, Zhu et al., 2003, Thomas et al., 2006, Vasudevan and Pfaff, 2007).
In the current study, we used two approaches to determine the role of classic nuclear AR in androgen reinforcement. To minimize possible activation of estrogen receptors (ER), we tested DHT self-administration. In the first experiment, rats with the testicular feminization mutation (Tfm) were tested for ICV self-administration of DHT. Tfm is a single base substitution which results in defective ARs with limited ligand binding (Yarbrough et al., 1990). Male Tfm rats exhibit an external female phenotype due to insufficient androgenic stimulation during development (Zuloaga et al., 2008b). If functional nuclear ARs are required for AAS reinforcement, Tfm rats should not self-administer DHT. Instead, Tfm rats were able to acquire DHT self-administration. In the second experiment, we tested ICV self-administration of membrane-impermeable forms of DHT in hamsters. When DHT is conjugated to bovine serum albumin (BSA), its actions are restricted to cell-surface receptors. If nuclear ARs are required for androgen reinforcement, hamsters should not self-administer DHT conjugated to BSA. On the contrary, the hamsters showed a clear preference for DHT conjugated to BSA. Together, these studies show that nuclear ARs are not required for androgen self-administration. Instead, androgen reinforcement may be mediated by membrane ARs.
Methods and Materials
Subjects
Rats
Adult male Tfm rats and wild-type (WT) littermates were obtained from a colony at Michigan State University. Their genotype was verified by PCR, similar to methods described previously (Fernandez et al., 2003). Briefly, ear clips were digested overnight at 55° C in lysis buffer containing proteinase K, then heat inactivated at 95° for 30 minutes. AR was amplified using forward primer 5′-GCAACTTGCATGTGGATGA-3′ and reverse primer 5′-TGAAAACCAGGTCAGGTGC-3′, yielding a 135bp product. Amplified samples were then digested with Sau96I restriction enzyme (R0165L, New England BioLabs, Ipswich, MA) overnight at 37° C and run on a 3% agarose gel. Only the WT AR is cut with this restriction enzyme, leaving two bands below 100bp, whereas the Tfm AR remains uncut. Tfm animals were also verified by phenotype, by the presence of nipples, feminine ano-genital distance and abdominal testes. Tfm rats have previously been used to demonstrate non-genomic androgen effects in hippocampus (MacLusky et al., 2006). At the start of the experiment, WT rats were between 75 to 140 day old, and Tfm rats were between 75 to 138 day old.
Hamsters
Adult male Syrian hamsters (130 – 150 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed singly on a reversed light cycle (14L:10D) with food and water available ad libitum. All experimental procedures were approved by institutional animal care and use committees of the respective institutions and conducted in accordance with the Guide for Care and Use of Laboratory Animals (NationalResearchCouncil, 1996).
Surgery
All animals were implanted with a 22g stainless steel guide cannulae (Plastic One, Roanoke, VA) into the lateral ventricle [rat: AP: 0.7, ML: -1.8, DV: -4.0 ∼ -5.0 (Paxinos and Watson, 1998); hamster: AP: +1.0, ML, +1.0, DV: -3.0 ∼ -5.0 (Morin and Wood, 2001), mm from bregma], under Na+ pentobarbital anesthesia (rat: 50 mg/kg, hamster: 100mg/kg) as described previously (Wood et al., 2004). All surgical procedures were carried out under aseptic conditions according to Principles of Laboratory Animal Care (NIH, 1985). Animals were allowed to recover for at least a week following the surgery before testing.
Drugs
DHT, DHT-carboxymethyl-oxime (CMO), DHT-CMO-BSA, DHT-hemisuccinate (Hemis), and DHT-Hemis-BSA were obtained from Steraloids (Newport, RI). In DHT-CMO-BSA, DHT is conjugated to BSA at the C3 position with CMO as the linker. Similarly, DHT is linked to BSA at the C17 position via Hemis to form DHT-Hemis-BSA. Both DHT-CMO-BSA (Gatson et al., 2006) and DHT-Hemis-BSA (Braun and Thomas, 2003) have previously been used to investigate possible effects of androgens at the plasma membrane. DHT was dissolved in an aqueous solution of 13% β-cyclodextrin (βCD, Sigma-Aldrich, St. Louis, MO) at 1μg/μl. As determined from our previous study in hamsters, this dose produces robust operant responding during ICV self-administration (DiMeo and Wood, 2006b). DHT derivatives were dissolved in the same vehicle at the molar equivalent concentration of DHT (DHT-CMO: 1.25 μg/μl, DHT-CMO-BSA: 8.7 μg/μl, DHT-Hemis: 1.34 μg/μl, DHT-Hemis-BSA: 8.83μg/μl). BSA (Sigma-Aldrich) was dissolved in the same vehicle at 7.45 μg/μl to achieve the molar equivalent concentration of BSA as in DHT-CMO-BSA and DHT-Hemis-BSA. BSA-containing drugs were prepared daily immediately before use to avoid degradation, and all solutions were filtered through a 0.22 μm filter. Previous studies have shown that only a small proportion of steroid dissociates from BSA (Stevis et al., 1999), and this quantity is insufficient to induce significant androgenic effects (Lieberherr and Grosse, 1994, Gatson et al., 2006). Likewise, our earlier study has shown that DHT is self-administered at 1.0 μg/μl, but not at 0.1 μg/μl (DiMeo and Wood, 2006b). Hence, it is unlikely that free DHT (>10%) dissociates from BSA in sufficient quantity to support self-administration.
Apparatus
Animals were allowed to self-administer drug or vehicle solution 4 hrs/day, 5 days/week in an operant chamber (Med Associates, St. Albans, VT) enclosed in a sound-attenuating chamber with forced ventilation. Each chamber was equipped with a house-light, 2 nose-poke holes, and a computer-controlled syringe pump connected to a liquid swivel on a balance arm. Solutions from a 100 μl glass syringe were delivered to the animal through Tygon tubing connected to the swivel. The tubing connecting the swivel and the ICV cannula was protected by a metal spring. Drug solution or vehicle was delivered via a 28-ga internal cannula inserted into the guide cannula immediately before testing. Each infusion delivered 1 μl of solution at 0.2 μl/s. Nose-poke holes were located 6 cm from the floor beneath the house light. One of the nose-poke holes was designated as the active nose-poke hole. A response on this hole was recorded as an active nose-poke (R: active-reinforced) and counted toward the response requirement (FR1 to 5) for triggering an infusion. Once an infusion was triggered, the house-light was extinguished and the active hole illuminated during the 5-s infusion to aid in discrimination of the active nose-poke hole. Nose-poking in the active hole during this 5-s timeout period was recorded but did not count toward further reinforcement (NR: active-non-reinforced). A response on the other nose-poke hole was recorded as an inactive nose-poke (I) but did not result in any infusion. The location of the active nose-poke hole to the front or the back of the chamber was balanced to control for side preferences. The data were recorded by WMPC software (Med Associate) on a Windows PC.
ICV self-administration
Rats
Self-administration of DHT in Tfm and WT rats followed an ascending fixed-ratio (FR) schedule from FR1 to FR5. The rats were initially trained on FR1, where each response on the active nose-poke was reinforced. Thereafter, the number of responses required to obtain an infusion was raised by one every 5 days. At FR5, five responses on the active nose-poke hole were required for an infusion. Overall, rats were tested on FR1 for 10 days and FR2 to FR5 (5 days each), for a total of 30 days. Rats from each genotype were randomly assigned to either DHT or vehicle (Veh) groups, and allowed to self-administer DHT or the βCD vehicle, respectively. Thirty six rats (nWT = 19, nTfm = 17) were used in this experiment.
Hamsters
Hamsters were tested under an FR1 schedule for 15 days. In previous studies, 15 days of ICV T self-administration is sufficient to acquire a preference for the active nose-poke. Hamsters were randomly assigned to DHT (n = 8), DHT-CMO (n = 9), DHT-CMO-BSA (n = 10), DHT-Hemis (n = 11), DHT-Hemis-BSA (n = 8), or BSA (n = 9) groups.
Data analysis
Rats
Daily preference scores for the active nose-poke were determined by subtracting inactive nose-pokes from the sum of active-reinforced and active non-reinforced nose-pokes (R+NR-I). The mean preference score was calculated for each animal from the last 5 days of FR1 and during FR2 to FR5. Additionally, the average number of reinforcements per session for each animal at each FR was compared.
Data were analyzed by 3-way ANOVA, with genotype (WT or Tfm), drug (DHT or vehicle) and FR schedule (1 ∼ 5) as between-subject factors. FR schedule was treated as a between factor, since some animals failed to complete the entire 30 days of testing due to the clogging of the ICV guide cannula. In those cases, only the data from the completed schedules were included in the analyses. The number of animals included in each condition is shown in Table 1. A three-way ANOVA was followed up by appropriate lower order ANOVAs for simple effects. The Newman-Keuls test for post-hoc pair-wise comparisons was used when necessary.
Table 1.
The body weight (mean ± SEM in g) and the number of rats used (n) at the start of each FR and the end of FR5. * Significantly different from FR1 (p < 0.05). # Significantly different from WT (p < 0.05).
| FR schedule | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | n | 2 | n | 3 | n | 4 | n | 5 | n | End | n | Ave. | n | |
| WT-DHT | 467.9 ±28.3 | 10 | 478.4 ±21.2 | 9 | 477.1 ±21.4 | 9 | 490.7 ±21.8 | 9 | 501.6 ±24.6 | 8 | 512.6 ±25.1 | 7 | 493.2 ±6.3 | 104 |
| WT-Veh | 442.2 ±21.6 | 9 | 500.2 ±14.7 | 9 | 502.7 ±17.7 | 9 | 514.3 ±19.2 | 9 | 521.0 ±19.0 | 8 | 524.8 ±16.4 | 8 | ||
| Tfm-DHT | 356.2 ±18.3 | 9 | 375.3 ±17.5 | 9 | 401.3 ±15.7 | 9 | 402.1 ±18.3 | 7 | 416.3 ±16.8 | 7 | 430.1 ±17.8 | 7 | 393.5 ±5.5# | 94 |
| Tfm-Veh | 351.5 ±23.4 | 8 | 382.6 ±15.5 | 8 | 392.3 ±12.9 | 8 | 405.8 ±17.3 | 8 | 412.3 ±22.4 | 7 | 415.6 ±22.5 | 7 | ||
| Ave. | 407.7 ±14.3 | 36 | 435.6 ±12.7 | 35 | 444.8 ±11.6* | 35 | 457.8 ±12.8* | 33 | 466.0 ±13.5* | 30 | 472.6 ±13.3* | 29 | 445.9 ±5.5 | 198 |
Hamsters
The individual means of R, NR, and I were used for data analysis. The preference score for each animal was determined by subtracting the mean inactive nose-poke (I) from the mean active nose-poke (R+NR-I). The mean preference score was analyzed with a one-sample t-test against 0 (i.e. no preference) for each group. Additionally, the number of reinforcements received was averaged for each animal. The mean reinforcement received for each drug group was compared against that of BSA controls with a 2 independent-samples t-test. Animals who failed to complete a minimum of 5 sessions were excluded from analysis (1 each from DHT-Hemis and DHT-Hemis-BSA groups).
All statistical analyses were conducted using SPSS 12 (SPSS Inc., Chicago, IL). For all analysis, p < 0.05 was considered statistically significant. The data are presented as mean ± SEM per 4h session.
Results
WT and Tfm rats self-administer DHT
Operant responding
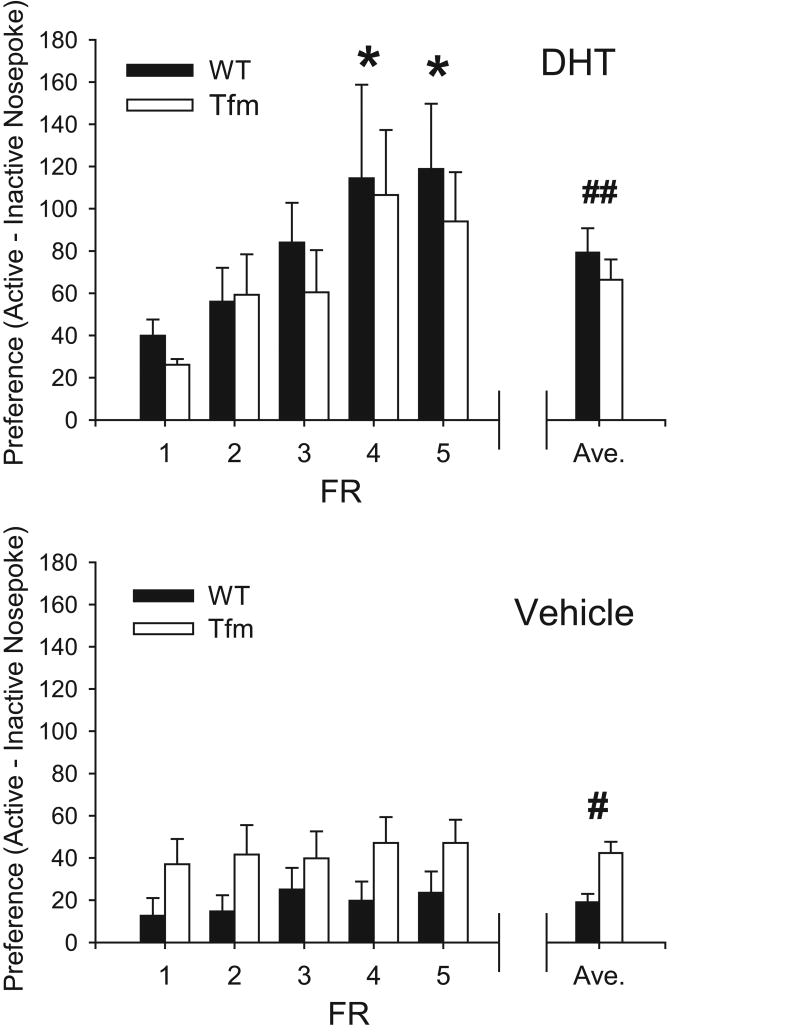
Figs. 1 illustrates the mean preference for active nose-poke (R + RN - I) at each FR for DHT and Veh groups. Rats self-administering DHT showed a greater preference for the active nose-poke (73.1±7.6 resp/4h), compared to vehicle controls (29.8±3.5 resp/4h; F1,145 = 31.77, p < 0.001). There was also a main effect of FR schedule (F4,145 = 4.25, p < 0.01), genotype-drug interaction (F1,145 = 5.27, p = 0.02), and a drug-FR schedule interaction (F4,145 = 2.60, p = 0.02). There was no main effect of genotype, and other interactions were not significant.
Figure 1.
Mean preference (active – inactive nose-pokes) for rats self-administering DHT (top) and vehicle (bottom). Means ± SEM for each FR are shown, along with the overall average ± SEM (right). * Significantly different from FR1 (p < 0.05). # Significantly different from WT (p < 0.05). ## Significantly from vehicle (p < 0.05). Note that preference increased only in rats receiving DHT as the FR requirement increased.
Post-hoc tests revealed that the rats self-administering DHT showed a significantly greater preference over the FR schedule (F4,73 = 4.18, p < 0.01), increasing preference from FR1 (33.4±4.4 resp/4h) to FR4 (110.8±26.7 resp/4h) and FR5 (106.4±18.9 resp/4h). No effect of genotype was observed in this group (genotype-FR schedule: F4,73 = 0.13, ns; genotype: F1,73 = 0.86, ns).
In contrast, the rats self-administering Veh showed no change in preference over the FR schedule (F4,72 = 0.31, ns), and no genotype-FR schedule interaction (F4,72 = 0.12, ns). Unlike for DHT, Tfm rats showed greater preference than WT in this group (42.3±5.3 and 19.1±4.0 resps/4h, respectively; F4,72 = 11.81, p < 0.01).
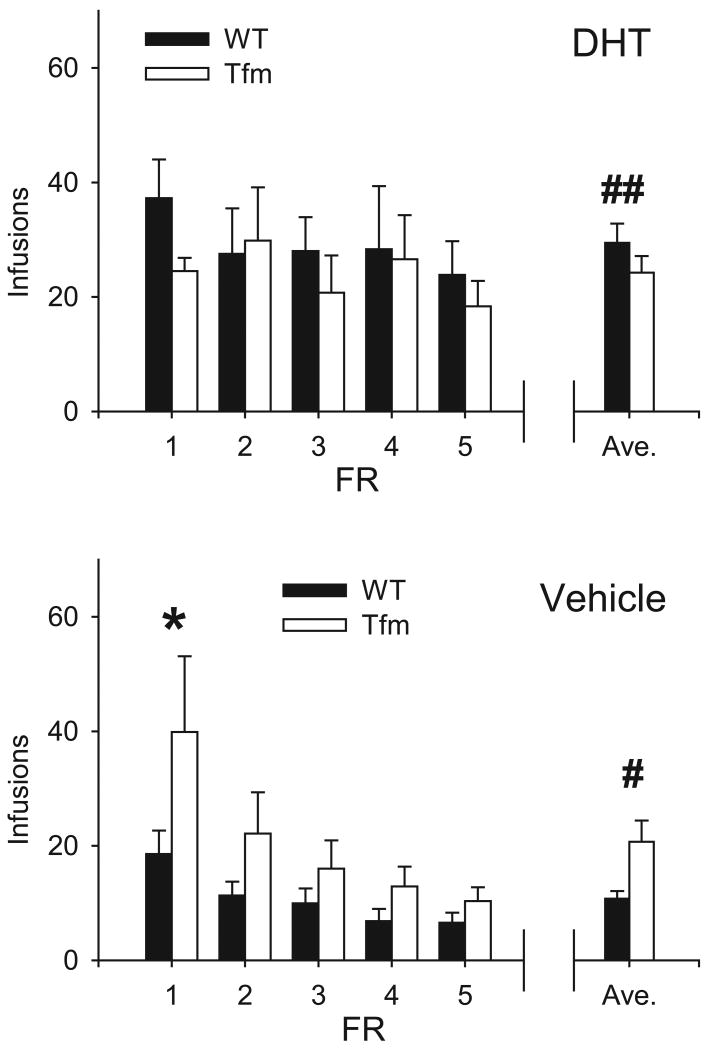
Infusions
The average number of DHT and Veh infusions received at each FR is shown in Fig. 2. Overall, rats received more infusions when allowed to self-administer DHT (26.9±2.2 μg/4h) vs. vehicle (15.4±1.9 μl/4h, F1,145 = 14.70, p < 0.001). There was also a main effect of FR schedule (F1,145 = 3.32, p = 0.01), and genotype-drug interaction (F1,145 = 6.41, p = 0.01). All other interactions and main effects were not significant.
Figure 2.
Mean infusions received by rats self-administering DHT (top) and vehicle (bottom). Means ± SEM for each FR are shown, along with the overall average ± SEM (right). * Significantly different from DHT FR1 (p < 0.05). # Significantly different from WT (p < 0.05). ## Significantly from vehicle (p < 0.05). Note that rats self-administering DHT received a relatively constant number of infusions, while rats self-administering vehicle received fewer infusions as the FR requirement increased.
Average daily intake of DHT across all FR schedules was similar in Tfm (24.3±2.9 μg/4hr) and WT (29.4±3.4 μg/4h) rats. In both groups, the drug intake remained constant as FR schedule increased (F4,73 = 0.54, ns). During FR1, Tfm and WT males self-administered DHT at 24.5±2.3 μg/4h and 37.3±6.7 μg/4h, respectively. Under an FR5 schedule, DHT self-administration averaged 18.3±4.5 μg/4h for Tfm and 23.9±5.9 μg/4h for WT rats. This group exhibited no genotype- or genotype-FR schedule-based differences (F1,73 = 1.17, ns; F4,73 = 0.34, ns, respectively).
By contrast, in both Tfm and WT rats, the number of vehicle infusions declined significantly as the FR requirement increased (F4,72 = 4.73, p < 0.01). Somewhat surprisingly, Tfm rats self-administered approximately twice as much vehicle (20.7±2.4 μl/4h) as WT rats (10.7±1.4 μl/4h, F1,72 = 7.77, p < 0.01). In Tfm rats at FR1, the number of vehicle infusions (39.9±13.2 μl/4h) exceeded the number of DHT infusions (24.5±2.3 μl/4h). However, by the end of the experiment, vehicle self-administration had declined to 10.3±2.4 μl/4h. Likewise, WT rats self-administered 18.6±4.1 μl/4h vehicle at FR1, which declined to 6.6±1.8 μl/4h at FR5.
The mean body weight at the start of each FR schedule and the number of animals in each condition is shown in Table 1. The WT rats were significantly heavier than Tfm rats (F1,174 = 144.62, p < 0.001), and all groups gained weight over time (F5,174 = 5.59, p < 0.001). There was no effect of drug condition (DHT v.s. Veh) on body weight (F1, 174 = 0.31, ns), or any interaction. DHT intake adjusted for body weight was similar in both genotypes at both FR1 (WT: 77.9 μg/kg, Tfm: 65.4 μg/kg) and FR5 (WT: 46.5 μg/kg, Tfm: 42.6 μg/kg).
Syrian hamsters self-administer DHT conjugated to BSA
Operant responding
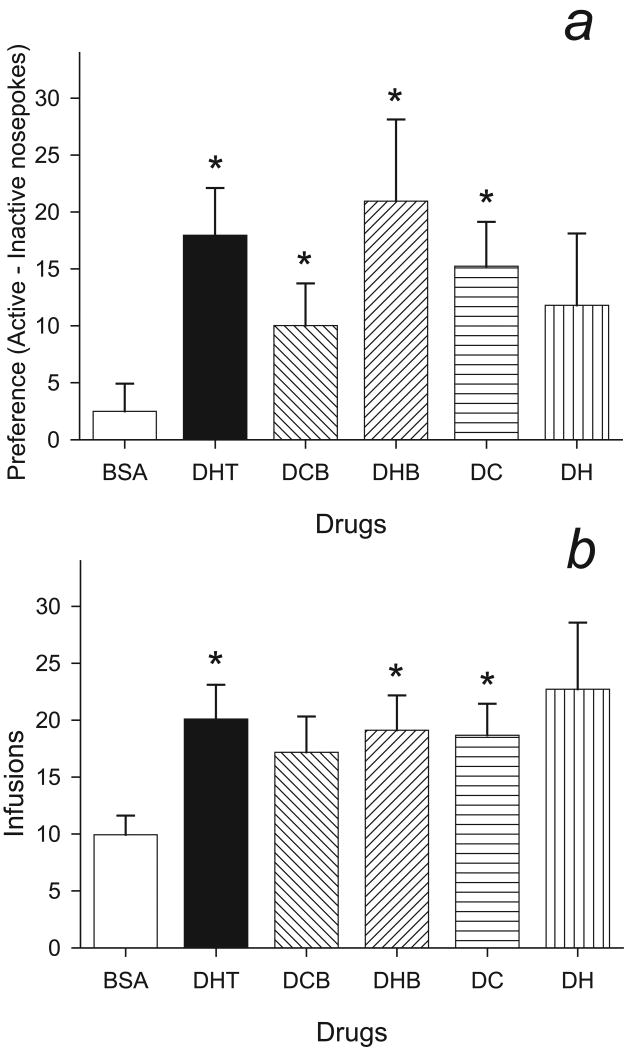
Hamsters self-administered DHT and DHT conjugated to BSA, but not BSA alone. Fig. 3a shows the mean preference (active - inactive nose-pokes) for DHT, BSA, DHT-CMO-BSA, and DHT-Hemis-BSA, DHT-CMO, DHT-Hemis. Consistent with our previous studies, hamsters developed a preference for the active nose-poke during DHT self administration (t7 = 4.34, p < 0.01), but showed no preference when self-administering BSA (t8 = 1.03, ns). Likewise, hamsters showed a preference for the active nose-poke with both DHT-CMO-BSA (t9 = 2.71, p = 0.02) and DHT-Hemis-BSA (t7 = 2.92, p = 0.02). With DHT attached to linkers alone, hamsters self-administered DHT-CMO (t8 = 3.91, p < 0.01), but did not show a significant preference when self-administering DHT-Hemis (t10 = 1.87, p = 0.09). With DHT-Hemis, responses on the active nose-poke (40.5±10.3 resp/4h) were similar to those for DHT-Hemis-BSA (41.2±11.4 resp/4h), but these males also showed increased responses for the inactive nose-poke (28.7±6.6 resp/4h) compared to those for DHT-Hemis-BSA (20.3±4.4 resp/4h).
Figure 3.
3a: Mean preference (active – inactive nose-pokes) for hamsters self-administering BSA (n = 9), DHT (n = 8), DHT-CMO-BSA (DCB, n= 10) and DHT-Hemis-BSA (DHB, n = 8), DHT-CMO (DC, n = 8), and DHT-hemis (DH, n = 11). * Significantly different from 0 (p < 0.05).
3b: Mean infusions received by hamsters self-administering DHT (n = 8), BSA (n = 9), DHT-CMO-BSA (DCB, n = 10), DHT-Hemis-BSA (DHB, n = 8)), DHT-CMO (DC, n = 9), and DHT-Hemis (DH, n = 11). * Significantly different from BSA (p < 0.05).
Infusions
The number of infusions received for each group is shown in Fig. 3b. Hamsters received significantly more DHT- than BSA-infusions (t15 = 3.04, p = 0.01). Similarly, hamsters received more infusions when allowed to self-administer DHT-Hemis-BSA (t15 = 2.72, p = 0.02) or DHT-CMO (t16 = 2.70, p = 0.02) compared to BSA. The numbers of infusions received for DHT-CMO-BSA (17.2±3.2 μl/4hr) and DHT-Hemis (22.7±5.9 μl/4hr) groups were similar to those self-administering DHT, DHT-Hemis-BSA, and DHT-CMO. Nonetheless, the hamsters did not receive significantly more DHT-CMO-BSA (t17 = 1.96, p = 0.07) or DHT-Hemis (t18 = 1.91, p = 0.07) compared to BSA.
Overdose
Eleven of 55 hamsters died before completing all 15 test sessions. Deaths due to androgen overdose during testosterone self-administration have previously been described (Peters and Wood, 2005). In the present study, 2 of 8 males (25%) died during DHT self-administration, similar to the 24% reported for testosterone overdose (Peters and Wood, 2005). Self-administration of DHT-CMO and DHT-Hemis were associated with the highest losses (each 3 of 8 per group, 38%), while there were few deaths among hamsters self-administering BSA (1 of 9, 11%) or DHT-Hemis-BSA (0 of 8). As with testosterone overdose, none of the hamsters in the present study died during self-administration. Instead, hamsters died several hours later in their home cages, with severe locomotor and respiratory depression.
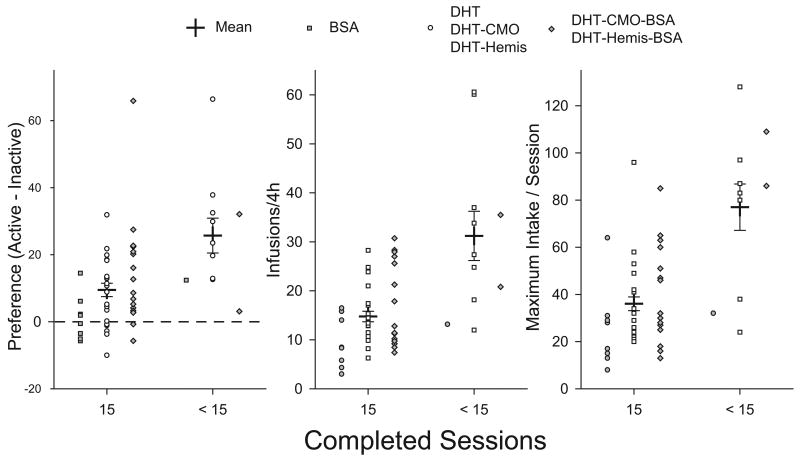
Testosterone overdose is closely correlated with testosterone intake, particularly maximum intake per session (Peters and Wood, 2005). Fig. 4 compares preference scores, the number of reinforcements received, and peak intake for hamsters who completed all 15 test sessions, and those who did not. Both groups showed a significant preference for the active nose-poke (p < 0.05). However, the preference was significantly greater in hamsters that died during self-administration (25.7 ± 5.2 resp/4h) compared with those that survived (9.5 ± 2.0 resp/4h, t53 = 3.42, p < 0.01). Hamsters that failed to complete 15 sessions received more than twice as many infusions per session (31.2 ± 5.0 inf/4h) as those who completed all sessions (14.8 ± 1.1 inf/4h, t53 = 5.05, p < 0.001). Furthermore, for hamsters that died during the study, the maximum intake per session was significantly higher (77.0 ± 9.8 inf/4h) than for males that survived (36.1 ± 2.9 inf/4h, t53 = 5.41, p < 0.001).
Figure 4.
Mean preference scores (left), infusions received (middle), and maximum intake per session (right) for hamsters who completed all 15 sessions (C15, n = 44) and those who did not (<15, n = 11). Group means ± SEM are shown as cross-hairs. Individual scores are shown as gray boxes (BSA), open circles (DHT and its derivatives), and gray diamonds (DHT-BSA conjugates. * Significantly different from C15 (p < 0.05).
Discussion
Androgen self-administration may be mediated by membrane-associated, but not by nuclear androgen receptors
The current study demonstrates that classical nuclear ARs are not essential for androgen self-administration. Both Tfm and WT rats developed a preference for the active nose-poke during DHT self-administration. Furthermore, they were able to respond to the ascending FR schedule by increasing active nose-pokes, thereby maintaining a steady level of drug intake regardless of the FR schedule. In contrast, rats receiving vehicle failed to respond to the changes in the FR schedule. Their active nose-pokes did not significantly increase in response to changes in FR schedule, and they received fewer infusions as the response requirement increased. Because ligand binding to the “classic” nuclear androgen receptor is compromised in Tfm mutants, this supports our hypothesis that androgen reinforcement is mediated via alternate pathways.
The unexpectedly high responding for vehicle by Tfm rats is unlikely to be due to the vehicle itself. We observed similar phenomena in a separate group of Tfm rats who were not receiving any infusions (data not shown). Instead, it may be related to feminized behavioral traits in the Tfm males. The increased nose-pokes by Tfm rats may be analogous to higher exploratory head dips observed in female rats (Brown and Nemes, 2008). Alternatively, the Tfm rats and mice are known to exhibit heightened anxiety-like behaviors (Zuloaga et al., 2006, Zuloaga et al., 2008a). Perhaps, the sedative/anxiolytic effects of DHT (Agren et al., 1999, Arnedo et al., 2000, Frye and Seliga, 2001, Berbos et al., 2002, Peters and Wood, 2005) blunted the anxiety-like behaviors when Tfm rats self-administered DHT.
In addition, self-administration of DHT-BSA conjugates in male hamsters provides evidence that androgens may act at the neuronal plasma membrane to have reinforcing action. Hamsters exhibited a significant preference for both DHT-BSA conjugates. The doses self-administered are in line with our previous studies on T, DHT, and commonly-abused steroids (Ballard and Wood, 2005, DiMeo and Wood, 2006b). In contrast, hamsters showed no preference for BSA alone. The data on mortality and drug intake demonstrate DHT and its derivatives can be lethal, extending our previous data on T overdose (Peters and Wood, 2005).
The current study reveals a species-specific pattern of operant responding. Hamsters did not prefer the active nose-poke while self-administering vehicle, as previously demonstrated (Johnson and Wood, 2001, Wood, 2002, DiMeo and Wood, 2004, Triemstra and Wood, 2004, Wood et al., 2004, Ballard and Wood, 2005, DiMeo and Wood, 2006b). In rats, however, there was a clear preference for active nose-poke regardless of the drug received. We observed a similar trend in our previous study on IV self-administration of T in rats, although it was not statistically significant (Wood et al., 2004). Based on such species-specific behavioral difference in self-administration, a caution must be taken when comparing behavioral data from rats and hamsters.
There are several caveats that need to be considered in interpretation of the current study. First, nuclear ARs with significantly impaired ligand binding are still present in Tfm rats (Yarbrough et al., 1990), unlike in Tfm mice (He et al., 1991). It is possible that these mutated nuclear ARs are sufficient for mediating effects of androgens at supra-physiological doses. Second, the DHT-BSA conjugates may degrade in vivo, resulting in free DHT. Although this does not appeared to be a significant issue in vitro (Lieberherr and Grosse, 1994, Gatson et al., 2006), the degree and the time-course of DHT-BSA degradation in vivo in the brain is currently unknown. Finally, DHT-BSA conjugates may not significantly penetrate into the brain tissue. DHT-BSA is significantly larger than DHT, thus the effects of DHT-BSA observed in the current study are likely to be mediated at sites close to the ventricles.
Despite these caveats, these two different approaches produced consistent results which argue strongly against the necessity for nuclear AR in androgen reinforcement. In addition, the self-administration of BSA conjugates suggests that androgens may act at the plasma membrane in androgen reinforcement. To our knowledge, the current study provides the first in vivo evidence for behaviorally relevant effects of androgens at the plasma membrane.
Androgens exert rapid nuclear AR-independent effects on reward
Several other studies on androgen reward have shown results consistent with non-genomic or plasma membrane effects. CPP develops within 30 min of systemic T injection (Alexander et al., 1994), a time-course consistent with acute non-genomic effects of T. CPP can be also induced with intra-Acb infusions of T or its metabolite (Packard et al., 1997, Frye et al., 2002), although Acb has few genomic AR. Furthermore, the VTA expresses Fos in response to ICV T-infusion (Dimeo and Wood, 2006a), despite the lack of significant classical AR expression there. The current study does not provide information regarding the site of action in the brain. Nonetheless, it does indicate that the relative lack of nuclear AR alone is not a sufficient reason to exclude structures such as Acb and VTA from the potential sites that may mediate androgenic effects.
Rapid plasma membrane effects of steroids in dorsal and ventral striatum are not limited to androgens. Progestins are known to induce CPP, possibly via gamma-aminobutyric acid (GABA) receptors in Acb (Frye, 2007). Estrogens also exert rapid, membrane receptor-mediated effects in the dorsal striatum (Mermelstein et al., 1996, Becker and Rudick, 1999). A membrane-associated receptor has already been isolated for progestins (Zhu et al., 2003), and evidence is accumulating for cell-surface receptors for estrogens (reviewed in Vasudevan and Pfaff, 2007) and androgens (reviewed in Thomas et al., 2006). While estrogens are also reinforcing (DiMeo and Wood, 2006b), the reinforcing effects of T appear to be predominantly androgenic. Hamsters self-administer non-aromatizable androgens, such as drostanolone and DHT (Ballard and Wood, 2005, DiMeo and Wood, 2006b). Additionally, the anti-androgen flutamide can block T self-administration (Peters and Wood, 2004). While this may appear to contradict the role of membrane AR reported in this study, flutamide has been reported to block membrane AR activation as well (Braun and Thomas, 2003, Braun and Thomas, 2004).
The properties of membrane androgen receptors
Historically, the effects of steroids, including androgens, were considered to be transduced by nuclear receptor-mediated processes. However, reports of rapid androgen effects, presumably mediated by membrane-associated receptors, have been available for several decades. For example, in the medial preoptic area, androgens can alter neuronal firing within seconds (Yamada, 1979) to minutes (Pfaff and Pfaffmann, 1969). Furthermore, Orsini and colleagues (Orsini, 1985, Orsini et al., 1985) have shown a rapid modification of neuronal firing frequency by androgens in the lateral hypothalamus (LHA). This effect of androgens in LHA may be of particular relevance to the present study, as the LHA is known to be involved in the reward circuitry (Olds and Milner, 1954) and LHA orexin/hypocretin is regulated by gonadal steroids (Muschamp et al., 2007).
The cell types with possible membrane ARs include glial (Gatson et al., 2006), gonadal (Braun and Thomas, 2003, Braun and Thomas, 2004), and immune cells (Benten et al., 1999, Guo et al., 2002), myocytes (Estrada et al., 2003), and osteoblasts (Lieberherr and Grosse, 1994). Although the molecular identity has yet to be determined, candidates for the membrane AR include membrane receptors with known steroid binding sites, such as GABA-A (reviewed in Lambert et al., 2003) and NR2 subunits of N-methyl-D-aspartic acid receptors (Malayev et al., 2002). Alternatively, Thomas and colleagues (2004) have reported evidence for a novel G-protein coupled receptor as a membrane AR. In addition, the effects of androgens unrelated to a specific receptor cannot be excluded in the current study.
Recent in vitro studies suggest that there are multiple membrane ARs, or more than one binding site on a single receptor, as proposed for the membrane progesterone receptor (Ramirez et al., 1996). In many cell types, the membrane AR appears to be a membrane receptor coupled to Gq/o (Lieberherr and Grosse, 1994, Benten et al., 1999, Zhu et al., 1999, Guo et al., 2002, Estrada et al., 2003). However, the steroid binding characteristics and the sensitivity to anti-androgens of the putative membrane AR vary greatly depending on the cell type. For instance, anti-androgens can block the effects of DHT on croaker ovarian cells (Braun and Thomas, 2003, Braun and Thomas, 2004), while they are not effective in other cell types (Lieberherr and Grosse, 1994, Benten et al., 2004, Gatson et al., 2006), or may even exert agonist-like effects in hippocampal cells (Pike, 2001, Nguyen et al., 2007) and several cancer cell lines (Peterziel et al., 1999, Zhu et al., 1999, Evangelou et al., 2000, Papakonstanti et al., 2003). Furthermore, different T-binding characteristics have been reported for different organs in fish (Braun and Thomas, 2004).
Our experience with commonly-abused AAS indicate that major modification(s) at the A-ring (at C2 and/or C3) and at C17 tend to interfere with self-administration (Ballard and Wood, 2005). For example, stanozolol, which has a major modification at C2 and C3 as well as a methyl group attached to C17, is not self-administered. In the current study, hamsters self-administered both BSA conjugated at C3 (DHT-CMO-BSA) and C17 (DHT-Hemis-BSA). Further research is required to elucidate the characteristics of androgens self-administered.
Clinical significance
AAS, especially T, are by far the most common performance enhancing agents used by athletes, accounting for nearly half the positive doping tests (World Anti-Doping Agency, 2006). Given such wide-spread use, AAS abuse has wide-ranging health consequences. Cardiac and hepatic side-effects of AAS abuse are well-established (Leshner, 2000). These and the anabolic effects of AAS have been thought to be mediated through nuclear AR. However, the possible nuclear AR-independent effects of androgens suggest that the influence of AAS may extend well beyond structures with nuclear AR expression.
As far as resemblance to other drugs of abuse, AAS produce different effects and have different mechanisms of action from stimulants. Unlike stimulants (Graybiel et al., 1990), AAS induce c-Fos activation only in the VTA and not in Acb (Dimeo and Wood, 2006a). Furthermore, AAS attenuate stimulant-induced Acb DA release (Birgner et al., 2006), and inhibit DA release acutely (Triemstra et al., 2008). Behaviorally, AAS do not induce locomotor activation characteristic of stimulants (Peters and Wood, 2005).
Instead, behavioral responses to acute AAS resemble those of opioids or benzodiazepines, possibly exerting additive effects when taken together. Acute exposure to AAS depresses autonomic functions, including respiration and body temperature (Peters and Wood, 2005). AAS-induced autonomic depression is reminiscent of the symptoms of opioid overdose, and is blocked by the opioid antagonist naltrexone (Peters and Wood, 2005). Furthermore, nandrolone, a commonly-used AAS, potentiates hypothermic effects of morphine and exacerbate naloxone-precipitated morphine withdrawal symptoms (Celerier et al., 2003). In addition, it is well-established that acute AAS are sedative/anxiolytic (Agren et al., 1999, Arnedo et al., 2000, Frye and Seliga, 2001, Berbos et al., 2002, Peters and Wood, 2005), possibly mediated by their direct effects on GABA-A receptors (Masonis and McCarthy, 1995, Masonis and McCarthy, 1996). Increased ethanol consumption in rats chronically treated with AAS may also be an reflection of altered GABAergic function (Johansson et al., 2000).
Our findings on overdose raise an additional health concern. Currently, the classification of AAS as control substances is based on their anabolic properties (Controlled Substances Act, 1991). However, the current study demonstrates that the anabolic efficacy of AAS does not necessarily correspond to their reinforcing properties and overdose risks. In addition to DHT-BSA conjugates, DHT-CMO used in this study is not a controlled substance, although its reinforcing properties and mortality from its overdose appears to be quite similar to DHT and T (Peters and Wood, 2005). The pattern of overdose also resembled that previously reported for T (Peters and Wood, 2005), where high intake resulted in mortality 24 to 48 hrs later. In light of these findings, the criteria used for scheduling a steroid as a controlled substance may require revisions to account for its abuse liability and toxicity, in addition to its anabolic potency.
The results of the current study suggest that nuclear AR, the only AR isolated so far, is not essential for the androgen reinforcement. Instead, the results suggest that androgen reinforcement is transduced at the plasma membrane. Thus, further inquiries into the identity of putative membrane AR, their functional characteristics and anatomical distribution are required to elucidate the underlying mechanism of AAS abuse and its clinical implications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agren G, Thiblin I, Tirassa P, Lundeberg T, Stenfors C. Behavioural anxiolytic effects of low-dose anabolic androgenic steroid treatment in rats. Physiol Behav. 1999;66:503–509. doi: 10.1016/s0031-9384(98)00323-0. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: implications for the biological basis of sexual motivation. Behav Neurosci. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol Biochem Behav. 2000;65:327–332. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- Ballard CL, Wood RI. Intracerebroventricular self-administration of commonly abused anabolic-androgenic steroids in male hamsters (Mesocricetus auratus): nandrolone, drostanolone, oxymetholone, and stanozolol. Behav Neurosci. 2005;119:752–758. doi: 10.1037/0735-7044.119.3.752. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Benten WP, Guo Z, Krucken J, Wunderlich F. Rapid effects of androgens in macrophages. Steroids. 2004;69:585–590. doi: 10.1016/j.steroids.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell. 1999;10:3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbos ZJ, Chu L, Wood RI. Acute behavioral effects of anabolic steroids: anxiety, stereotypy and locomotor activity. Horm Behav. 2002;41:457. [Google Scholar]
- Birgner C, Kindlundh-Hogberg AM, Nyberg F, Bergstrom L. Altered extracellular levels of DOPAC and HVA in the rat nucleus accumbens shell in response to sub-chronic nandrolone administration and a subsequent amphetamine challenge. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Braun AM, Thomas P. Androgens inhibit estradiol-17beta synthesis in Atlantic croaker (Micropogonias undulatus) ovaries by a nongenomic mechanism initiated at the cell surface. Biol Reprod. 2003;69:1642–1650. doi: 10.1095/biolreprod.103.015479. [DOI] [PubMed] [Google Scholar]
- Braun AM, Thomas P. Biochemical characterization of a membrane androgen receptor in the ovary of the atlantic croaker (Micropogonias undulatus) Biol Reprod. 2004;71:146–155. doi: 10.1095/biolreprod.103.025825. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatr Rep. 2002;4:377–387. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic-androgenic steroid dependence. Br J Addict. 1991;86:759–768. doi: 10.1111/j.1360-0443.1991.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Brown GR, Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav Processes. 2008;78:442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Yazdi MT, Castane A, Ghozland S, Nyberg F, Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur J Pharmacol. 2003;465:69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Choi PY, Pope HG., Jr Violence toward women and illicit androgenic-anabolic steroid use. Ann Clin Psychiatry. 1994;6:21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- Title 21, Chapter 13 - Drug Abuse Prevention and Control Controlled Substances Act. 1991 [Google Scholar]
- DiMeo AN, Wood RI. Circulating androgens enhance sensitivity to testosterone self-administration in male hamsters. Pharmacol Biochem Behav. 2004;79:383–389. doi: 10.1016/j.pbb.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Dimeo AN, Wood RI. ICV testosterone induces Fos in male Syrian hamster brain. Psychoneuroendocrinology. 2006a;31:237–249. doi: 10.1016/j.psyneuen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- DiMeo AN, Wood RI. Self-administration of estrogen and dihydrotestosterone in male hamsters. Horm Behav. 2006b;49:519–526. doi: 10.1016/j.yhbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- Evangelou A, Jindal SK, Brown TJ, Letarte M. Down-regulation of transforming growth factor beta receptors by androgen in ovarian cancer cells. Cancer Res. 2000;60:929–935. [PubMed] [Google Scholar]
- Fernandez R, Collado P, Garcia Doval S, Garcia-Falgueras A, Guillamon A, Pasaro E. A molecular method for classifying the genotypes obtained in a breeding colony from testicular feminized (Tfm) rats. Horm Metab Res. 2003;35:197–200. doi: 10.1055/s-2003-39081. [DOI] [PubMed] [Google Scholar]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Rosellini R, Svare B. The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5alpha-reduced metabolites. Pharmacol Biochem Behav. 2002;74:119–127. doi: 10.1016/s0091-3057(02)00968-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cognitive, Affective & Behavioral Neuroscience. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Benten WP, Krucken J, Wunderlich F. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem. 2002;277:29600–29607. doi: 10.1074/jbc.M202997200. [DOI] [PubMed] [Google Scholar]
- He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19:2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Lindqvist A, Nyberg F, Fahlke C. Anabolic androgenic steroids affects alcohol intake, defensive behaviors and brain opioid peptides in the rat. Pharmacol Biochem Behav. 2000;67:271–279. doi: 10.1016/s0091-3057(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73:285–292. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40:73–79. doi: 10.1016/0376-8716(95)01192-7. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Leshner AI. (NIDA Research Report Series).Anabolic Steroid Abuse. 2000:1–8. [Google Scholar]
- Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem. 1994;269:7217–7223. [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–2398. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masonis AE, McCarthy MP. Direct effects of the anabolic/androgenic steroids, stanozolol and 17 alpha-methyltestosterone, on benzodiazepine binding to the. gamma-aminobutyric acid(a) receptor. Neurosci Lett. 1995;189:35–38. doi: 10.1016/0304-3940(95)11445-3. [DOI] [PubMed] [Google Scholar]
- Masonis AE, McCarthy MP. Effects of the androgenic/anabolic steroid stanozolol on GABAA receptor function: GABA-stimulated 36Cl- influx and [35S] TBPS binding. J Pharmacol Exp Ther. 1996;279:186–193. [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of The Golden Hamster Brain. Academic Press; San Diego: 2001. [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR, editor. NationalResearchCouncil. Guide for the care and use of laboratory animals. National Research Council; Washington, DC: 1996. [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- NIH. Principle of laboratory animal care. National Institute of Health; Bethesda, Maryland: 1985. [Google Scholar]
- Olds J, Milner PM. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Orsini JC. Direct effects of androgens on lateral hypothalamic neuronal activity in the male rat: II. A pressure ejection study. Brain Res Bull. 1985;15:547–552. doi: 10.1016/0361-9230(85)90203-5. [DOI] [PubMed] [Google Scholar]
- Orsini JC, Barone FC, Armstrong DL, Wayner MJ. Direct effects of androgens on lateral hypothalamic neuronal activity in the male rat: I. A microiontophoretic study. Brain Res Bull. 1985;15:293–297. doi: 10.1016/0361-9230(85)90154-6. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci. 1997;111:219–224. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- Packard MG, Schroeder JP, Alexander GM. Expression of testosterone conditioned place preference is blocked by peripheral or intra-accumbens injection of alpha-flupenthixol. Horm Behav. 1998;34:39–47. doi: 10.1006/hbeh.1998.1461. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol. 2003;17:870–881. doi: 10.1210/me.2002-0253. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: in sterotaxic coordinates. 4th. Academic Press; New York: 1998. [Google Scholar]
- Peters KD, Wood RI. Anabolic-androgenic steroid addiction involves androgen and opioid receptors. Proceedings of the 34th Annual Meeting of the Society for Neuroscience; San Diego, CA. 2004. [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Pfaffmann C. Olfactory and hormonal influences on the basal forebrain of the male rat. Brain Res. 1969;15:137–156. doi: 10.1016/0006-8993(69)90315-1. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Research. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology. 1999;140:5455–5458. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71:310–316. doi: 10.1016/j.steroids.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Triemstra JL, Sato SM, Wood RI. Testosterone and nucleus accumbens dopamine in the male Syrian hamster. Psychoneuroendocrinology. 2008;33:386–394. doi: 10.1016/j.psyneuen.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triemstra JL, Wood RI. Testosterone self-administration in female hamsters. Behav Brain Res. 2004;154:221–229. doi: 10.1016/j.bbr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Wood RI. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm Behav. 2002;41:247–258. doi: 10.1006/hbeh.2002.1769. [DOI] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl) 2004;171:298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol. 1999;39:359–370. doi: 10.1002/(sici)1097-4695(19990605)39:3<359::aid-neu3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- WorldAnti-DopingAgency. Adverse analytical findings reported by accredited laboratories. World Anti-Doping Agency; Montreal, Canada: 2006. [Google Scholar]
- Yamada Y. The effects of testosterone on unit activity in rat hypothalamus and septum. Brain Res. 1979;172:165–169. doi: 10.1016/0006-8993(79)90906-5. [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- Yesalis CE, Kennedy NJ, Kopstein AN, Bahrke MS. Anabolic-androgenic steroid use in the United States. JAMA. 1993;270:1217–1221. [PubMed] [Google Scholar]
- Zhu X, Li H, Liu JP, Funder JW. Androgen stimulates mitogen-activated protein kinase in human breast cancer cells. Mol Cell Endocrinol. 1999;152:199–206. doi: 10.1016/s0303-7207(99)00031-3. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008a;54:758–766. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation. Horm Behav. 2008b;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DZ, Jordan CL, Breedlove SM. Rats with the testicular feminization mutation (TFM) show increased indices of anxiety. Proceedings of the 36th Annual Meeting of the Society for Neuroscience; Atlanta, GA. 2006. Program # 152.118. [Google Scholar]