Abstract
Although early studies demonstrated that exogenous estrogen lowered a woman’s risk of cardiovascular disease, recent trials indicate that HRT actually increases the risk of coronary heart disease or stroke. However, there is no clear explanation for this discrepancy. Is estrogen a helpful or a harmful hormone in terms of cardiovascular function? This review discusses some recent findings that propose a novel mechanism which may shed significant light upon this controversy. We propose that nitric oxide synthase (NOS) expressed within the vascular wall is a target of estrogen action. Under normal conditions in younger women, the primary product of estrogen action is NO, which produces a number of beneficial effects on vascular biology. As a woman ages, however, there is evidence for loss of important molecules essential for NO production (e.g., tetrahydrobiopterin, L-arginine). As these molecules are depleted, NOS becomes increasingly “uncoupled” from NO production, and instead produces superoxide, a dangerous reactive oxygen species. We propose that a similar uncoupling and reversal of estrogen response occurs in diabetes. Therefore, we propose that estrogen is neither “good” nor “bad”, but simply stimulates NOS activity. It is the biochemical environment around NOS that will determine whether estrogen produces a beneficial (NO) or deleterious (superoxide) product, and can account for this dual and opposite nature of estrogen pharmacology. Further, this molecular mechanism is consistent with recent analyses revealing that HRT produces salutary effects in younger women, but mainly increases the risk of cardiovascular dysfunction in older postmenopausal women.
Keywords: estrogen, hormone replacement therapy, coronary, nitric oxide, superoxide
In the late 20th century estrogens, particularly as hormone replacement therapy (HRT), were highly prescribed to alleviate and/or prevent common complications of female menopause. The popularity of HRT was due in large part to the ability of estrogen to prevent or possibly reverse menopausal complaints (e.g., osteoporosis, hot flushes, thinning of vaginal epithelium). Exogenous estrogen is highly efficacious at treating these symptoms. In addition, at this time it was a commonly-held belief that estrogens could protect against the most serious health risk in Western society: cardiovascular disease [1, 2]. The Nurses’ Health Study realized a 40% reduction in coronary heart disease events over time in women who used HRT after menopause [3]. In addition, laboratory studies demonstrated that estrogen lowered coronary vascular resistance and enhanced coronary blood flow either in vitro [4] or in vivo [5], and ameliorated myocardial ischemia or improved coronary vasodilatory responses in both female [6-8] and male [9] patients. Furthermore, estrogens reduced coronary heart disease by 40-50% through multiple mechanisms, including favorable effects on plasma lipoproteins, antioxidant effects, preservation of vascular endothelium function, upregulation of nitric oxide synthase, enhanced NO production, inhibition of platelet aggregation, increased prostacyclin production via upregulation of cyclooxygenase-2 activity, decreased cell adhesion molecules, and decreased inflammatory factors [1, 10-12].
Despite the prevalence of findings that estrogen supported and even enhanced cardiovascular function, data from large-scale clinical trials, notably the Women’s Health Initiative (WHI), indicated that postmenopausal women receiving HRT actually exhibited an increase in the incidence of both coronary heart disease and stroke [13]. These trials substantiated the findings that estrogen could lessen the impact of osteoporosis, genitourinary, and other menopausal complications; but, in light of the unexpected findings that estrogens could actually exacerbate cardiovascular dysfunction, physicians have become hesitant to prescribe HRT for their patients. In addition, the American Heart Association continues to recommend that women avoid HRT as a possible means of preventing cardiovascular disease. In light of these trials, reports, and opinions, the majority of menopausal women are today avoiding the use of HRT.
Recent review of clinical trials is now supporting the idea that the age of a woman, particularly how many years since the onset of menopause, may be a critical factor in predicting the eventual outcome of HRT. The mean age of women receiving HRT (estrogen + progesterone) in the WHI was 63 years, with an overall outcome of 40% increased risk of stroke and 24% increased risk of coronary heart disease. Similar increases in risk were noted in women receiving only estrogen therapy; however, younger women in this same group (age 50-59) actually experienced a 44% reduction in coronary heart disease (CHD) compared to the placebo control group -- a finding similar to that of the Nurses’ Health Study [3]. In contrast, estrogen therapy actually increased the risk of CHD for older women (70-79 years) compared to those receiving only placebo. Interestingly, these findings are consistent with other clinical trials as well. For example, a recent commentary on two HRT arms of the WHI concluded that women initiating HRT at a younger age (i.e., closer to onset of menopause) exhibited a lower CHD event rate compared to older subjects (i.e., > 60 years of age), indicating that HRT is indeed a safe and effective therapeutic option for the primary prevention of CHD in postmenopausal women, provided the treatment is started at a suitable (i.e., younger) postmenopausal time and at a suitable dose [14].
Therefore, there is increasing evidence that the age of a woman appears to be a critical factor in determining the overall outcome of HRT. Specifically, there looks to be a clear inverse correlation with age and potential HRT benefit. In other words, the older a woman is when HRT is initiated, the less likely she will experience beneficial effects of estrogen on the cardiovascular system; or instead may actually experience deleterious effects of HRT, with possible increases in the risk of coronary heart disease and/or stroke [12]. Despite this apparent relationship between age and HRT effectiveness, experimental evidence proposing a cellular/molecular basis for this phenomenon remains controversial. It is still a mystery as to why “younger” women tend to respond more positively to exogenous estrogen treatment compared to “older” women. In face of this uncertainly, we have proposed a mechanism residing in the wall of blood vessels (and other tissues as well) that could conceivably account, at least in part, for this dual and opposite effect of estrogen on cardiovascular function [15].
Estrogen as a coronary vasodilator
Estrogen is a well-known vasodilator, both vivo and in vitro. Nonetheless, acute effects of estrogens on vascular resistance and blood flow continue to be controversial. Clinical studies reveal that acutely administered (15 - 60 min) estrogen induces coronary vasodilation in both male and female patients. For example, sublingual estrogen delays the onset of exercise-induced myocardial ischemia in women [16], and intravenous estrogen attenuates abnormal coronary vasomotor responses in men [9] or postmenopausal women [6] with established CHD. Clearly, estrogen exerts a rapid, probably non-genomic, vasodilatory effect on the coronary circulation; however, our understanding of the molecular basis of how estrogen affects coronary arteries is far from complete.
It is often assumed that estrogen-induced relaxation of coronary artery smooth muscle (CASM) is primarily indirect, i.e., mediated by endothelium-derived vasodilatory substances. While it is clear that estrogen can indeed stimulate the release of NO from endothelial cells to relax the underlying CASM, estrogen can also induce endothelium-independent relaxation of human coronary arteries [17, 18]. We have demonstrated directly that estrogen opens K+ channels in isolated human CASM cells by stimulating production of NO in the absence of endothelium [19]. Therefore, there appears to be a redundancy of estrogen target cells in the coronary vascular wall: endothelial and CASM cells, both of which express estrogen receptors. Because of the ability of steroids to penetrate lipid bilayers, it is likely that estrogen can affect both cell types under normal conditions. Moreover, the direct effect of estrogen on human CASM cells may be an important mechanism for helping to counteract pathological coronary vasoconstriction in arteries with a damaged or diseased intima, as evidenced by clinical studies demonstrating that acute (15-30 minute) administration of estrogen to postmenopausal women with coronary artery disease relieves myocardial ischemia [7, 8] and increases coronary blood flow [6]. These and other studies have established the fact that acute exposure to estrogen induces a relaxation of coronary arteries. In vivo, such relaxation would enhance coronary blood flow, and should thereby help alleviate the symptomology associated with coronary ischemia. Therefore, one would assume that the primary outcome of HRT would most likely be a lower risk of myocardial ischemia and/or infarction. As noted above, however, initiating HRT in older menopausal women actually increases the risk of coronary heart disease in test subjects.
Estrogen and vascular smooth muscle: signal transduction leading to rapid relaxation
We had originally demonstrated that estrogen-induced relaxation of endothelium-denuded coronary arteries involves activity of the large-conductance, calcium- and voltage-activated potassium (BKCa) channel via stimulation of NO production in CASM cells [20]. We have now established that the majority of estrogen-stimulated NO production in CASM is from NOS-1 (nNOS). Inhibiting NO production prevents estrogen from increasing BKCa channel currents in CASM cells [21], and estrogen enhances NO-stimulated fluorescence in human CASM cells [22]. Moreover, we recently demonstrated that overexpression of NOS-1 enhances BKCa channel activity in human CASM cells, and that estrogen-stimulated channel activity was inhibited by Nω-propyl-L-arginine (LNPA), which inhibits NOS-1 selectively [23]. Therefore, we have identified NOS-1 as a probable target of estrogen action in CASM.
Dual and opposite effects of estrogen in the vasculature: one mechanism, two outcomes
Production of NO in the vasculature is generally considered to be a “good” thing, leading to vasodilation, attenuated release of endothelin-1 and thromboxane A2, inhibition of platelet aggregation, and inhibition of smooth muscle proliferation, and stimulation of endothelial cell proliferation / angiogenesis [24]. Therefore, we hypothesized that the “good” cardiovascular effects of estrogen were likely mediated via NO produced in the endothelial and vascular smooth muscle cells (and primarily in smooth muscle cells when endothelial cells are damaged by aging, atherosclerosis, diabetes, etc.), while the “bad” effects must be due to a secondary, NO-independent mechanism. Laboratory and clinical studies supported the idea of beneficial effects of estrogen-stimulated NO production. For example, aortae from females release more NO than vessels from males [25], suggesting a role for estrogen in elevated NO production. Furthermore, NO production is increased during pregnancy [26] and during the preovulatory phase of the menstrual cycle when estrogen levels are highest [27]. Thus, there is considerable sexual dimorphism in the production of NO which correlates positively with estrogen levels -- at least for women during their childbearing years, who, in general, exhibit a significantly lower risk of cardiovascular disease compared to males of similar age. On the other hand, the identity and molecular nature of a mechanism whereby estrogen could promote cardiovascular dysfunction were still unknown.
A potential clue into this dual nature of estrogen action was gained from studies investigating the mechanisms of estrogen-induced artery coronary relaxation. While attempting to block estrogen-induced relaxation of endothelium-denuded coronary arteries, we uncovered an unexpected effect of estrogen: coronary artery constriction. We were able to not simply attenuate estrogen-stimulated coronary relaxation by inhibiting L-arginine binding to NOS-1, but instead converted the expected vasodilatory response of estrogen into a powerful (and very unexpected) estrogen-induced coronary constriction [15]. Here was a novel mechanism whereby estrogen could induce vasoconstriction and thereby limit blood flow to vital organs – and possibly contribute to an increased risk of CHD and stroke. Interestingly, the contractile effect of estrogen on coronary arteries could be completely reversed by attenuating superoxide production with either tempol (superoxide dismutase mimetic) or tiron (superoxide scavenger), strongly suggesting that this vasoconstriction was likely mediated by reactive oxygen species (e.g., superoxide). Fluorescence studies then confirmed estrogen-stimulated superoxide production in CASM cells when NO production had been inhibited by blocking access of L-arginine to NOS-1 [15]. Furthermore, the vascular effect of estrogen was completely dependent upon the presence of L-arginine: with L-arginine, estrogen was a coronary vasodilator; when L-arginine binding to NOS-1 was inhibited, estrogen was a coronary vasoconstrictor. These studies strongly suggested that NOS-1 was the primary site of estrogen-stimulated superoxide production, although we did not conclusively rule out other potential sites of action (e.g., NADPH oxidase, xanthine oxidase). Therefore, we now had evidence linking a single enzyme, NOS-1, to both estrogen-induced coronary relaxation and constriction: a single molecular mechanism of estrogen action that could possibly account for two completely different physiological outcomes.
NOS-1 as a source of both NO and superoxide
NOS is a multifunctional enzyme, possessing both oxygenase and reductase domains with binding sites for several cofactors. Interestingly, it is the disposition of the microenvironment around NOS which will determine its primary product. For example, NOS can catalyze an “uncoupled” NADPH oxidation to generate superoxide rather than NO [28], and O2- has been suggested to play a role in age-related increased coronary vascular resistance due to its oxidative potential and ability to further reduce NO bioavailability [29]. Interestingly, among the NOS isoforms it is NOS-1 that exhibits the greatest tendency to generate superoxide under uncoupled conditions [28, 30]; “uncoupled”, in this sense, meaning the inability to produce NO due to lack of substrate and/or cofactors. Because NOS-1 is the only NOS isoform detected in CASM and NOS-1 is clearly a target of estrogen action in these cells, we propose that NOS-1 is an important source of estrogen-stimulated superoxide production in CASM. Furthermore, all NOS isoforms have the ability to produce superoxide. Therefore, NOS-3 (eNOS) should also be a potential mechanism for estrogen-stimulated superoxide production in the vasculature, and might also contribute to deleterious effects of estrogen on cardiovascular function in vessels.
Aging and decreased NO availability
There is evidence that the aging process could be associated with increased uncoupling of NOS activity, leading to enhanced production of reactive oxygen species as a woman ages. For example, aging reduces the levels of L-arginine and tetrahydrobiopterin (BH4), both of which are critical cofactors required to sustain NO production from NOS activity. As a woman ages, her serum L-arginine levels and NO production decrease [31, 32], leading to enhanced oxidative stress on the cardiovascular system [33]. In addition, arginase activity is upregulated in the aging vasculature, thus lowering the availability of L-arginine for NOS activity in either endothelium or vascular smooth muscle [34]. Concomitant with age-dependent loss of L-arginine, BH4 synthesis also declines with age [35]. BH4, which is essential for generating NO and inhibiting O2- production from nNOS [36], is decreased by nearly 30-fold by diet-induced hyperlipidemia [37]. Therefore, lower levels of BH4 would increase the tendency to uncouple NOS activity as atherosclerosis progresses to further predispose arteries to oxidative damage. In summary, it appears increasingly likely that as women age and atherosclerosis progresses there is reduced NO bioavailability and enhanced oxidative stress.
Our findings that estrogen can stimulate NOS activity, yet produce two very different products, suggest that a NOS stimulator (such as estrogen given to women in HRT) would very likely accelerate the normal age-related decline in cardiovascular function via enhanced generation of reactive oxygen species. In addition, and maybe somewhat surprisingly, estrogen should also modulate vascular reactivity in men. Aromatase is expressed in arterial smooth muscle [38], thus providing a local mechanism for de novo synthesis of estrogen from androgens in the vasculature of both males and females in a manner completely independent of plasma estrogen levels. In fact, higher plasma levels of testosterone might actually enhance the overall production of estrogen within the vascular wall (assuming sufficient expression and activity of aromatase). Regardless of the source of estrogen (circulating or autocrine in origin), our findings now provide a novel molecular mechanism whereby intravascular synthesis of estrogen could impact cardiovascular function in both women and men to produce either physiological or pathophysiological effects.
Age and the WHI: uncoupling the controversy?
As discussed above, findings from the WHI demonstrated an overall increased risk of cardiovascular disease when taking HRT; however, when age of the participants was taken into consideration, it was apparent that the younger women in the trial (age <60 years) actually obtained a reduction in cardiovascular risk from HRT. It was primarily the older women (age > 60 years) who experienced the greater risk after receiving HRT. Thus, there was an age-dependency to the outcome of HRT, with younger women exhibiting more beneficial outcomes. Could this difference be explained, at least in part, by HRT producing predominantly NO in these younger women, whereas reactive oxygen species were the primary products of HRT in the older subjects? If so, this could help explain the confusing dichotomy of responses to estrogen, and is also consistent with findings that younger women receiving estrogen-containing oral contraceptives seldom experience cardiovascular complications (other than possibly an increased risk of venous thromboembolism in some women, especially if they are smokers).
An interesting study by Chen et al. [35] has demonstrated that a woman’s brain can lose most (~81.5%) of the enzymatic machinery to synthesize BH4 as she ages. In fact, these studies suggest that by the age of 50 her neuronal level of GTP-cyclohydrolase-I (GTPCH; the key rate-limiting enzyme in BH4 synthesis) is reduced by nearly 50%. Based on our model, we would predict that estrogen might promote cognitive function in “younger” menopausal women, but would have no (or possibly a negative) effect on learning and memory in older menopausal women whose NOS would exist primarily in the uncoupled state (i.e., HRT might promote oxidative stress and thereby impair cognitive function in older subjects). This concept, though admittedly speculative at present, is entirely consistent with the so-called “critical period hypothesis” which proposes that HRT can enhance cognitive function in women, but only in the early (i.e., middle-age) menopausal years [39]. Estrogen treatment after this therapeutic window appears to be largely ineffective in preventing age-related cognitive decline. We speculate that such a “critical period” also exists for estrogen-associated protection against age-related cardiovascular decline, and that once this time frame has passed, HRT could further accelerate such a decline. Interestingly, these findings also support the novel idea that menopause, far from being simply a deleterious consequence of aging, might actually be a beneficial adaptation to prevent excessive oxidative stress otherwise produced by estrogen in older women. Could menopause be nature’s way of protecting women from potential age-related harmful effects of estrogen?
Women, estrogen, and diabetes: a model of premature aging?
Although the deleterious consequences of diabetes cut across all races, cultures, and genders, it is becoming increasing clear that women are at greater risk for diabetes-associated cardiovascular disease than men. During childbearing years women are relatively protected against significant cardiovascular dysfunction compared to men of similar age, but diabetes blunts this potential benefit of female gender [40]. Despite the fact that cardiovascular mortality continues to decline for both non-diabetic men and women, and also for diabetic men, age-adjusted cardiovascular mortality has actually increased 23% for diabetic women [41]. Diabetes produces particularly disparate outcomes for coronary heart disease, with risk of fatal CHD 50% higher in diabetic women than in men [42]. Similar studies indicate that diabetes more than doubles the rate of death due to CHD in women compared to men (90% vs. 40%, respectively) [43]. It is not known, however, why diabetic women tend to suffer more cardiovascular disease compared to diabetic men.
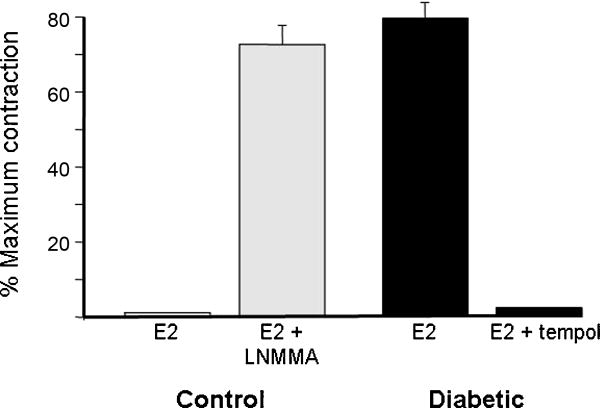
In some respects diabetes can be considered to mimic premature aging. This is particularly true for diabetic cardiovascular disease, which is characterized by a higher incidence of atherosclerosis, hypertension, and CHD. In addition, it is well-known that oxidative stress is increased in diabetes. Diabetes reduces expression of GTPCH [44], as occurs during the normal aging process in females. Further, diabetes substantially increases the activity of arginase, which depletes L-arginine [45]. Diabetes-associated loss of BH4 and L-arginine would promote NOS uncoupling, and further elevate the potential for oxidative stress and decreased NO bioavailability in diabetes. Such stresses most likely contribute to the increased cardiovascular dysfunction associated with both type 1 and type 2 diabetes. Recent findings from our laboratory suggest that NOS-1 expressed in CASM from diabetic pigs exists primarily in the uncoupled state, and thus is a mechanism for estrogen-stimulated production of superoxide (Figure 1).
Figure 1.
Estrogen contracts coronary arteries when NOS is uncoupled pharmacologically or by a disease state (diabetes). Isometric contractile force recordings demonstrate that 100nM 17β-estradiol (20 min), a known coronary vasodilator, contracts endothelium-denuded coronary arteries from normal pigs after treatment with 100μM N-monomethyl-L-arginine (LNMMA) to uncouple NOS activity (n=10; gray bars). Interestingly, estrogen produced the same level of contraction in untreated coronary arteries from diabetic pigs (n=5; black bars). Estrogen-induced contraction of these “diabetic” arteries was completely reversed by further addition of 1mM tempol, a superoxide dismutase mimetic.
In our studies Yorkshire swine were rendered diabetic by injection with streptozotocin and maintained as a model of humanoid diabetes, as described previously [46]. Coronary arteries were harvested and prepared for contractile-force recordings, as described previously [15]. We found that estrogen had little effect on arteries from control swine; however, when these control arteries were first pretreated (30 minutes) with 100μM L-NMMA in order to “uncouple” NOS activity from NO production, estrogen (100nM) now produced a significant contractile effect (Figure 1). This contractile response could be reversed by inhibiting accumulation of superoxide with either tempol or tiron, indicating that estrogen-stimulated coronary artery contraction resulted from superoxide production during these artificial “uncoupled” conditions. Most interestingly, we observed that 100nM 17β-estradiol contracted coronary arteries from diabetic pigs in the absence of any other agents. This contractile effect was inhibited by tempol, strongly suggesting that estrogen stimulates production of superoxide in these vessels to induce coronary contraction. Thus, it was apparent that diabetes can transform “normal” estrogen-induced coronary artery relaxation into a “pathological” vasoconstrictor effect dependent upon generation of reactive oxygen species. Our previous studies demonstrated that we could make control arteries respond like “diabetic” arteries when we had uncoupled the activity of NOS-1, and thereby allowed estrogen to stimulate production of superoxide instead of NO [15].
These findings raise the interesting possibility that diabetes transforms estrogen from a “helpful” into a “harmful” hormone, and also suggest a potential mechanism that could help explain why women suffer greater cardiovascular risk in diabetes compared to men: estrogen-stimulated oxidative stress in diabetic women. This hypothesis of “premature cardiovascular aging in diabetes” is very consistent with the clinical findings that estrogen appears to produce mainly deleterious consequences in older postmenopausal women, but is largely beneficial in younger women. We suggest that diabetes may be thought to accelerate the normal aging process, particularly in women, and thus promote cardiovascular dysfunction, at least in part, by estrogen-stimulated oxidative stress in the female vascular system. Our findings indicate a critical role for NOS uncoupling in this process, and suggest this enzyme as a new potential therapeutic target for preventing diabetic cardiovascular complications in women (and possibly men).
Conclusions
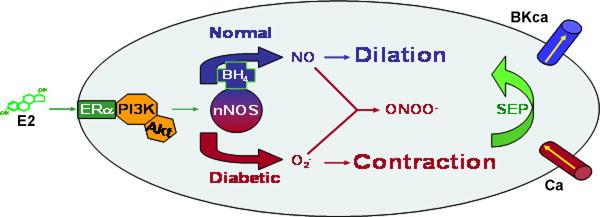
We propose that, in reality, estrogen is neither “good” nor “bad”, and that estrogen signaling mechanisms remain rather constant over a woman’s lifespan. Instead, our findings suggest that it is the biochemical microenvironment around NOS (NOS-1 in vascular smooth muscle; NOS-3 in endothelial cells) that will ultimately determine whether the overall effects of estrogen and HRT are either “good” or “bad” in terms of cardiovascular function (Figure 2). Our model further suggests that if important precursors and cofactors that decline with normal aging (e.g., L-arginine, BH4) could be supplemented exogenously, then the overall health benefits of HRT might be restored such that women, no matter their age, would experience a reduced risk of cardiovascular disease when taking HRT. Further, if premature uncoupling of NOS activity is a contributing factor to a woman’s greater risk of suffering cardiovascular disease in diabetes, then such supplementation might also aid in reversing the morbidity and mortality associated with this disease state. Of course, more research must be done to further test the proposed model; but if subsequent studies can confirm that estrogen stimulates NOS to produce both NO and superoxide, then possibly a new therapeutic window could be opened to help lower the risk of cardiovascular disease. Such measures would be particularly important as our population experiences increasing lifespans due to the advances in biomedical research.
Figure 2.
Summary Hypothesis: Diabetes transforms the “normal” vasodilatory response of CASM to estrogen into coronary vasoconstriction by enhancing ROS production. Because BH4 and L-arginine levels are depleted by diabetes, there should be greater potential for “uncoupled” NOS to produce mainly superoxide (and peroxynitrite). This increased oxidative stress opens Ca channels and/or closes BKCa channels, thus leading to increased [Ca2+], and coronary artery contraction.
Acknowledgments
We would like to thank our sources of support for this work: grants from the National Heart, Lung, and Blood Institute (HL-64779 and HL07389, R. White; HL-68026, S. Barman) and the American Heart Association (Scientist Development Grant, G. Han; Southeast Affiliate 0555149B, S. Barman). Sadly, we must report that our colleague, Dr. Ross Gerrity, passed away in July of 2008 due to diabetes-related coronary heart disease. We dedicate this article to his memory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 2.White RE. Estrogen and vascular function. Vascular Pharmacoloy. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- 3.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–61. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 4.Raddino R, Manca C, Poli E, Bolognesi R, Visioli O. Effects of 17 beta-estradiol on the isolated rabbit heart. Arch Int Pharmacodyn Ther. 1986;281:57–65. [PubMed] [Google Scholar]
- 5.Node K, Kitakaze M, Kosaka H, Minamino T, Sato H, Kuzuya T, Hori M. Roles of NO and Ca2+-activated K+ channels in coronary vasodilation induced by 17beta-estradiol in ischemic heart failure. Faseb J. 1997;11:793–9. doi: 10.1096/fasebj.11.10.9271364. [DOI] [PubMed] [Google Scholar]
- 6.Reis SE, Gloth ST, Blumenthal RS, Resar JR, Zacur HA, Gerstenblith G, Brinker JA. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- 7.Alpaslan M, Shimokawa H, Kuroiwa-Matsumoto M, Harasawa Y, Takeshita A. Short-term estrogen administration ameliorates dobutamine-induced myocardial ischemia in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1997;30:1466–71. doi: 10.1016/s0735-1097(97)00346-x. [DOI] [PubMed] [Google Scholar]
- 8.Rosano GM, Caixeta AM, Chierchia S, Arie S, Lopez-Hidalgo M, Pereira WI, Leonardo F, Webb CM, Pileggi F, Collins P. Short-term anti-ischemic effect of 17beta-estradiol in postmenopausal women with coronary artery disease. Circulation. 1997;96:2837–41. doi: 10.1161/01.cir.96.9.2837. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal RS, Heldman AW, Brinker JA, Resar JR, Coombs VJ, Gloth ST, Gerstenblith G, Reis SE. Acute effects of conjugated estrogens on coronary blood flow response to acetylcholine in men. Am J Cardiol. 1997;80:1021–4. doi: 10.1016/s0002-9149(97)00596-1. [DOI] [PubMed] [Google Scholar]
- 10.White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- 11.Hodis HN, Mack WJ, Lobo R. Postmenopausal hormone replacement therapy as antiatherosclerotic therapy. Curr Atheroscler Rep. 2002;4:52–8. doi: 10.1007/s11883-002-0062-y. [DOI] [PubMed] [Google Scholar]
- 12.Harman SM. Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS. Gend Med. 2006;3:254–69. doi: 10.1016/s1550-8579(06)80214-7. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson JC. Hormone replacement therapy and cardiovascular disease revisited. Menopause Int. 2009;15:55–7. doi: 10.1258/mi.2009.009018. [DOI] [PubMed] [Google Scholar]
- 15.White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1468–75. doi: 10.1152/ajpheart.01173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosano GM, Sarrel PM, Poole-Wilson PA, Collins P. Beneficial effect of oestrogen on exercise-induced myocardial ischaemia in women with coronary artery disease. Lancet. 1993;342:133–6. doi: 10.1016/0140-6736(93)91343-k. [DOI] [PubMed] [Google Scholar]
- 17.Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–42. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 18.Chester AH, Jiang C, Borland JA, Yacoub MH, Collins P. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron Artery Dis. 1995;6:417–22. doi: 10.1097/00019501-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 19.White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–61. doi: 10.1016/s0008-6363(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 20.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–42. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272:H2765–73. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 22.White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Sneed C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–61. doi: 10.1016/s0008-6363(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 23.Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol. 2007;293:H314–21. doi: 10.1152/ajpheart.01342.2006. [DOI] [PubMed] [Google Scholar]
- 24.Frishman WH, Helisch A, Naseer N, Lyons J, Hays RM. Nitric oxide donor drugs in the treatment of cardiovascular disease. In: Frishman WH, Sonnenblick EH, Sica DA, editors. Cardiovascular Pharmacotherapeutics. McGraw-Hill; New York: 2003. [Google Scholar]
- 25.Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci U S A. 1992;89:11259–63. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. Faseb J. 1993;7:566–71. [PubMed] [Google Scholar]
- 27.Kharitonov SA, Logan-Sinclair RB, Busset CM, Shinebourne EA. Peak expiratory nitric oxide differences in men and women: relation to the menstrual cycle. Br Heart J. 1994;72:243–5. doi: 10.1136/hrt.72.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 30.Gorren AC, Mayer B. The versatile and complex enzymology of nitric oxide synthase. Biochemistry (Mosc) 1998;63:734–43. [PubMed] [Google Scholar]
- 31.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 32.Reckelhoff JF, Kellum JA, Jr, Racusen LC, Hildebrandt DA. Long-term dietary supplementation with L-arginine prevents age-related reduction in renal function. Am J Physiol. 1997;272:R1768–74. doi: 10.1152/ajpregu.1997.272.6.R1768. [DOI] [PubMed] [Google Scholar]
- 33.Yu BP, Chung HY. Oxidative stress and vascular aging. Diabetes Res Clin Pract. 2001;54(Suppl 2):S73–80. doi: 10.1016/s0168-8227(01)00338-2. [DOI] [PubMed] [Google Scholar]
- 34.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–6. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 35.Chen EY, Kallwitz E, Leff SE, Cochran EJ, Mufson EJ, Kordower JH, Mandel RJ. Age-related decreases in GTP-cyclohydrolase-I immunoreactive neurons in the monkey and human substantia nigra. J Comp Neurol. 2000;426:534–48. [PubMed] [Google Scholar]
- 36.Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–80. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 37.Vasquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol. 2002;22:1655–61. doi: 10.1161/01.atv.0000029122.79665.d9. [DOI] [PubMed] [Google Scholar]
- 38.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999;84:1285–91. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 39.Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, Speizer FE, Nathan DM, Manson JE. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161:1717–23. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 41.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. Jama. 1999;281:1291–7. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 42.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. Bmj. 2006;332:73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuanetti G, Latini R, Maggioni AP, Santoro L, Franzosi MG. Influence of diabetes on mortality in acute myocardial infarction: data from the GISSI-2 study. J Am Coll Cardiol. 1993;22:1788–94. doi: 10.1016/0735-1097(93)90758-s. [DOI] [PubMed] [Google Scholar]
- 44.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes. 2001;50:1654–65. doi: 10.2337/diabetes.50.7.1654. [DOI] [PubMed] [Google Scholar]