Abstract
The omentum, an important peritoneal tissue, is studded with a high number of immune aggregates, or “milky spots,” the number, function, and phenotype of which is largely unknown. We have analyzed the immune composition on the normal omentum and also have shown that both free immune cells and tumor cells in the peritoneal fluid bind preferentially to these immune aggregates. This binding may be mediated by the network of collagen I fibers, which overlay these areas. In addition, we have shown that not only do omental vessels express vascular endothelial growth factor receptor 3 (VEGFR3), a receptor that is only found on angiogenic blood vessels, but that tumor cells co-localize with these vessels, possibly increasing the ability of tumor to induce neovascularization and therefore thrive.
Keywords: Omentum, Tumor, Peritoneal cavity, Metastasis, Angiogenesis
Introduction
Ovarian cancer is the most common cause of death from gynecological cancers with an estimated 21,650 deaths expected in 2008 [1] primarily because the disease has reached an advanced stage before it is discovered [2]. Dissemination from the primary tumor occurs mainly by exfoliation of cells through the surface of the ovarian capsule, which are then spread by the circulation of the peritoneal fluid. Although tumor cells may attach to any intraperitoneal surface, the omentum appears to be a selective site of attachment of the initial disseminated cells and aggressive tumor growth [3] and subsequent seeding to the rest of the peritoneal cavity. The omentum, a sheath of protective tissue overlying the stomach and pancreas [4], is composed of well-vascularized adipose tissue embedded with clusters of immune cells originally termed “milky spots”. These immune aggregates were first described in rabbits by Ranvier [5], and have since been shown to exist in other mammalian species [6]. This tissue is thought to serve as a cushion and acts to filter peritoneal fluid providing protection from infection. In initial experiments using mouse tumor cell lines injected intraperitoneally, we have found that multiple cell lines, including five mouse lines and one human ovarian line, bound preferentially to these immune cell clusters [5]. Given that tumor cells appear to preferentially localize to these immune aggregates on the omentum, it is important to understand their cellular composition, structure, and function. In our studies, we have used a recently developed mouse ovarian tumor model called ID8 [7] and a whole mount fluorescence microscopy technique [8] to study the metastasis of tumor cells within the peritoneal cavity. These studies indicate that attachment of tumor cells to these immune aggregates is promoted by the normal physiological function of these sites, and that the aggressive growth of the peritoneal metastases may be due to the unique, proangiogenic vasculature present at these sites.
Immune cell composition and phenotype of immune aggregates on the naïve omentum
Due to the ease of injections and the ability to induce potent immune responses, the peritoneal cavity is often used as a site for immunizations, although the cell composition of this space differs considerably from that of the spleen, lymph nodes, or peripheral blood, sites most often analyzed for immune responses. The omentum is one tissue that is thought to provide the peritoneal cavity with immune cells. Some reports have likened the omentum to an “intestinal thymus” [9]. For example, the majority of B cells in the peritoneal cavity are a distinct lineage of cells known as B1 lymphocytes that have a limited receptor repertoire and respond primarily to carbohydrate antigens and self antigens [10]. Interestingly, the omentum has been shown to be a site in which these B1 lymphocytes are generated [11]. Other immune populations exist in the peritoneal cavity such as macrophages, T cells, NK cells, and DC, however, the presence of these subsets on the omentum has not been fully characterized. We have used flow cytometry to examine the cellular composition of the mouse omentum and compared it to that of the peritoneal cavity. The peritoneal cavity immune cells were removed from normal C57BL/6 mice by rinsing the cavity with 3 ml of PBS. The omentum was removed, and immune aggregate cells were isolated by mincing the tissue in 2 ml of 0.2% collagenase and rotating at 37°C for 45 min. The dissociated omental cells were stained with anti-CD45 to identify the hematopoietic cells, and with antibodies specific for additional markers including CD8 or CD4 (T cell subsets), CD3 and γδ TCR (γδ T cells), CD3 and NK1.1 (NKT cells), CD19 (B cells), CD19 and CD11b (B1 cells), NK1.1 (NK cells), GR-1 (granulocytes), CD11c (DC), and F4/80 (monocyte/macrophages). Results are given in Table 1 as a percentage of each subset out of the total CD45+ population. The different subsets of immune cells, which contain both innate and adaptive immune populations, were similar between the omentum and the peritoneal lavage, although the omentum contained significantly more CD4+ T cells. We next examined the activation phenotype of CD4+ and CD8+ T cells for the omentum and other immune sites. The omentum contained a high percentage of activated (CD44+, CD62L− and CD44+, CD62L+) CD4+/CD8+ T cells when compared to the other sites such as the spleen and lymph nodes (Table 2). Interestingly, the omental T cells seem to more closely resemble cells from the intestinal lamina propria, which are known to contain activated lymphocytes [12, 13]. This may be indicative of a constitutive role of these cells in dealing with bacteria.
Table 1.
Percentages of immune subsets within the naïve omentum and peritoneal lavage
| T cells (CD8+) |
T cells (CD4+) |
γδ T cells (γδTCR+) |
NKT cells (NK1.1+, CD3+) |
B1 cells (CD19+, CD11b+) |
|
|---|---|---|---|---|---|
| Omentum | 4 ± 0.3 | 13 ± 1 | 2.5 ± 0.4 | 1.6 ± 0.6 | 21 ± 2.6 |
| Peritoneal lavage | 2 ± 0.7 | 5 ± 0.6 | 0.6 ± 0.2 | 0.5 ± 0.2 | 30 ± 1.9 |
|
| |||||
| B2 cells (CD19+, CD11b−) |
NK cells (NK1.1+) |
Dendritic cells (CD11c+) |
Granulocytes (GR-1+, F4/80−) |
Macrophages (F4/80+, CD11b+) |
|
|
| |||||
| Omentum | 25 ± 2.8 | 5 ± 0.5 | 4 ± 0.3 | 0.5 ± 0.04 | 14 ± 2.6 |
| Peritoneal lavage | 17 ± 2.7 | 2 ± 0.3 | 3 ± 0.1 | 2 ± 0.1 | 34 ± 2.4 |
Table 2.
T cell activation surface marker phenotype within the naïve omentum, peritoneal lavage, lymph node, and spleen
| CD8+ T cells | |||||
|---|---|---|---|---|---|
| Activated |
Naïve |
||||
| CD44+ CD62L− | CD44+ CD62L+ | CD44− CD62L+ | VLA-4lo LFA1lo | VLA-4hi LFA1hi | |
| Inguinal LN | 3.5 ± 0.1 | 11.4 ± 1.4 | 74 ± 1.2 | 98.6 ± 0.4 | 1.3 ± 0.4 |
| Spleen | 6 ± 0.6 | 15.9 ± 1.0 | 73.4 ± 1.1 | 93.4 ± 1.0 | 6.4 ± 1.0 |
| Omentum | 13.8 ± 21.3 | 34.6 ± 3.9 | 49 ± 3.2 | 63.9 ± 4.3 | 35.8 ± 4.4 |
| Peritoneal lavage | 28.4 ± 3.9 | 34.5 ± 7.5 | 23.6 ± 1.7 | 50.8 ± 8.1 | 48.8 ± 8.3 |
| CD4+ T cells | |||||
| Activated |
Naïve |
||||
| CD44+ CD62L− | CD44+ CD62L+ | CD44− CD62L+ | VLA-4lo LFA1lo | VLA-4hi LFA1hi | |
|
| |||||
| Inguinal LN | 9.6 ± 0.5 | 7.6 ± 1.8 | 71 ± 2.1 | 96.4 ± 0.8 | 3.53 ± 0.9 |
| Spleen | 21.4 ± 2.5 | 7.86 ± 2.1 | 63 ± 3.8 | 85 ± 0.9 | 14 ± 0.6 |
| Omentum | 27.4 ± 4.3 | 19 ± 5.0 | 50.9 ± 3.0 | 54 ± 4.1 | 45.3 ± 4.2 |
| Peritoneal lavage | 46.3 ± 1.9 | 15 ± 1.7 | 26.5 ± 1.6 | 48.7 ± 8.6 | 51.1 ± 8.7 |
Tumor cells and peritoneal lymphocytes bind preferentially to omental immune aggregates
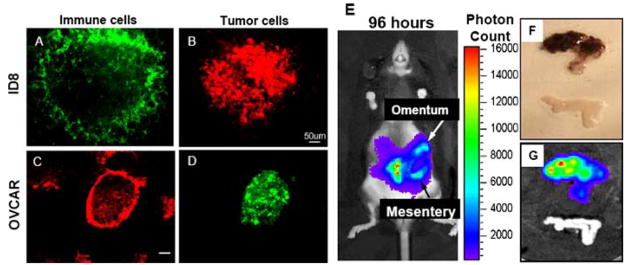
Previously, our lab and others [4] have shown that intraperitoneal injection of B16 melanoma tumor cells resulted in macroscopic foci of black-pigmented tumor cells on the omentum. Upon closer microscopic analysis using the whole mount technique and GFP expressing B16 tumor cells injected i.p., we found that the tumor foci were localized to distinct areas on the omentum, which coincided with immune cells. Five additional GFP+ or mCherry+ [14] tumor cell lines including ID8, an ovarian line, bound preferentially to these areas (Fig. 1a, b). Interestingly, a human ovarian tumor cell line (OVCAR-3), labeled with the fluorescent marker CFSE, also selectively bound to these same areas of the mouse omentum (Fig. 1c, d). This result suggests that direct peritoneal metastatic seeding is similar in a xenograft system. Next, we monitored tumor burden over time within the same mouse by injecting B16 cells transfected with luciferase i.p. Following infusion with luciferin and using an in vivo imaging system (IVIS), the photon count, which represents tumor burden, was determined at both 24 h (data not shown) and 96 h (Fig. 1e). At 96 h, the tumor-bearing omentum, along with a control omentum from a naïve mouse, was excised and digital images obtained (Fig. 1f, g). Even through the pigmented skin of a C57BL/6 mouse, light from the tumor growing on the omental tissue is clearly visible in an intact mouse. These data demonstrate the possibility of monitoring the presence and subsequent growth of peritoneal metastases on the omentum, which can be utilized to examine potential tumor immunotherapy.
Fig. 1.
Both mouse and human ovarian tumor cells bind selectively to the immune aggregates of the omentum and the IVIS system can be utilized to visualize growth of tumors on the omentum. 2 × 105 mouse ID8/Cherry (a, b) or CFSE-labeled human OVCAR-3 (b, c) tumor cells were injected i.p. into C57BL/6 mice. The omentum was removed 6 h later and stained for CD45 to label immune cells (a, c). For each tumor, images from the same field illustrate an immune aggregate (a or c) that contains numerous ID8/Cherry (b) or CFSE-labeled OVCAR-3 (d) tumor cells. Interestingly, these tumor cells were not found elsewhere on the omentum. Bars represent 50 μm (a, b) and 100 μm (c, d). 8 × 105 B16/Luciferase cells were injected i.p. The same mouse was then monitored for tumor growth at either 24 (data not shown), or 96 h (e) later using the IVIS system. After this timepoint, the omentum with tumor was removed along with an omentum from an uninjected mouse (f), and again analyzed using the IVIS system (g). Clearly, the tumor grows initially on the omentum and mesentery as illustrated in (g), and tumor growth can be monitored over time based on emitted light intensity
The omentum, like the rest of the peritoneum, is reported to be covered by a layer of mesothelial cells [15]. We have been able to identify these cells using whole mount by staining for the adhesion molecule, VCAM-1 (CD106) [4], and these VCAM-1+ cells are selectively found over the immune aggregates. Therefore, either the mesothelial cells do not cover the entire omentum, or alternatively, the mesothelial cells on the immune aggregates express much higher levels of VCAM-1. Either of these possibilities makes these mesothelial cells strong candidates for initiating the selective adhesion of tumor cells on the immune aggregates. Indeed, confocal microscopy studies have revealed that the tumor cells initially bind to these outer mesothelial cells (data not shown).
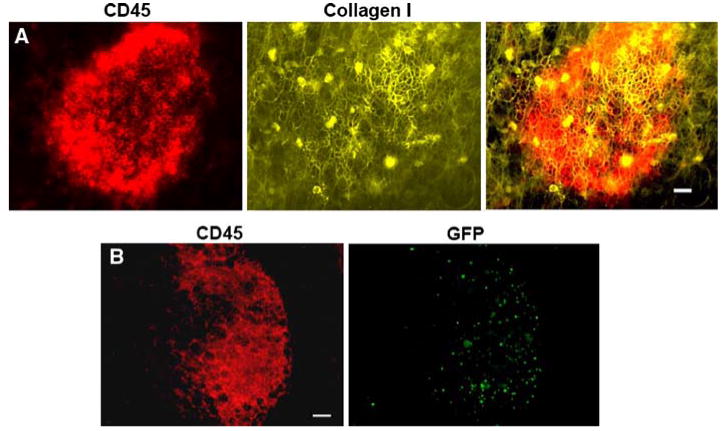
We have identified another unique feature of the immune aggregates, which is the presence of an intricate extracellular matrix that appears to be absent from the surrounding areas of the omentum. The whole mount technique provides an excellent visualization of this as shown in Fig. 2a, which depicts an omental immune aggregate stained for collagen I. This extracellular matrix also expresses collagen III (data not shown) and is only seen in this dense configuration within the immune aggregates. Since we have observed openings in the overlying layer of mesothelial cells, this matrix is probably accessible to metastatic tumor cells, and thus could also be involved in the attachment process.
Fig. 2.
GFP splenocytes home to immune aggregates, which possess a collagen matrix overlay. Omenta were removed and stained with anti-CD45 to label immune cells (red) and with anti-collagen type I (yellow). Overlaid images from the same field of view illustrate an immune aggregate and the co-localization of collagen I, which directly overlays on the immune aggregate. The bar represents 25 μm (a). 6 × 105 GFP+ splenocytes obtained from a β-actin GFP mouse were injected i.p. into syngenic C57BL/6 mice. The omentum was removed 1 h after injection and stained for CD45 to label immune cells. Images from the same field illustrate a CD45+ immune aggregate (red) that contains numerous transferred GFP+ splenocytes (green). Interestingly, these splenocytes were not found elsewhere on the omentum. Bar represents 50 μm (b)
The ability of the cells within these aggregates to generate adaptive immunity to i.p. antigens has not received extensive study, and there is very little information available on whether or not these immune cells respond to the presence of metastatic tumor cells. However, there is evidence that some ovarian cancer patients have T cells present in the peritoneal cavity that are capable of reacting with tumor cells [16, 17]. This raises the possibility that the preferential homing of tumor-reactive cells to the immune aggregates on the omentum and other peritoneal tissues might provide an advantage for adoptive immunotherapy of metastatic disease. We have found that lymphocytes injected into the peritoneal cavity, like tumor cells, also preferentially localize to the immune aggregates (Fig. 2b). In addition, the percentage of T cells found in the peritoneal lavage and on the omentum that expresses the integrin adhesion molecules VLA-4 (α4β1) and LFA-1 (αLβ2), which bind to VCAM-1 and ICAM-1, respectively, is much larger than in lymphoid organs (Table 2). Therefore, VLA-4 and LFA-1 expressing immune cells may preferentially home to omental immune aggregates due to the presence of VCAM-1+/ICAM-1+ mesothelial cells covering the immune aggregates.
Specific immunization strategies targeting omental immune aggregates elicit potent immune responses
We have shown that the omentum is the primary site of free tumor attachment within the peritoneal cavity and that the omental immune aggregates contain both adaptive and innate immune cells. Therefore, it may be possible to induce an immune response to a tumor-associated antigen. To test this, we used an ID8 cell line transfected with ovalbumin (Ova) as a model tumor-associated antigen and quantified the peptide-specific response using an IFN-γ ELISpot assay. Mice were primed i.p. with 5 × 106 irradiated ID8/Ova tumor cells and 6.5 weeks later spleen, omentum, and peritoneal lavage cells were collected and assayed for their ability to produce IFN-γ in response to Ova peptides. Following immunization with irradiated tumor cells, specific spots were undetectable in the spleens and only a minimal number were recovered from the peritoneal cavity (Table 3). A similar result was obtained when mice were injected with viable ID8/Ova and the tumor allowed to grow for 2 weeks before collection and assay of the cells (data not shown). It is possible that this result might be because the i.p. route is a poor route of delivery, so we tested the ability to prime with antigen delivered in other forms into the peritoneal cavity. As clearly shown in Table 3 below, this route of delivery is very effective in generating Ova-specific responses when the antigen is conjugated to beads or pulsed on DC. In fact, omental cells were superior in generating an immune response when compared to spleen or peritoneal lavage. The ineffectiveness of tumor cells to generate immune responses may be a result of immunosuppressive effects inherent to tumor cells or the amount of antigen delivered. Nonetheless, our data indicates that the omentum can respond strongly to antigen, suggesting that alternative vaccine strategies, such an alteration of the mode and location of antigen delivery, may be more effective than others in generating potent immune responses.
Table 3.
C57BL/6 mice were immunized i.p. with tosyl activated beads alone, or beads coated with 230 μg Ova/108 beads or with 5 × 105 bone marrow derived DC pulsed with Ova peptides, and 5 days later the organs removed, dissociated and the cells cultured ± Ova peptide overnight and the ELISpot assay developed
| Immunization | Spleen cells | Omental cells | Peritoneal lavage cells |
|---|---|---|---|
| Beads alone | 0 | 0 | 10 |
| Beads + Ova | 43 | >700 | 253 |
| Ova peptide pulsed BMDC | 231 | 208 | 468 |
| ID8/Ova | 0 | 10 |
In the case of ID8/Ova, 5 × 106 ID8/Ova were injected i.p. and 6.5 weeks later, peritoneal lavage and omentum dissociated and pooled and cultured with tumor lysate overnight and the Elispot assay developed. Results are given as the number of Ova specific IFN-γ ELISpots/1.25 × 105 cells
Omental vessels are proangiogenic in both naïve and tumor-bearing mice
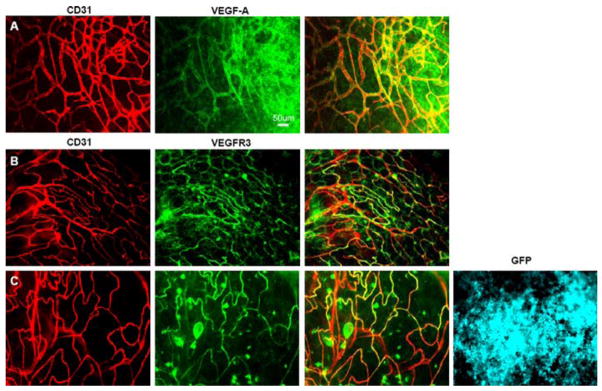
We have previously shown that the presence of tumor cells on the omentum alters the normal architecture of the omentum by disrupting the immune aggregates and inducing vascular morphological alteration indicative of angiogenesis [4]. Some of these changes may be a result of tumor-secreted factors. Indeed, message for several angiogenic growth factors including VEGF-A and VEGF-C were detected in ID8 tumor cells in vitro (Table 4). We next used whole mount microscopy to determine if VEGF-A, which is predominantly secreted as a soluble molecule, but can be detected bound to the ECM, could be visualized in tumor-bearing omental immune aggregates. Although not detected visually on naïve omenta (data not shown), VEGF-A was seen perivascularly within the immune aggregates when tumor was present (Fig. 3a).
Table 4.
Presence in ID8 cells of mRNA for factors involved in angiogenesis

|
Using RT-PCR mRNA was obtained from ID8 cells grown in vitro. Negative controls done in the absence of RT had no bands
Fig. 3.
Omental vessels from tumor-bearing mice show VEGF-A and express VEGFR3. Naïve omenta were removed and stained with anti-CD31 (red) to label vessels and either VEGF-A (green) (a) or VEGFR3 (green) (b). The overlay of the images is shown in yellow (a, b). 2 × 105 B16/GFP tumor cells were injected i.p. into C57BL/6 mice. The omentum was removed 6 days later, stained and whole mount performed. All images are from the same field of an immune aggregate stained for anti-CD31 to label vessels (red) and VEGFR3 (green) or showing the B16/GFP tumor cells (blue) (c). Bars represent 50 μm
Our lab has previously reported that VEGFR3 is induced on tumor vasculature and may be an indicator of adult angiogenesis [18]. Although VEGFR3 is positive on both blood and lymphatic vessels in utero, it is downregulated on blood vessels after birth but can be upregulated in the rare cases of adult angiogenesis, such as chronic wound healing and tumor growth [19–21]. Surprisingly, naïve omental vessels located within the immune aggregates were found to be positive for VEGFR3, while negative for the lymphatic marker LYVE-1 (Fig. 3b), further reinforcing the idea that these vessels are continuously undergoing angiogenesis. This data reinforces our previous findings that vessels within immune aggregates are constitutively high for the proangiogenic marker CD105 [4]. As expected, omental vessels from mice injected with B16/GFP i.p. are also VEGFR3 positive (Fig. 3c).
Concluding remarks
The peritoneal cavity is a unique immunological microenvironment. In addition to lymphocytes, we have shown that tumor cells injected into the peritoneal cavity preferentially bind to and grow on the immune aggregates of the omentum. This binding may be a result of the presence of VCAM-1+ mesothelial cells or the collagen network that overlays the immune aggregates, whereas tumor growth may be accelerated due to constitutively angiogenic vessels within the aggregates. Ovarian cancer, which is associated with this type of intraperitoneal metastatic spread, is the most lethal type of gynecologic malignancy due to its asymptomatic nature at early stages and the lack of effective treatments for the advanced stage disease. Using a recently derived ovarian cancer cell line, ID8, we have been able to monitor tumor growth over time both ex vivo and in vivo. In addition, using a cell line, which expresses ovalbumin as a model antigen, we have shown that the type of intraperitoneal immunization given can affect the immune response to that antigen. Intraperitoneal administration of antigen-coated beads stimulated a strong response by omental cells. However, the local and systemic response to antigen produced by viable tumor cells was found to be low, either because of suppressive factors produced by the tumor cells or limited availability of antigen. Elucidating the mechanisms of both the immune response of the immune aggregates, as well as tumor binding to the omentum may enhance our ability to prevent peritoneal metastasis and induce a clinical immune response to ovarian cancer and other peritoneal malignancies.
References
- 1.Society AC: Cancer Facts & Figs. 2008 Available at: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
- 2.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, et al. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–74. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 4.Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–52. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranvier H. Du developpement t de l’accroissement des vaisseaux sanguins. Arch Physiol. 1874;1:429. [Google Scholar]
- 6.Shimotsuma M, Shields JW, Simpson-Morgan MW, Sakuyama A, Shirasu M, Hagiwara A, et al. Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology. 1993;26:90–101. [PubMed] [Google Scholar]
- 7.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–91. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 8.Gerber SA, Moran JP, Frelinger JG, Frelinger JA, Fenton BM, Lord EM. Mechanism of IL-12 mediated alterations in tumour blood vessel morphology: analysis using whole-tissue mounts. Br J Cancer. 2003;88:1453–61. doi: 10.1038/sj.bjc.6600907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koten JW, den Otter W. Are omental milky spots an intestinal thymus? Lancet. 1991;338:1189–90. doi: 10.1016/0140-6736(91)92043-2. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Honjo T. B-1 cells and autoimmunity. Ann NY Acad Sci. 1995;764:402–9. [PubMed] [Google Scholar]
- 11.Kearney JF, Bartels J, Hamilton AM, Lehuen A, Solvason N, Vakil M. Development and function of the early B cell repertoire. Int Rev Immunol. 1992;8:247–57. doi: 10.3109/08830189209055577. [DOI] [PubMed] [Google Scholar]
- 12.Resendizz-Albor AA, Esquivel R, Lopez-Revilla R, Verdin L, Moreno-Fierros L. Striding phenotypic and functional differences in lamina propria lymphocytes from the large and small intestine of mice. Life Sci. 2005;76:2783–803. doi: 10.1016/j.lfs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Seibold F, Seibold-Schmid B, Cong Y, Shu FY, McCabe R, Weaver C, et al. Regional differences in L-selectin expression in murine intestinal lymphocytes. Gastroenterology. 1998;114:965–74. doi: 10.1016/s0016-5085(98)70316-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Jackson WC, Steinbach PA, Tsien RY. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc Natl Acad Sci USA. 2004;101:16745–9. doi: 10.1073/pnas.0407752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beelen RH. The greater omentum: physiology and immunological concepts. Neth J Surg. 1991;43:145–9. [PubMed] [Google Scholar]
- 16.Freedman RS, Tomasovic B, Templin S, Atkinson EN, Kudelka A, Edwards CL, et al. Large-scale expansion in interleukin-2 of tumor-infiltrating lymphocytes from patients with ovarian carcinoma for adoptive immunotherapy. J Immunol Methods. 1994;167:145–60. doi: 10.1016/0022-1759(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 17.Ioannides CG, Platsoucas CD, Rashed S, Wharton JT, Edwards CL, Freedman RS. Tumor cytolysis by lymphocytes infiltrating ovarian malignant ascites. Cancer Res. 1991;51:4257–65. [PubMed] [Google Scholar]
- 18.Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000;156:1499–504. doi: 10.1016/S0002-9440(10)65021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–12. [PubMed] [Google Scholar]
- 20.Clarijs R, Schalkwijk L, Hofmann UB, Ruiter DJ, de Waal RM. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res. 2002;62:7059–65. [PubMed] [Google Scholar]
- 21.Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201:93–9. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]