Abstract
Synechococcus sp. IU 625 is one of the freshwater cyanobacteria responsible for harmful algal blooms (HAB). Cyanophages can serve as natural control agents and may be responsible for algal bloom prevention and disappearance. Cyanophage AS-1, which infects Synechococcus sp. IU 625 (Anacystis nidulans) and Synechococcus cedrorum, plays an important role in the environment, significantly altering the numbers of its hosts. Since seasonal (temperature-dependent) lytic induction of cyanobacterial prophage has been proposed to affect seawater algal blooms, we investigated if the AS-1 lytic cycle could be induced by a shift to high temperature. Our hypothesis was confirmed, as more phages were released at 35°C than at 24°C, with maximal induction observed with a shift from 24°C to 35°C. Furthermore, transmission electron microscopy (TEM) images provide direct evidence of lysogenic to lytic conversion with temperature shift. Thus, temperature is an important inducer for AS-1 conversion from lysogenic to lytic cycle and could have applications in terms of modulating cyanobacterial populations in freshwater aquatic environments. The study gives insight into the effect of climate change on the interaction between cyanophage and cyanobacteria in freshwater ecosystems.
Keywords: Cyanophage AS-1, Synechococcus, cyanobacteria, temperature, prophage induction
Introduction
Dense harmful algal blooms of cyanobacteria (formerly known as blue-green algae) in freshwater can affect human and animal health due to acute intoxicants or allergic reactions (Carmichael, 1991, 1992; Carmichael and Li, 2006; Carmichael and Schwartz, 1984; Codd et al., 1989). They also have a huge impact on aquatic biodiversity caused by oxygen depletion that threatens aquatic ecosystems. Algal blooms are likely to become an even greater concern due to the increased rainfall and water temperatures associated with global climate change as evidenced by the increased frequency of cyanobacterial blooms in Europe, China, Korea, Japan, and the United States of America (Babin et al., 2005; Fastner et al., 1999; Hunter, 2003; Skulberg et al., 1984).
Run-off of fertilizer into freshwater lakes can lead to their eutrophication (Slonczewski and Foster, 2008). In this process, nitrogen in fertilizer leads to a burst of cyanobacterial growth, which initially increases oxygen levels in the water. After the algal bloom depletes nitrogen, cyanobacteria die and sink to deeper levels of the lake, where heterotrophic bacteria metabolize organic material from the decomposing cyanobacteria, multiplying to high numbers and depleting oxygen levels. Low levels of oxygen in the lower levels of the lake results in greatly decreased numbers of aquatic life. Hence, lake eutrophication is a major problem in the agricultural regions of developed nations, such as the Midwestern United States.
Synechococcus sp. IU 625 is one of the freshwater cyanobacteria that are responsible for harmful algal blooms (Beardall, 2008). It is a prokaryotic unicellular rod-shaped cyanobacterium with a Gram-negative bacterial cell wall structure. It is a non-motile, obligate photoautotroph with a low salt tolerance. Synechococcus sp. IU 625 can serve as an environmental pollution indicator because of its simplicity, size, ease of growth and heavy metal response. Many heavy metal studies have been done using Synechococcus sp. IU 625 as a model system to study the response of organisms to heavy metal stresses (Lee et al., 1992a; Lee and Lustigman, 1996; Lee et al., 1992b; Lee et al., 1991; Lee et al., 1994; Lee et al., 1993; Lee et al., 1999; Lee et al., 2002; Lustigman et al., 2000).
Cyanophages are viruses that infect cyanobacteria and can be found in most marine and freshwater ecosystems. They play important roles in the environment to regulate cyanobacterial populations. Cyanophages serve as natural control agents for cyanobacterial populations and may be responsible for algal bloom prevention and disappearance. In fact, cyanophages were responsible for the vanishing of algal blooms in the Gulf of Mexico, Tampa Bay (Florida), Georgia coastal rivers, and Rhode Island’s coastal waters (Lu et al., 2001; McDaniel et al., 2002; Okunishi et al., 2003). Viruses can affect microbial populations by either going through a lytic cycle, causing destruction of the host cell, or a lysogenic stage, in which the viral genome is inserted and maintained as prophage within its host cell (Waterbury and Valois, 1993). Seasonal changes in temperature could cause prophage to enter the lytic cycle thus leading the disappearance of algal blooms (Paul and Jiang, 2001; Williamson et al., 2002; Wilson et al., 1993). Lysogens can also be induced to the lytic cycle by pollutants and other factors (Lee et al., 2006; Ortmann et al., 2002). For freshwater ecosystems, very few studies have been done on the prophage induction from a lysogenic stage to a lytic cycle.
Cyanophage AS-1 was named after its two hosts Anacystis nidulans (now named Synechococcus sp. IU 625) and Synechococcus cedrorum. The structure of AS-1 resembles the T-even bacteriophage that replicate in E. coli. AS-1 has a proteinaceous icosahedral head of 90 nm diameter and a long contractile tail (22 nm × 244 nm) (Safferman et al., 1972). Bisen et al suggested the possibility of temperate AS-1 lysogeny in Synechococcus sp. IU 625 in 1985 (Bisen et al., 1985), but no direct evidence was provided. In a recent study, copper was found to significantly increase lytic induction of AS-1 prophage (Lee et al., 2006).
Here, we report that lytic induction of AS-1 prophage from Synechococcus sp. IU 625 can be induced by high temperature, as confirmed with both plaque forming units (PFU) measurements and transmission electron microscopy (TEM) images. This study gives insight into the interaction between cyanophages and cyanobacteria with climate change in the ecosystem.
Material and methods
Culture, maintenance, and growth of Synechococcus sp. IU 625 (SYN-N), lysogenic Synechococcus sp. IU 625 (SYN-L), and Cyanophage AS-1
Cyanobacterium Synechococcus sp. IU 625 (SYN-N) was obtained from Dr. Roy McGowan at Brooklyn College, City University of New York. The lysogenic Synechococcus sp. IU 625 (SYN-L) was isolated, cultured and maintained at Montclair State University. The cultures were inoculated aseptically in Mauro’s modified medium (3M medium) at pH 7.9 and grown at ambient temperature (24°C) with constant fluorescent light and continuous agitation at 100 rpm in an Innova™ 4340 incubator shaker (New Brunswick Scientific, Edison, NJ). The growth of the cells was monitored by direct cell count using hemacytometers and by turbidity study using a Spectronic GENESYS 20 at OD750nm. The purity of cultures was checked periodically by plating on nutrient agar plates. The stock cultures were maintained on 3M agar plates and/or slants (Lee et al., 2006).
The growth of lysogenic SYN-L at 24°C, 35°C, and temperature switch from 24°C to 35°C
Three sets of 100 ml of SYN-L cultures were inoculated into 250 ml flasks with OD750nm at approximately 0.5. Two sets of the cultures were grown at 24°C and 35°C respectively. A third set of cultures was grown at 24°C for 7 days, and on day 8 was switched to 35°C for continued growth. The cell count, turbidity and viral plating count for cultures from 24°C and 35°C were monitored on days 1, 4, 8, 11, 15, and 18. For the temperature switched cultures, the same parameters were also monitored on days 1, 4, 8 (day 1 of switch), 11 (day 4 of switch), and 15 (day 8 of switch) respectively.
Culture, maintenance, and titering of Cyanophage AS-1
AS-1 was cultured aseptically and fed with exponentially growing Synechococcus sp. IU 625 (SYN-N). The infected cultures were then incubated at room temperature under a continuous “cool-white” fluorescent light. The plaque plating method was used to monitor the released AS-1 as described by (Lee et al., 2006). Ten ml SYN-N culture was centrifuged at 5,000 rpm for 10 minutes and the cell pellet was collected. At different time points, the 2.5 ml of SYN-L, grown under various conditions, was added into the cell pellet and mixed well. One ml of pre-warmed (liquid) 1% soft 3M agar was added into the mixture and was vortexed. The mixture was then poured onto a 3M agar plate. After the soft top agar solidified, the plate was placed under continuous “cool-white” fluorescent light for 5–7 days until the plaques (clear zones) were formed, and counted (Lee et al., 2006). Although the optical densities on days 4, 8, and 11 were similar, cell size can vary with temperature (data not shown). Therefore, release of efficiency (RE) and percentage of increase of release (PIR) were calculated (see below) which take into account the PFU released per normalized CFU of 1 × 107. PFU, RE and PIR were determined by using the following formulas:
T-tests were used to determine of significance of differences between conditions for PFU/ml, RE, and PIR on day 8; p<0.05 was accepted as significant.
Morphological study of temperature switched SYN-L by transmission electron microscopy (TEM)
One ml of temperature switched SYN-L culture was collected at different time intervals (4, 8, 12 and 24 hours) after shift from 24°C to 35°C. The samples were spun down by centrifugation at 14,000 rpm for 5 minutes and the samples were re-suspended in 5% glutaraldehyde for 1 hour to process the primary fixation of cells. The cells were then pelleted by centrifuging the cells at 14,000 rpm and then re-suspended in 50 μl of 4% sodium cacodylate agarose (2 grams of agarose dissolved in 50-mL of 0.1 M sodium cacodylate buffer). After it solidified, the agarose containing the cells was dehydrated by incubating the samples in the processing machine under the following conditions: 1) 0.1 M sodium cacodylate buffer for 10 minutes; 2) 0.1 M sodium cacodylate buffer for 10 minutes/0.1 M sodium cacodylate buffer for 10 minutes; 3) 2% osmium tetroxide (in 0.2 M sodium cacodylate buffer) for 120 minutes; 4) 0.1 M sodium cacodylate buffer for 10 minutes; 5) 0.1 M sodium cacodylate buffer for 10 minutes; 6) 8 % tannic acid (0.05 M sodium cacodylate buffer) for 20 minutes. 7) 0.1 M sodium cacodylate buffer wash for 15 minutes; 8) 0.1 M sodium cacodylate buffer wash for 10 minutes; 9) 50% ethanol for 10 minutes; 10) 70% ethanol for 10 minutes; 11) 95% ethanol for 10 minutes; 12) 95% ethanol for 10 minutes; 13) 100% ethanol for 10 minutes; 14) 100% ethanol for 10 minutes; 15) 50% ethanol/50% propylene oxide mixture for 10 minutes; 16) 100% propylene oxide for 10 minutes; 17) 100% propylene oxide for 10 minutes; 18) 2 : 1 propylene oxide : Epon mixture for 3 hours; 19) 1:1 propylene oxide : Epon mixture for 6 hours; 20) 1 : 2 propylene oxide : Epon mixture for 9 hours; 21) pure Epon for 3 hours; 22) pure Epon for 3 hours. After dehydration, the samples were transferred into pure Epon and cured at 60°C in a vacuum oven for 48 hours. The solid samples were sliced into 1 μM thick sections in a Porter Blum MT-2, and then into 120 nm thin sections in RMC MTXL Ultramicrotome. Uranyl acetate and lead citrate were used for sample staining. All the samples were examined using a Hitachi H7500 transmission electron microscope, and photographed using an AMT1 Meg CCD Camera.
Results
Growth kinetics of lysogenic Synechococcus sp. IU 625 (SYN-L) at 24°C, 35°C, and temperature shift from 24°C to 35°C
In order to determine if different temperatures, or raising the temperature, could affect the release of AS-1 from lysogenic cells (SYN-L), we first monitored the growth of Synechococcus sp. IU 625 (SYN-L) with direct cell count and spectrophotometry at different temperatures. Synechococcus sp. IU 625 is typically grown under ambient light at 24°C. Figure 1 shows that Synechococcus sp. IU 625 exhibits slightly improved growth at 35°C compared to 24°C (Fig. 1a and 1b). The growth pattern of temperature shifted cultures from 24°C to 35°C at day 8 (log phase of cell growth) is very similar to the growth at 35°C (Fig. 1c). The temperature study suggests that Synechococcus sp. IU 625 grows slightly faster at 35°C than at 24°C.
Figure 1.
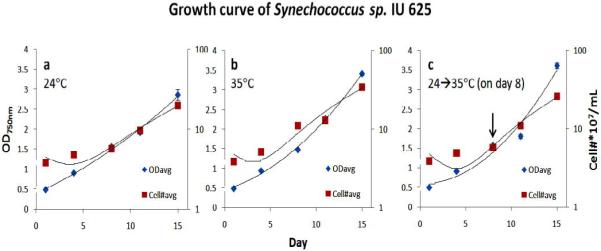
Growth of lysogenic Synechococcus sp. IU 625 (SYN-L) at a) 24°C; b) 35°C; and c) temperature shift from 24°C to 35°C on day 8 (log phase).
High temperature induces Cyanophage AS-1 lysogenic to lytic conversion
To determine if high temperature induces AS-1 lysogenic to lytic conversion, Synechococcus sp. IU 625 cultures were grown at 24°C, 35°C, or switched from 24°C to 35°C. Cyanophage AS-1 release was monitored by plaque forming units per ml (PFU/ml) as shown in Table 1. There is a constant release of virus from lysogenic SYN-L cultures, which has also been reported to occur with marine cyanobacteria (Ortmann, 2002). On day 4, similar amounts of PFU/ml were observed in all three conditions 10.50±2.121 (24°C), 13.00±5.657 (35°C) and 10.50±2.121 (24°C → 35°C). On day 8 significant induction of lytic phage was observed, as the PFU/ml at 24°C was 81.50±7.778 (p<0.05) and the PFU/ml at 35°C was 382.5±14.85 (p<0.05). An even higher titer, 742.5±14.85 (p<0.05), was observed on day 8 after a temperature shift from 24°C to 35°C. On day 11, PFU/ml of 247.0±5.657 and 540.0±11.31 were observed at 24° and 35°C respectively. The temperature switched culture at day 11 (the 4th day of switch), PFU/ml was too numerous to count (TNTC). These results demonstrate that although the growth was similar in the 35°C and temperature switched cultures, PFU/ml was significantly higher in the temperature switched cultures (p<0.05). Thus, this is the first report of high temperature as an inducer of Cyanophage AS-1 lysogenic to lytic conversion.
Table 1.
Summary of temperature induction study. The growth of SYN-L at temperature 24°C, 35°C, and switch from 24°C to 35°C monitored by turbidity, direct count, PFU, and Release Efficiency (RE)
| Temperature (°C) |
Days | OD750nm | Cell Numbers (*107)/ml |
Plaque Formation Unit (PFU/ml) |
Release Efficiency (RE) |
|---|---|---|---|---|---|
| 24 | 1 | 0.490±0.014 | 3.835±0.092 | 0 | 0 |
| 4 | 0.912±0.011 | 4.850±0.495 | 10.50±2.121 | 2.199±0.662 | |
| 8 | 1.565±0.049 | 5.760±0.198 | 81.50±7.778 | 14.18±1.838 | |
| 11 | 1.925±0.049 | 9.660±0.764 | 247.0±5.657 | 25.63±1.440 | |
| 15 | 2.865±0.134 | 19.80±0.424 | * | NA | |
| 35 | 1 | 0.490±0.014 | 3.835±0.092 | 0 | 0 |
| 4 | 0.945±0.021 | 5.150±0.636 | 13.00±5.657 | 2.612±1.421 | |
| 8 | 1.470±0.099 | 11.20±0.566 | 382.5±14.85 | 34.23±3.055 | |
| 11 | 2.265±0.035 | 13.05±0.636 | 540.0±11.31 | 41.41±1.152 | |
| 15 | 3.410±0.156 | 34.45±1.061 | * | NA | |
| 24 → 35 | 1 | 0.490±0.014 | 3.835±0.092 | 0 | 0 |
| 4 | 0.912±0.011 | 4.850±0.495 | 10.50±2.121 | 2.199±0.662 | |
| 8 | 1.565±0.049 | 5.760±0.198 | 742.5±14.85 | 128.9±1.854 | |
| 11 | 1.805±0.064 | 10.95±1.061 | * | NA | |
| 15 | 3.615±0.262 | 25.70±1.556 | * | NA |
Too numerous to count; NA: Not Applicable.
Since the PFU/ml results described above were normalized by similar optical density but not CFU/ml, we next normalized PFU released per 1 × 107 CFU. This is important, because temperature has been reported to change bacterial cell size (Shehata and Marr, 1975). In order to quantitatively analyze the viral release from lysogenic cultures at various temperatures, RE assays, which measure PFU/ml per 107 bacterial cells, were performed. On day 4, the RE was similar, 2.199±0.662 and 2.612±1.421 at 24°C and 35°C respectively (Table 1). However, on day 8, significant viral release was observed at 35°C. The RE was 14.18±1.838 at 24°C and was 34.23±3.055 at 35°C (Table 1). A high RE (128.9±1.854) was observed with temperature shift (24°C →35°C on day 8) (Table 1). These results clearly indicate that RE is correlated with temperature, as the ratio of viral release was increased 2.420±0.098 times on day 8 at 35°C compared to 24°C (p<0.05) (Table 2). Moreover, when temperature was increased from 24° to 35°C on day 8, the RE increased 9.161±1.056 times compared to 24°C (p<0.05) and 3.780±0.283 times compared to 35°C (p<0.05) (Table 2).
Table 2.
Comparison by using Release Efficiency (RE) ratio and Percentage of Increase of Release (PIR) using data on day 8 (during log phase)
| Condition | RE ratio | PIR (%) |
|---|---|---|
| 35°C/24°C | 2.420±0.098 | 142.0±9.824 |
| (24→35°C)/24°C | 9.161±1.056 | 816.1±105.6 |
| (24→35°C)/35°C | 3.780±0.283 | 278.0±28.31 |
The PIR was also calculated (Table 2). The PIR on day 8 at 35°C was 142.0±9.824 percent higher (p<0.05) than that of cultures at 24°C. The PIR of temperature switched culture (from 24° to 35°C) was 816.1±105.6 percent higher (p<0.05) than the cultures at 24°C, and 278.0±28.31 percent higher (p<0.05) than the cultures at 35°C. Thus, high temperature significantly induces lysogenic to lytic conversion of Cyanophage AS-1.
Transmission electron microscopy confirms temperature-dependent lysogenic to lytic conversion of Cyanophage AS-1 prophage
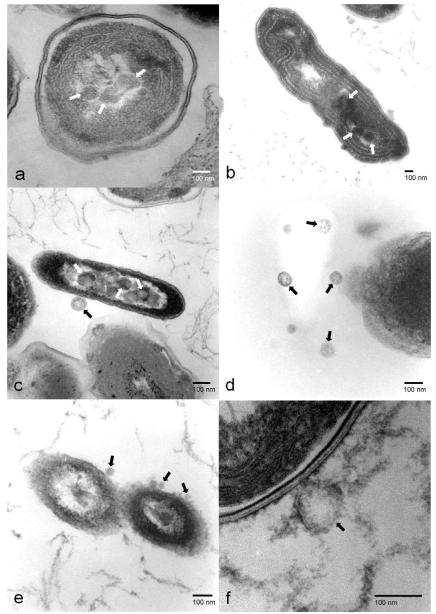
Since our data show that high temperature induces lysogenic to lytic conversion of Cyanophage AS-1, we used TEM to follow this process. Figure 2 shows the morphology and subcellular structures of SYN-L with no temperature induction of the AS-1 prophage. SYN-L with prophage is indistinguishable from SYN-N without prophage (not shown). However, once the SYN-L cells were shifted to high temperature, lysogenic to lytic conversion can be observed after 4 hours, with dense areas (see arrows) forming within the SYN-L cytoplasm (compare Figures 3a and 2a). These dense areas represent the first stage of viral biosynthesis. Figure 3b, with a sample taken at 8 hours, shows a later stage of biosynthesis, with the dense areas (denoted with arrows) growing both in size and intensity. These dense areas are significantly smaller than the size of the AS-1 capsid, which has a diameter of 90 nm compared to the 60 nm diameter of these dense areas. As seen in Figure 3c, AS-1 capsid proteins finally appear at 12 hours (see arrows). After 24 hours, new AS-1 particles are released. In Figures 3e and 3f, we observe newly synthesized AS-1 particles reabsorbing to SYN-L cells that contain AS-1 prophage that were not induced by high temperature. Thus, we demonstrate that the AS-1 phages that are produced by high-temperature-induced lysogenic to lytic conversion are fully functional, virulent particles.
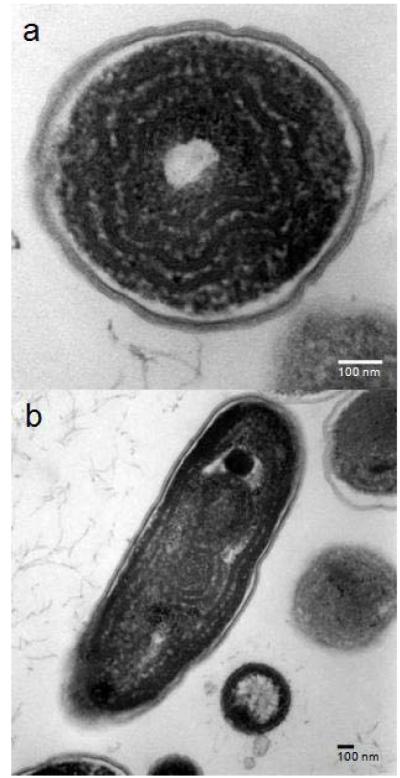
Figure 2.
Transmission electronic microscopic images of Synechococcus sp. IU 625 (uninduced SYN-L). a) cross section; and b) long section.
Figure 3.
Induction of prophage AS-1. Arrows indicate the locations of pre-viral particles (3a & 3b) or viral particles (3c, 3d, 3e, & 3f). a) Biosynthesis - early stage, 4 hours; b) Biosynthesis – late stage, 8 hours; c) Assembly, 12 hours; d) Release, 24 hours; e) Free AS-1 virus readsorption, 24 hours; and f) Enlarged adsorption, 24 hours.
Discussion
Here, we report that Cyanophage AS-1 prophage can be induced by high temperature. This observation has significant ecological implications. Okunishi et al correlated the disappearance of algal blooms in the Gulf of Mexico with the induction of prophage in marine (salt water) cyanobacteria (Okunishi et al., 2003). As heat waves become more common with global warming, we need to better understand the biological implications of climate change.
To our knowledge, this is the first report of high temperature induction of a freshwater cyanobacterial prophage. Synechococcus sp. IU 625 is a ubiquitous freshwater cyanobacterium that has been implicated in algal blooms (Beardall, 2008). Significantly, we have established that a freshwater (cyano)prophage, like its marine counterparts, can be induced with high temperature.
Since many algal blooms are composed of toxin-secreting cyanobacteria, induction of prophage may provide a means of biocontrol. We have now reported two inducers of Cyanophage AS-1 prophage: high temperature (this study) and copper (Lee et al., 2006). Our goal is to identify non-toxic biocontrol molecules that can be added to bodies of water that can induce prophage, which can limit or prevent the ecological damage caused by algal blooms.
Acknowledgements
TC was supported by SHU Biological Sciences Research Fund; SRM was supported by NIH Grant SC2 GM084860-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babin M, Cullen JC, Roesler CS, Donaghay PL, Doucette GJ, Kahru M, Lewis MR, Scholin CA, Sieracki ME, Sosik HM. New approaches and technologies for observing harmful algal blooms. Oceanography. 2005;18:210–27. [Google Scholar]
- Beardall J. Blooms of Synechococcus: An analysis of the problem worldwide and possible causative factors in relation to nuisance blooms in the Gippsland Lakes. Monash University; 2008. [Google Scholar]
- Bisen PS, Audholia S, Bhatnagar AK. Mutation to resistance for virus AS-1 in the cyanobacterium Anacystis nidulans. Microbios Letters. 1985;29:7–13. [Google Scholar]
- Carmichael WW. Blue-Green Algae: An Overlooked Health Threat. Health & Environment Digest, Freshwater Foundation. 1991;5:1–8. [Google Scholar]
- Carmichael WW. Cyanobacteria secondary metabolites--the cyanotoxins. J Appl Bacteriol. 1992;72:445–59. doi: 10.1111/j.1365-2672.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Carmichael WW, Li R. Cyanobacteria toxins in the Salton Sea. Saline Systems. 2006;2:5. doi: 10.1186/1746-1448-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael WW, Schwartz LD. Preventing livestock deaths from blue-green algae poisoning: Farmer’s Bulletin. U.S. Department of Agriculture; Washington, D.C.: 1984. p. 11. [Google Scholar]
- Codd GA, Bell SG, Brooks WP. Cyanobacterial toxins in water. Water Science & Technology. 1989;21:1–13. [Google Scholar]
- Fastner J, Neumann U, Wirsing B, Weckesser J, Wiedner C, Nixdorf B, Chorus I. Microcystins (hepatotoxic heptapeptides) in German fresh water bodies. Environmental Toxicology. 1999;14:13–22. [Google Scholar]
- Hunter PR. Climate change and waterborne and vector-borne disease. J Appl Microbiol. 2003;94(Suppl):37S–46S. doi: 10.1046/j.1365-2672.94.s1.5.x. [DOI] [PubMed] [Google Scholar]
- Lee HL, Lustigman B, Schwinge V, Chiu IY, Hsu S. Effect of mercury and cadmium on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1992a;49:272–8. doi: 10.1007/BF00191766. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lui D, Platner PJ, Hsu SF, Chu TC, Gaynor JJ, Vega QC, Lustigman BK. Induction of temperate cyanophage AS-1 by heavy metal--copper. BMC Microbiol. 2006;6:17. doi: 10.1186/1471-2180-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LH, Lustigman B. Effect of barium and nickel on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1996;56:985–92. doi: 10.1007/s001289900142. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Chu IY, Hsu S. Effect of lead and cobalt on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1992b;48:230–6. doi: 10.1007/BF00194376. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Chu IY, Jou HL. Effect of aluminum and pH on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1991;46:720–6. doi: 10.1007/BF01689958. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Dandorf D. Effect of manganese and zinc on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1994;53:158–65. doi: 10.1007/BF00205154. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Maccari J. Effect of copper on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1993;50:600–7. doi: 10.1007/BF00191252. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Murray S, Koepp S. Effect of selenium on the growth of the cyanobacterium Anacystis nidulans. Bull Environ Contam Toxicol. 1999;62:591–9. doi: 10.1007/s001289900916. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman BK, Murray SR. Combined effect of mercuric chloride and selenium dioxide on the growth of the cyanobacteria, Anacystis nidulans. Bull Environ Contam Toxicol. 2002;69:900–7. doi: 10.1007/s00128-002-0144-0. [DOI] [PubMed] [Google Scholar]
- Lu J, Chen F, Hodson RE. Distribution, isolation, host specificity, and diversity of cyanophages infecting marine Synechococcus spp. in river estuaries. Appl Environ Microbiol. 2001;67:3285–90. doi: 10.1128/AEM.67.7.3285-3290.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustigman B, Lee LH, Morata J, Khan F. Effect of thallium on the growth of Anacystis nidulans and Chlamydomonas reinhardtii. Bull Environ Contam Toxicol. 2000;64:565–73. doi: 10.1007/s001280000040. [DOI] [PubMed] [Google Scholar]
- McDaniel L, Houchin LA, Williamson SJ, Paul JH. Lysogeny in marine Synechococcus. Nature. 2002;415:496. doi: 10.1038/415496a. [DOI] [PubMed] [Google Scholar]
- Okunishi S, Yoshimura N, Maeda H, Yoshikawa T, Sakata T. A Virulent Cyanophage Affects the Seasonal Abundance of Cyanobacterial Picoplankton (Synechococcus sp.) in Kagoshima Bay. Microbes and Environments. 2003;18:10–5. [Google Scholar]
- Ortmann AC, Lawrence JE, Suttle CA. Lysogeny and lytic viral production during a bloom of the cyanobacterium Synechococcus spp. Microb Ecol. 2002;43:225–31. doi: 10.1007/s00248-001-1058-9. [DOI] [PubMed] [Google Scholar]
- Paul JH, Jiang SC. Lysogeny and Transduction. In: Pauls JH, editor. Marine Microbiology (Methods in Microbiology) Academic Press; 2001. p. 666. [Google Scholar]
- Safferman RS, Diener TO, Desjardins PR, Morris ME. Isolation and characterization of AS-1, a phycovirus infecting the blue-green algae, Anacystis nidulans and Synechococcus cedrorum. Virology. 1972;47:105–13. doi: 10.1016/0042-6822(72)90243-7. [DOI] [PubMed] [Google Scholar]
- Shehata TE, Marr AG. Effect of temperature on the size of Escherichia coli cells. J Bacteriol. 1975;124:857–62. doi: 10.1128/jb.124.2.857-862.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulberg OM, Codd GA, Carmichael WW. Toxic Blue-Green Algal Blooms in Europe: A Growing Problem. Ambio: Journal of Human Environment. 1984;13:244–7. [Google Scholar]
- Slonczewski JL, Foster JW. Microbiology: An Evolving Science. W. W. Norton & Co.; New York: 2008. [Google Scholar]
- Waterbury JB, Valois FW. Resistance to Co-Occurring Phages Enables Marine Synechococcus Communities To Coexist with Cyanophages Abundant in Seawater. Appl Environ Microbiol. 1993;59:3393–9. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SJ, Houchin LA, McDaniel L, Paul JH. Seasonal variation in lysogeny as depicted by prophage induction in Tampa Bay, Florida. Appl Environ Microbiol. 2002;68:4307–14. doi: 10.1128/AEM.68.9.4307-4314.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, Joint IR, Carr NG, Mann NH. Isolation and Molecular Characterization of Five Marine Cyanophages Propagated on Synechococcus sp. Strain WH7803. Appl Environ Microbiol. 1993;59:3736–43. doi: 10.1128/aem.59.11.3736-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]