Abstract
Directional asymmetry, the systematic differences between the left and right body sides, is widespread in human populations. Changes in directional asymmetry are associated with various disorders that affect craniofacial development. Because facial dysmorphology is a key criterion for diagnosing fetal alcohol syndrome (FAS), the question arises whether in-utero alcohol exposure alters directional asymmetry in the face. Data on the relative position of 17 morphological landmarks were obtained from facial scans of children who were classified as either FAS or control. Shape data obtained from the landmarks was analyzed with the methods of geometric morphometrics. Our analyses showed significant directional asymmetry of facial shape, consisting primarily of a shift of midline landmarks to the right and a displacement of the landmarks around the eyes to the left. The asymmetry of FAS and control groups differed significantly and average directional asymmetry was increased in those individuals exposed to alcohol in utero. These results suggest that the developmental consequences of fetal alcohol exposure affect a wide range of craniofacial features in addition to those generally recognized and used for diagnosis of FAS.
Keywords: directional asymmetry, discriminant function, facial dysmorphology, fetal alcohol syndrome, Procrustes superimposition
Introduction
Directional asymmetry is defined as systematic differences between the left and right sides of the body. Examples are the conspicuous asymmetry of internal organs and the more subtle asymmetry of the human brain (e.g. Toga and Thompson, 2003). With the advent of powerful methods of geometric morphometrics, it has become apparent that subtle, but significant directional asymmetry is nearly ubiquitous even for apparently symmetric features, such as craniofacial features or limbs, and is found in a wide range of organisms (Auffray et al., 1996; Klingenberg, 2002; Klingenberg et al., 2002; Klingenberg et al., 1998), including humans (e.g. DeLeon, 2007; Ercan et al., 2008; Schaefer et al., 2006).
While subtle facial directional asymmetry is present in healthy individuals (DeLeon, 2007; Ercan et al., 2008; Schaefer et al., 2006), stronger directional asymmetry is often associated with conditions that disrupt normal craniofacial development, such as cleft lip and palate (Bock and Bowman, 2006), deformational plagiocephaly or craniosynostosis (Netherway et al., 2006). Changed patterns of directional asymmetry have been reported among individuals diagnosed with disorders in which facial changes may be a secondary consequence of abnormal brain development, such as schizophrenia (Hennessy et al., 2004) and autism spectrum disorder (Hammond et al., 2008).
Specific facial dysmorphology, resulting from prenatal exposure to alcohol, remains the key diagnostic feature of fetal alcohol syndrome (FAS) (Astley and Clarren, 2000; Hoyme et al., 2005). Anthropometric measurements of facial changes have been shown to correctly distinguish FAS from non-FAS in different ethnic populations (Moore et al., 2007). In addition, modern techniques of geometric morphometrics have confirmed that prenatal alcohol exposure has significant effects on facial shape (Mutsvangwa and Douglas, 2007). Thus far, little attention has been paid to the effects of prenatal alcohol exposure on facial asymmetry, although Kieser (1992) reported that maternal alcohol consumption correlates with fluctuating asymmetry in the teeth of children. Although asymmetry is normal in most populations (Ercan et al., 2008; Kimmerle and Jantz, 2005; McIntyre and Mossey, 2002; Shaner et al., 2000), no study has investigated the effects of prenatal exposure to alcohol on directional asymmetry.
Here, we report changes in the pattern of directional asymmetry between individuals prenatally exposed to alcohol and those who were not exposed. We have included ethnically distinct samples (Moore et al., 2007). We used sensitive morphometric methods specifically developed for measuring asymmetry of shape (Bock and Bowman, 2006; Klingenberg et al., 2002; Klingenberg and McIntyre, 1998). These methods detected subtle, but statistically significant directional asymmetry in the face and revealed that asymmetry is not the same for individuals who were prenatally exposed to alcohol as compared to those without prenatal alcohol exposure.
Material and Methods
Study design
Participants were assessed as part of an ongoing international consortium, the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Participants were from two sites: Cape Town, South Africa and Helsinki, Finland. This study was approved by the Institutional Review Board at each site and at the grantee institutions (Indiana University School of Medicine, Wayne State University School of Medicine, and Biomedical Sciences, San Diego State University). All participants and/or their parent(s)/legal guardian(s) provided written informed consent and assent.
As part of the study visit, each participant was examined by members of the CIFASD Dysmorphology Core, who completed a standardized, uniform assessment as described in Jones et al. (2006). Details of the study visit are provided in Moore et al. (2007). Briefly, a standard classification system, based solely on structural features and growth deficiency, was used to determine a preliminary classification of FAS, not FAS, or deferred (Hoyme et al., 2005; Jones et al., 2006). Analyses were limited to participants with either a diagnosis of FAS from the Dysmorphology Core, or to participants with a diagnosis of “control” from the Dysmorphology Core who were not exposed to alcohol during pregnancy, according to maternal interview data. Individuals who were known to have been exposed to alcohol in-utero but did not receive a diagnosis of FAS were labeled “deferred.” Because the focus of this paper was to determine whether asymmetry differs between individuals with FAS and controls that were not exposed to alcohol, we excluded these deferred individuals from the current analyses. Due to potential differences in morphometric facial structure between ethnicities and sample size considerations, only participants reported to be Finnish Caucasian (FC: 40 FAS, 50 control) or Cape Coloured (CC: 49 FAS, 29 control) were included in the analysis. Demographic data are provided in Table 1.
Table 1.
Subject Demographics. The tabled information is for those subjects included in the analyses. FC, Finnish Caucasian; CC, Cape Coloured; SD, standard deviation.
| # subjects | # males | Mean age (SD) | Mean IQ (SD) | ||
|---|---|---|---|---|---|
| FC | FAS | 40 (41%) | 17 (42%) | 13.2 (3.6) | 90.7 (16.0) |
| Control | 50 (59%) | 20 (40%) | 13.7 (3.6) | 99.9 (17.7) | |
| CC | FAS | 49 (60%) | 22 (48%) | 5.2 (1.3) | 84.1 (11.5) |
| Control | 29 (39%) | 15 (52%) | 4.4 (1.0) | 86.9 (13.3) |
Collection of 3D Images
Facial images were captured using a commercially available laser scanner, the Minolta Vivid 910fw (Konica Minolta Sensing Americas, Inc., Boulder, CO). The scanner shines a low-intensity “eye safe” laser on the participant. Details describing calibration assessment of the scanners are provided in Moore et al. (2007). Participants were seated approximately 660 mm from the scanner and a trained operator located seven soft tissue landmarks (bilateral: frontotemporale, tragion, gonion; unilateral: menton) by inspection and/or palpation, and marked them on the skin using an eye-liner pencil. Two frontal and two lateral left and right scans were obtained of each subject. For the lateral scans, the participant faced at near right angles to the scanner. Collected images were processed using a commercially available software package, Rapidform™ 2006 (INUS Technology Incorporated, Seoul Korea).
Image processing and measurement
Rapidform™, a reverse modelling software package that scans physical objects and creates a digital version of the object, was used to merge the best lateral and frontal scans into a single, 3D model of the participant's face. For each subject, the better of the two scans of each of the three positions was determined such that neutral expressions were present in all scans, lighting was optimal, and the number of visible landmarks was maximized. Each 3D facial image was analyzed using a customized software plug-in, written by one of the authors (JLR) using Visual C++ and the Rapidform™ API. An anthropologist (EM, RW) identified a series of landmarks on the 3D model, of which 17 are included in this study (Fig. 1). The software required double redundant measurement accuracy. This redundant approach required the user to identify at least two sets of facial landmarks resulting in less than 2 mm difference per linear measurement. If the user failed to identify redundant landmarks, she or he was forced to pick a third set or re-pick the existing landmarks until accuracy met the 2 mm specification. Analyses were based on two configurations of landmarks per subject, excluding any divergent measurements. Due to problems with hair around the face and ears, some individuals did not have complete landmark data. These individuals were omitted from all analyses.
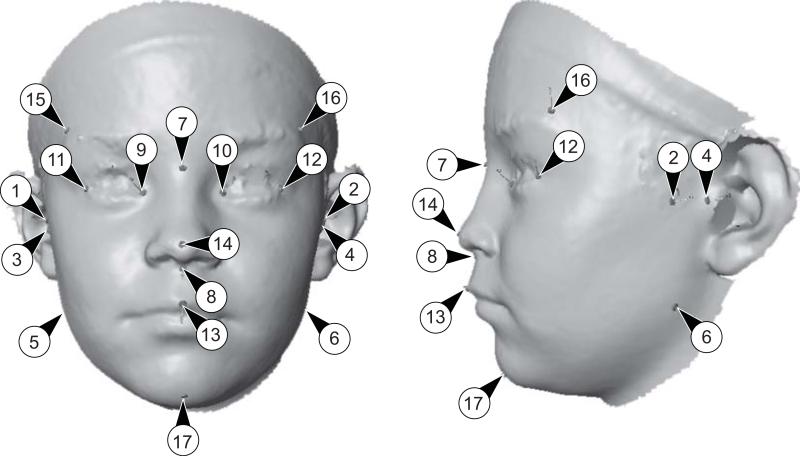
Figure 1.
The set of landmarks used in this study. Five of the 17 landmarks are in the median plane, whereas the other 12 occur in six pairs on both sides of the face.
Landmarks: 1, right zygonion; 2, left zygonion; 3, right tragion; 4, left tragion; 5, right gonion; 6, left gopnion; 7, nasion; 8, subnasale; 9, right endocanthion; 10; left endocanthion; 11; right exocanthion; 12, left exocanthion; 13, labiale superior; 14, pronasale; 15; right frontotemporale; 16, left frontotemporale; 17, menton.
Morphometric analysis
Geometric morphometrics is based on a definition of shape as the complete geometric information about an object, except its size, position, and orientation (e.g., Dryden and Mardia, 1998). The variation between shapes can be extracted from information on the landmark positions by a Procrustes fit, which removes variation of size, position and orientation (Dryden and Mardia, 1998). The criterion for an optimal fit is the sum of squared distances between corresponding landmarks. This measure, which is minimized in the Procrustes fit, can also be used as a measure of the absolute magnitude of the difference between two shapes, and is known as Procrustes distance (Dryden and Mardia, 1998). The coordinates of the landmarks after the Procrustes fit contain only shape variation and can be used for further analyses with the methods of multivariate statistics. These coordinates define a multidimensional shape space; because some information was removed by eliminating variation in size, position and orientation, this space has 7 dimensions fewer than the number of landmark coordinates that were used (in our example, 44 = (17 × 3) – 7).
For objects that are symmetric, such as the human face, a special version of this approach is required, which can characterize components of symmetric shape variation and asymmetry (Bock and Bowman, 2006; Klingenberg et al., 2002; Mardia et al., 2000). For each landmark configuration, this method used the original configuration and a copy that was reflected to its mirror image; the labels of the paired landmarks of the reflected copy were then exchanged; and finally, all original configurations and their reflected and relabelled copies were then included in a joint Procrustes fit (for details, see Klingenberg et al., 2002). After this Procrustes fit, the asymmetry of shape was computed for each individual as the deviation in the landmark coordinates of the original configuration from the symmetric shape obtained by averaging the original and reflected copies for that individual. These computations were carried out automatically in the MorphoJ software package (Klingenberg, 2008). This method uses information from all landmarks equally to determine the plane of symmetry and makes no assumptions regarding the symmetry or asymmetry of any landmarks. In the shape space defined by the Procrustes fit, the components of symmetric variation and asymmetry occupy mutually orthogonal subspaces (for our data, the symmetric component has 24 dimensions and the asymmetry component has 20 dimensions; Klingenberg et al., 2002).
Three subjects had measurements with large Mahalanobis distances from the average shape (a multivariate measure of distance relative to within-sample variation; Mardia et al., 1979). This set of landmarks for the three subjects were excluded from the study, and only a single set was included in the analysis for the respective subjects. Individuals with known craniofacial disorders (e.g., Williams Syndrome) were also removed from the study.
Measurement error has been previously identified as an important consideration when subtle biological effects are the focus of interest, such as in studies of asymmetry (Palmer and Strobeck, 1986; Robinson et al., 2002; Ward and Jamison, 1991). To quantify measurement error and the amount of variation due to the various factors in the study design, we used Procrustes ANOVA (Klingenberg et al., 2002; Klingenberg and McIntyre, 1998). This method is an extension of the one-way ANOVA proposed by Goodall (1991) for landmark data. It adds up sums of squares and mean squares over the coordinates of the landmarks after the Procrustes fit, and can therefore quantify the amounts of shape variation in units of squared Procrustes distance as an easily interpretable measure of the magnitude of the effects in the ANOVA. The method has been generalized to more complex designs, including asymmetry (Klingenberg et al., 2002; Klingenberg and McIntyre, 1998). For this Procrustes ANOVA, the degrees of freedom from the ANOVA design are multiplied with the dimensionality of the shape space. In the context of analyses of asymmetry for symmetric objects, the multiplier for the degrees of freedom depends on whether an effect is part of the symmetric or asymmetric component of variation (Klingenberg et al., 2002). A fully multivariate MANOVA approach has been developed, which provides additional information for interpreting the results (Klingenberg et al., 2002).
Asymmetry in a configuration of landmarks is computed from the difference between the original configuration and a copy that has been reflected and relabelled (swapping the labels of paired landmarks; e.g. Klingenberg et al., 2002). Thus, the overall directional asymmetry was computed as the average asymmetry across all individuals included in the analysis. Directional asymmetry was also estimated separately for each ethnic group (CC and FC).
Statistical comparisons of the mean asymmetries of the two ethnic groups (CC, FC) were done with permutation tests (Good, 2000; Manly, 2007) using both Procrustes distance and the T2 statistic (equivalent to using Mahalanobis distance) as test statistics. Procrustes distance is a measure of the absolute magnitude of shape differences that does not take into account the direction of the mean differences. In contrast, the T2 statistic does take into account the extent of variation in different directions of shape space (Klingenberg and Monteiro, 2005).
To assess whether differences in directional asymmetry were sufficient to distinguish children in the alcohol exposure groups, we used linear discriminant functions (separately for the CC and FC samples). The reliability of the classification was assessed with cross-validation that left out each individual in turn and evaluated whether the discriminant function computed with the remaining sample obtained a correct classification for the subject that was ‘left out’. From cross-validation results, sensitivity (number of correctly classified FAS cases / total number of FAS cases) and specificity (number of correctly classified controls / total number controls) was computed for each ethnic group.
Morphometric analyses were conducted with the MorphoJ software (Klingenberg, 2008). To visualize the shape changes identified by the analyses, we morphed the surface from a single facial scan to fit the required landmark positions (using the Landmark software; Wiley et al., 2005). This morphing used the thin-plate spline method (Bookstein, 1989) to interpolate shape changes at the landmark positions to every point on the facial surface between the landmarks.
Results
The overall Procrustes ANOVA (Table 2) shows highly significant effects on shape of the sample (CC versus FC), of prenatal alcohol exposure, and a significant effect of sex. The Procrustes ANOVA also indicated that the main effect of side (original vs. reflected copies) was highly significant (P < 0.0001), revealing the presence of directional asymmetry. The analysis also indicated that measurement error is small relative to the individual-by-side interaction, and was therefore not a confounding factor in this study. Because we focused our analyses on directional asymmetry, which is the vector of average asymmetry in a sample, the effect of measurement error is further reduced by the large sample size. The MANOVA results for the analysis were similar, except for the fact that the effect of sex was only significant for the symmetric component of shape variation, but not for the asymmetry component (P = 0.48). This indicates that sex dimorphism of facial shape does not interfere wit the analyses of asymmetry. Separate analyses for the CC and FC samples produced similar results.
Table 2.
Procrustes ANOVA for facial shape (Klingenberg et al., 2002). The effect of individuals represents the variation among individuals in the symmetric component of shape. The main effect of side is directional asymmetry (the systematic difference between the original and reflected copies of each individual). The individual × side effect quantifies fluctuating asymmetry; both of the latter effects concern the asymmetry component of shape. The residual variation is due to measurement error and includes both symmetric and asymmetric components of shape variation.
| Effect | Sums of squares | Mean squares | Degrees of freedom | F | P |
|---|---|---|---|---|---|
| Country |
0.2231 |
0.009296 |
24 |
32.74 |
< 0.0001 |
| FAS vs Control |
0.2117 |
0.008819 |
24 |
31.06 |
< 0.0001 |
| Sex |
0.0115 |
0.000481 |
24 |
1.69 |
0.0185 |
| Individual | 1.1176 | 0.000284 | 3936 | 5.15 | < 0.0001 |
| Side | 0.0354 | 0.001770 | 20 | 32.10 | < 0.0001 |
| Individual × side | 0.11842 | 0.000055 | 3340 | 5.08 | < 0.0001 |
| Residual | 0.0764 | 0.000011 | 7040 |
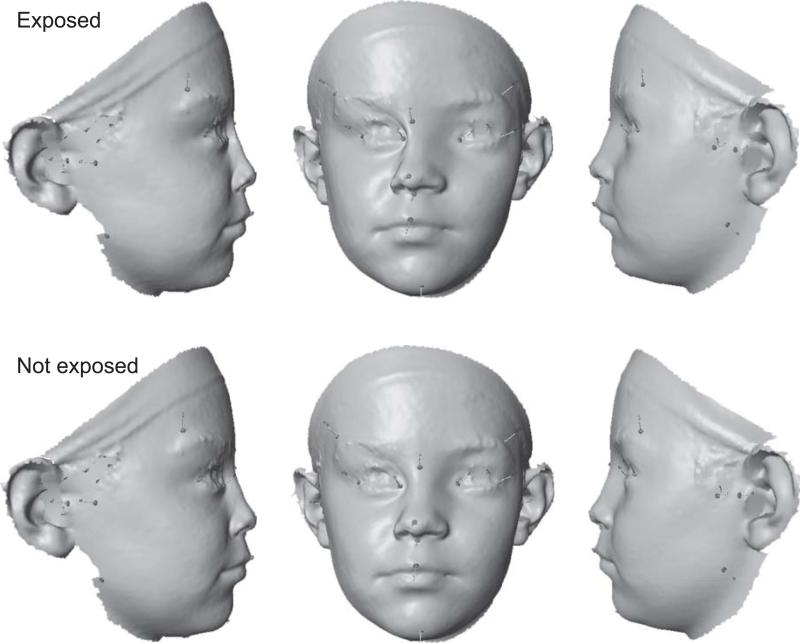
Analyses of asymmetry were then performed in each ethnic group separately. The CC FAS and control individuals had directional asymmetries that were significantly different for both test statistics (Procrustes distance 0.0091, P = 0.029; T2 = 58.41, P = 0.017). The principal shape features associated with the directional asymmetry were a shift of the midline landmarks to the right and a shift of the eyes to the left (Fig. 2). Moreover, the eye and the frontotemporale were displaced anteriorly on the right side and a posteriorly on the left side. Smaller asymmetries were observed for the zygonion (higher and slightly more anterior on the left than on the right side), the tragion (more anterior and slightly higher on the left than on the right side) and for the gonion (more posterior and slightly higher on the left than on the right side). Comparison according to alcohol exposure indicated that these features were much more accentuated for the FAS group exposed to alcohol than for the non-exposed control group (Fig. 2). The cross-validation of the linear discriminant classification rule using the asymmetries resulted in a sensitivity of 69% and specificity of 52%.
Figure 2.
Directional asymmetry in the FAS and control subjects in the CC sample. For better visibility, the observed asymmetry has been exaggerated 5-fold. The facial surfaces were generated by morphing a single facial scan according to the landmark positions for the mean asymmetries (Wiley et al., 2005).
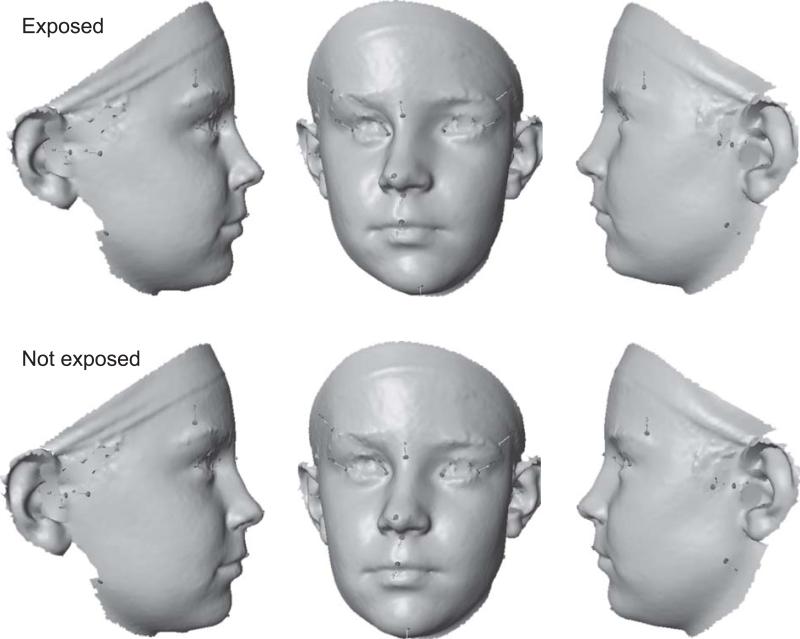
The directional asymmetries in the FC sample also differed significantly between the FAS and control-groups for both test statistics (Procrustes distance 0.0066, P = 0.043; T2 = 55.59, P = 0.008). Similar to the results in the CC sample, the main features of directional asymmetry were a shift of the midline landmarks (except menton) to the right and a shift of the eyes to the left. In addition, the FC shows substantial directional asymmetry of the frontotemporale, which is in a more posterior and somewhat lower position on the left than on the right side (particularly noticeable in relation to exocanthion; Fig. 3). Differences in directional asymmetry between the control and FAS groups, as in the CC sample, included a stronger rightward shift of the midline landmarks for the exposed group. Unlike the CC population, however, there was relatively little change in the directional asymmetry of the eyes or of frontotemporale. In contrast, there was a marked difference in directional asymmetry at gonion (more posterior and lateral position on the left than on the right side in the exposed group; in addition, substantially higher on the left than on the right side in the control group). Moreover, the control group also showed a parallel shift of zygonion and tragion (more anterior and medial on the left than on the right side), whereas the exposed group showed opposite anterior-posterior shifts (zygonion more posterior and tragion more anterior on the left than on the right side). The cross-validation of the discriminant function in the FC population resulted in a sensitivity of 60% and specificity of 66%.
Figure 3.
Directional asymmetry in the FAS and control subjects in the FC sample. For better visibility, the observed asymmetry has been exaggerated 10-fold.
Discussion
The finding of significant directional asymmetry in all groups agrees with most published analyses (Ercan et al., 2008; Kimmerle and Jantz, 2005; McIntyre and Mossey, 2002; Shaner et al., 2000). The average directional asymmetry was dominated by a shift of the midline landmarks to the right and of the eyes to the left (Fig. 2, 3). This is consistent with the strong tendency for a left dominance in facial distance measurements found in some published studies (Ercan et al., 2008; McIntyre and Mossey, 2002; Ras et al., 1994), but contrasts with the right dominance reported by other investigators (Ferrario et al., 1995; Shaner et al., 2000). It is unclear to what extent the inconsistency of published results corresponds to true differences between the populations under study or to differences in the methods used for measurements and analyses. For this study, the crucial results concern the changes of the directional asymmetry between groups with or without alcohol exposure in each of the two ethnic samples (i.e. the bottom versus top row in Figs. 2, 3).
Our analyses demonstrated significant differences in facial asymmetry between individuals with a diagnosis of FAS and individuals not exposed to alcohol prenatally (controls) in both populations. Differences in directional asymmetry were large enough to classify subjects into the correct group. The cross-validation results indicated facial asymmetry can classify individuals with a fair degree of accuracy. Although this performance approaches the accuracy of diagnoses by pediatricians after a two-day training program (Jones et al., 2006), it is unlikely that facial asymmetry is practical as a diagnostic tool. The effects of alcohol exposure on facial asymmetry are very subtle, and it is impossible with the present data, to quantify the degree of asymmetry due to amount or timing of alcohol exposure in utero. It is possible, however, that facial asymmetry discreetly provides complementary information to dysmorphologists, and may provide further insight into the pathophysiology of alcohol on the developing fetus. The CIFASD project is now collecting data on brain structure and function, which will allow for exploratory analysis to identify principal features of covariation between brain and the dysmorphology of the face.
Sample sizes were sufficiently large for detailed analyses of the shape features that differ between the directional asymmetry of FAS and control groups (Fig. 3). In both samples, changes of directional asymmetry were associated with a strengthening of features that were already present in the overall average directional asymmetry: the shift of midline landmarks to the right and, less strongly so, the opposite displacement of both eyes. Overall, children prenatally exposed to alcohol with an FAS diagnosis tend to have stronger directional asymmetry than the children in the respective non-exposed control groups. This strengthening of overall directional asymmetry for children with alcohol exposure is not the only change, however, because other aspects of directional asymmetry are affected as well.
Prenatal alcohol-induced alterations in directional asymmetry are similar in the two study sites overall, and differ only in relatively minor ways (Fig. 3). It is not possible to assign these differences to a particular cause since the two samples differ in several factors, including age and ethnicity. Although there is a substantial age difference between the samples: the Finnish sample consists of older individuals and has a greater age range than the Cape Town sample, a regression analysis of facial asymmetry on age did not indicate a systematic trend of directional asymmetry to change with age (results not shown). Accordingly, it is not possible with the present data to disentangle completely the effects of ethnic composition, age, and other possible factors.
Because craniofacial development of the face is intimately tied to the development of the brain, the question arises whether the increase of facial directional asymmetry associated with alcohol exposure correlates with a change in the asymmetry of the brain. Sowell et al. (2002) reported that individuals with severe prenatal alcohol exposure have reduced asymmetry of the cortical surface and gray matter density. Normal asymmetry is right-biased and particularly accentuated in the posterior inferior temporal lobes (Sowell et al., 2002). This region is not directly adjacent to the region of the face considered in this study; thus, it is unlikely that there is a direct developmental link as has been suggested for the facial and brain asymmetry in autism spectrum disorder (Hammond et al., 2008). On the other hand, Sulik and Johnston (1983) have demonstrated direct links between damage to the frontal lobes and craniofacial dysmorphology in mice prenatally exposed to ethanol, and Johnson et al. (1996) observed a similar association in human subjects. It is likely that a range of developmental processes, and their disruption by various disorders, can produce associations between the structure or function of the brain and facial shape and asymmetry. Examples include altered facial shape and asymmetry in schizophrenia (Hennessy et al., 2004) and correlations between facial features and cognitive performance in healthy subjects (Hennessy et al., 2006).
This study has demonstrated a clear difference in facial asymmetry between individuals with prenatal exposure to alcohol and those with no prenatal exposure. The powerful methods of geometric morphometric analysis will allow us to study the association of facial asymmetry with other brain-related functions as well as with neuropsychological and behavioural deficits that have been shown to exist in children with prenatal alcohol exposure (Mattson et al, 1998; McGee et al, 2008). Identification of patterns of asymmetry with brain morphology, function, or neuropsychological deficits will present health care providers with expanded opportunities for effective intervention.
Acknowledgements
The authors acknowledge the following people for assistance with this project: Leena Neuvonen, Kirsi Mallea, and Leena Loimu (Helsinki); and Christopher D. Molteno, Joseph L. Jacobson, Margaret September, Mandy van Niekerk, Mariska Pienaar, Jan Chamberlain, and Lisa Aitken (Cape Town).
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Dr. E. Riley is the PI of the Administrative Core of the CIFASD (AA014811) and can be contacted at eriley@mail.sdsu.edu or Center for Behavioral Teratology, 6363 Alvarado Court, 209, San Diego, CA 92120.
This international collaborative study is supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): U24AA014809 and U01AA014809 (to T.F.), U01AA014790 (to S.J.), U24AA014815 (to K.J.), U01AA014834 (to S.M.), U24AA014828 (to E.R.), U24AA014830 (to E.R.), and an administrative supplement to R01AA09524 (to S.J.). Additional funds were provided by the National Institutes of Health Office of Research on Minority Health, the Indiana Genomics Initiative (INGEN), and the Joseph Young, Sr., Fund from the State of Michigan (to S.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: Introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Auffray J-C, Alibert P, Renaud S, Orth A, Bonhomme F. Fluctuating asymmetry in Mus musculus subspecific hybridization: traditional and Procrustes comparative approach. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE, editors. Advances in morphometrics. Plenum Press; New York: 1996. pp. 275–283. [Google Scholar]
- Bock MT, Bowman AW. On the measurement and analysis of asymmetry with applications to facial modelling. Appl. Statist. 2006;55:77–91. [Google Scholar]
- Bookstein FL. Principal warps: thin-plate splines and the decomposition of deformations. IEEE Transactions on Pattern Analysis and Machine Intelligence. 1989;11:567–585. [Google Scholar]
- DeLeon VB. Fluctuating asymmetry and stress in a Medieval Nubian population. Am. J. Phys. Anthropol. 2007;132:520–534. doi: 10.1002/ajpa.20549. [DOI] [PubMed] [Google Scholar]
- Dryden IL, Mardia KV. Statistical shape analysis. Wiley; Chichester: 1998. [Google Scholar]
- Ercan I, Turan Ozdemir S, Etoz A, Sigirli D, Tubbs RS, Loukas M, Guney I. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J. Anat. 2008;213:663–669. doi: 10.1111/j.1469-7580.2008.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Miani A, Jr., Serrao G. A three-dimensional evaluation of human facial asymmetry. J. Anat. 1995;186:103–110. [PMC free article] [PubMed] [Google Scholar]
- Good P. Permutation tests: a practical guide to resampling methods for testing hypotheses. 2nd edn Springer; New York: 2000. [Google Scholar]
- Goodall CR. Procrustes methods in the statistical analysis of shape. J. R. Statist. Soc. B. 1991;53:285–339. [Google Scholar]
- Hammond P, Forster-Gibson C, Chudley AE, Allanson JE, Hutton TJ, Farrell SA, McKenzie J, Holden JJA, Lewis MES. Face–brain asymmetry in autism spectrum disorders. Mol. Psychiatry. 2008;13:614–623. doi: 10.1038/mp.2008.18. [DOI] [PubMed] [Google Scholar]
- Hennessy RJ, Laneb A, Kinsella A, Larkin C, O'Callaghan E, Waddington JL. 3D morphometrics of craniofacial dysmorphology reveals sex-specific asymmetries in schizophrenia. Schizophr. Res. 2004;67:261–268. doi: 10.1016/j.schres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hennessy RJ, McLearie S, Kinsella A, Waddington JL. Facial shape and asymmetry by three-dimensional laser surface scanning covary with cognition in a sexually dimorphic manner. J. Neuropsychiatry Clin. Neurosci. 2006;18:73–80. doi: 10.1176/jnp.18.1.73. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am. J. Med. Genet. 1996;61:329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118:e1734–e1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Kieser JA. Fluctuating odontometric asymmetry and maternal alcohol consumption. Ann. Hum. Biol. 1992;19:513–520. doi: 10.1080/03014469200002342. [DOI] [PubMed] [Google Scholar]
- Kimmerle EH, Jantz RL. Secular trends in craniofacial asymmetry studied by geometric morphometry and generalized Procrustes methods. In: Slice DE, editor. Modern Morphometrics in Physical Anthropology. Kluwer Academic/Plenum; New York: 2005. pp. 247–263. [Google Scholar]
- Klingenberg CP. Morphometrics and the role of the phenotype in studies of the evolution of developmental mechanisms. Gene. 2002;287:3–10. doi: 10.1016/s0378-1119(01)00867-8. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. MorphoJ. Faculty of Life Sciences, University of Manchester; Manchester: 2008. [Google Scholar]
- Klingenberg CP, Barluenga M, Meyer A. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution. 2002;56:1909–1920. doi: 10.1111/j.0014-3820.2002.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, McIntyre GS. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution. 1998;52:1363–1375. doi: 10.1111/j.1558-5646.1998.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, McIntyre GS, Zaklan SD. Left-right asymmetry of fly wings and the evolution of body axes. Proc. Royal Soc. Lond. B, Biol. Sci. 1998;265:1255–1259. doi: 10.1098/rspb.1998.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP, Monteiro LR. Distances and directions in multidimensional shape spaces: implications for morphometric applications. Syst. Biol. 2005;54:678–688. doi: 10.1080/10635150590947258. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, bootstrap and Monte Carlo methods in biology. Chapman & Hall/CRC; Boca Raton, FL: 2007. [Google Scholar]
- Mardia KV, Bookstein FL, Moreton IJ. Statistical assessment of bilateral symmetry of shapes. Biometrika. 2000;87:285–300. [Google Scholar]
- Mardia KV, Kent JT, Bibby JM. Multivariate analysis. Academic Press; London: 1979. [Google Scholar]
- McIntyre GT, Mossey PA. Asymmetry of the parental craniofacial skeleton in orofacial clefting. J. Orthod. 2002;29:299–305. doi: 10.1093/ortho/29.4.299. [DOI] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Wetherill LF, Rogers JL, Autti-Rämö I, Fagerlund Å, Jacobson SW, Robinson LK, Hoyme HE, Mattson SN, et al. Unique facial features distinguish fetal alcohol syndrome patients and control in diverse ethnic populations. Alcohol. Clin. Exp. Res. 2007;31:1707–1713. doi: 10.1111/j.1530-0277.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- Mutsvangwa T, Douglas TS. Morphometric analysis of facial landmark data to characterize the facial phenotype associated with fetal alcohol syndrome. J. Anat. 2007;210:209–220. doi: 10.1111/j.1469-7580.2006.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherway DJ, Abbott AH, Gulamhuseinwala N, McGlaughlin KL, Anderson PJ, Townsend GC, David DJ. Three-dimensional computed tomography cephalometry of plagiocephaly: Asymmetry and shape analysis. Cleft Palate Craniofac. J. 2006;43:201–210. doi: 10.1597/04-174.1. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Strobeck C. Fluctuating asymmetry: measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986;17:391–421. [Google Scholar]
- Ras F, Habets LLMH, van Ginkel FC, Prahl-Andersen B. Facial left-right dominance in cleft lip and palate: Three-dimension evaluation. Cleft Palate Craniofac. J. 1994;31:461–465. doi: 10.1597/1545-1569_1994_031_0461_flrdic_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Blackwell PG, Stillman EC, Brook AH. Impact of landmark reliability on the plana Procrustes analysis of tooth shape. Arch. Oral Biol. 2002;47:545–554. doi: 10.1016/s0003-9969(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Schaefer K, Lauc T, Mitteroecker P, Gunz P, Bookstein FL. Dental arch asymmetry in an isolated Adriatic community. Am. J. Phys. Anthropol. 2006;129:132–142. doi: 10.1002/ajpa.20224. [DOI] [PubMed] [Google Scholar]
- Shaner DJ, Peterson AE, Beattie OB, Bamforth JS. Assessment of soft tissue facial asymmetry in medically normal and syndrome-affected individuals by analysis of landmarks and measurements. Am. J. Med. Genet. 2000;93:143–154. doi: 10.1002/1096-8628(20000717)93:2<143::aid-ajmg12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002;17:1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC. Sequence of developmental alterations following acute ethanol exposure in mice: craniofacial features of the fetal alcohol syndrome. Am. J. Anat. 1983;166:257–269. doi: 10.1002/aja.1001660303. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Ward RE, Jamison PL. Measurement precision and reliability in craniofacial anthropometry: Implications and suggestions for clinical applications. J. Craniofac. Gen. Dev Biol. 1991;11:156–164. [PubMed] [Google Scholar]
- Wiley DF, Amenta N, Alcantara DA, Ghosh D, Kil YJ, Delson E, Harcourt-Smith W, Rohlf FJ, St. John K, Hamann B. Evolutionary Morphing, Paper presented at: Proceedings of the IEEE Visualization 2005 (VIS'05) 2005.