Abstract
Age-related hearing loss - presbycusis - is the most common communication problem and third most prevalent chronic medical disorder of the aged. The CBA and C57BL/6 mouse strains are useful for studying features of presbycusis. The CBA loses its hearing slowly, like most humans. Because the C57 develops a rapid, high-frequency hearing loss by middle age, it has an “old” ear but a relatively young brain, a model that helps separate peripheral (cochlear) from central (brain) etiologies. This field of sensory neuroscience lacks a good mouse model for the 5-10% of aged humans with normal cochlear sensitivity, but who have trouble perceiving speech in background noise. We hypothesized that F1 (CBA × C57) hybrids would have better hearing than either parental strain. Measurements of peripheral auditory sensitivity supported this hypothesis, however, a rapid decline in the auditory efferent feedback system, did not. Therefore, F1s might be an optimal model for studying cases where the peripheral hearing is quite good in old age; thereby allowing isolation of central auditory changes due to brain neurodegeneration.
Keywords: Mouse auditory system, C57BL/6, Presbycusis, Medial olivocochlear bundle, DPOAE, ABR, CBA, aging, hearing loss, deafness, mouse, cochlea, central auditory system, efferent feedback system, olivocochlear bundle, neurophysiology
1. INTRODUCTION
Mammals suffer reduced auditory sensitivity and/or complex sound processing abilities as they age. This neurodegenerative deficit, presbycusis (age-related hearing loss), is the most prevalent age-linked decline in a sensory system. Several very useful animal models have emerged for studying the neurophysiological and molecular bases of presbycusis. Among mammals, the gerbil has proved to be a good model for one of the main etiologies characteristic of presbycusis in humans. Specifically, the gerbil exhibits a well-understood age-correlated decrease in the endocochlear potential and in the structural integrity of the specialized cochlear organ that produces endolymph: the stria vascularis (Schulte and Schmiedt 1992; Schmiedt 1993, 1996; Schmiedt et al. 2002).
Given the many advantages of genetically engineered mice, including transgenics and knockouts, for understanding the bases of sensory neurodegenerative processes, there has been a strong need for well-characterized mouse models of presbycusis. Thus far, the most useful have been the CBA and C57BL/6 strains (e.g., Frisina and Walton 2001a, 2001b; Ohlemiller and Frisina 2008). The CBA strain has a slow, progressive hearing loss, as in most humans, when one corrects for the absolute lifespan differences of mice and men, and shows relatively good auditory sensitivity and moderate hair cell loss in old age (Willott 1991; Spongr et al., 1997; Guimaraes et al. 2004; Sha et al. 2008). In addition, like most aging humans, the CBA exhibits complex sound processing deficits starting in middle age and progressing into old age (Walton et al. 1997, 1998, 2002; Simon et al., 2004). These include temporal processing declines that are linked to communication sound perceptual deficits of the aging auditory system, including difficulty processing and understanding speech (Gordon-Salant and Fitzgibbons 1993; Fitzgibbons and Gordon-Salant 1996).
The C57 strain possesses a mutation in the ahl gene that disrupts the development of stereocilia on the apical surfaces of cochlear hair cells, particularly involving deficiencies in the cadherin 23 protein. This is particularly harmful because the ion channels that mediate transduction of sound waves into the electrophysiological responses of hair cells are found in the membranes of the stereocilia. The net effect is that the C57 has an accelerated, peripheral, age-related hearing loss, as reflected in rapid elevations in auditory brainstem response (ABR) thresholds and declines in distortion-product-otoacoustic emission (DPOAE) levels (Henry and Chole 1980; Willott, 1991; Jimenez et al. 1999; Zhu et al. 2007) . This rapid age-related hearing loss resembles a key feature of human presbycusis, in that the loss starts in the high frequencies, and with age progresses to lower frequencies. Specifically, the C57 has a severe-to-profound high-frequency hearing loss by one year of age.
In terms of laboratory investigations of the biological underpinnings of presbycusis, it is important to identify or develop mouse models that capture the various aspects of presbycusis occurring in higher mammals and humans. As we attempt to unravel the different clinical etiologies of presbycusis that lead to translational goals of preventing, slowing down or reversing this common sensory disorder, we have lacked a good model, specifically for aged humans who have very good peripheral hearing, i.e., those that have audiograms within the normal hearing range of young adults. These so called “Golden Ears”, comprising 5-10% of our aged population (mostly women), even with their cochleae that in many ways resemble those of young adults, still have trouble with suprathreshold complex sound processing, particularly the coding of sound temporal features (Fitzgibbons and Gordon-Salant 1996; Snell et al. 2002; Pichora-Fuller et al. 2006) and perception of speech in background noise (Frisina and Frisina, 1998; Snell and Frisina 2000).
The present study aimed to identify a suitable mouse model for aged individuals who have exceptionally acute peripheral sensitivity. It was hypothesized that F1 hybrid offspring from existing strains, in this case the CBA and C57, would yield superior hearing in old age. The results of the present investigation support this hypothesis relative to peripheral sensitivity, but not for the efferent feedback system extending from the auditory brainstem to the outer hair cell system, i.e., the medial olivocochlear bundle (MOC).
2. METHODS
2.1. Subjects
All mice were bred in-house, housed according to institutional protocols, with original breeding pairs obtained from Jackson Laboratories. For this cross-sectional study, the numbers of mice and age ranges for each subject group are provided in Table 1. The young adult and middle age CBA data are from one of our previous investigations (Jacobson et al. 2003), and some of the young adult C57 subjects are from Zhu et al. (2007). All animal procedures were approved by the University of Rochester Committee on Animal Resources and are consistent with NIH Guidelines.
Table 1.
Numbers and ages of mice for the present study.
| F1 (CBA × C57) |
CBA |
C57 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject Group | Male |
Female |
Age |
Male |
Female |
Age |
Male |
Female |
Age |
| Young Adult | 9 | 9 | 2.1-4.0 | 11 | 10 | 2.1-4.1 | 8 | 8 | 3 |
| Middle-aged | 9 | 9 | 14.0-16.4 | 8 | 5 | 14.0-16.4 | 4 | 4 | 14.5 |
| Old-aged | 6 | 5 | 24.0-28.0 | 9 | 8 | 24.3-28.0 |
Age is given in months.
2.2. Auditory brainstem response methodologies
The ABR and DPOAE testing procedures were similar to our previous reports, and are summarized here (Jacobson et al. 2003; Guimaraes et al. 2004; Varghese et al. 2005; Frisina et al. 2007; Zhu et al. 2007). Prior to data acquisition, individual mice were microscopically examined for evidence of external ear canal and middle ear obstruction. Mice with clearly visualized, healthy tympanic membranes were included. ABRs were recorded with subcutaneous platinum needle electrodes placed at the vertex (non-inverting input), right mastoid prominence (inverted input) and tail (indifferent site). Calibrated tone pips, 5 ms duration, 0.5 ms rise–fall time (phase alternating 90°) were utilized. Electroencephalographic (EEG) activity was differentially amplified (50 or 100 k; Grass [Quincy, MA] model P511 EEG amplifier), then input to an A/D converter (Tucker-Davis Technologies [TDT, Alachua, FL] AD1), and digitized at 50 kHz. Each averaged response was based on 300–500 stimulus repetitions recorded over 10-ms epochs. Contamination by muscle or cardiac activity was prevented by rejecting data epochs in which the single-trace EEG contained peak-to-peak amplitudes exceeding 50 μV. The threshold was defined as the first level that did not evoke a response to a measured frequency, i.e., no difference from the baseline. During this procedure, a general anesthetic, Avertin (Tribomoethanol, 5 mg/10 g body weight intraperitoneal), was used to immobilize the mice. Normal body temperature was maintained at 38° C with a servo heating pad. The ABR was recorded in a small sound attenuating chamber (IAC).
2.3. Distortion-product otoacoustic emission stimulus and recording procedures
Mice were anesthetized with a mixture of ketamine/xylazine (120 and 10 mg/kg body weight, respectively) by intraperitoneal injection before experimental sessions. All recording sessions were completed in a soundproof acoustic chamber (IAC lined with Sonex) with body temperature maintained with a heating pad. Before recording, the stimulus probe and microphone coupler were placed in the test ear near the tympanic membrane. The coupler for the speaker for the contralateral noise source was placed close to the tympanic membrane in the opposite ear canal.
Ipsilateral acoustic stimulation and simultaneous measurement of DPOAEs was accomplished with a Tucker–Davis (TDT) BioSig System III. Stimuli were digitally synthesized at 200 kHz using SigGen software with the ratio of f2/f1 constant at 1.25, and L1=65 dB and L2=50 dB SPL, as calibrated in a 0.1 cc coupler simulating the mouse ear canal. After synthesis, f1 and f2 were each passed through an RP2.1 D/A converter to PA5 programmable attenuators. Following attenuation, the signals went to ED1 speaker drivers which fed into the EC1 electrostatic loudspeakers coupled to the ear canal via short flexible tubes with rigid plastic tapering tips. For DPOAE measurements, resultant ear-canal sound pressure was recorded with an ER10B+ low noise microphone and probe (Etymotic, Elk Grove Village, IL) housed in the same coupler as the f1 and f2 speakers. The output of the ER10B+ amplifier was input to an MA3 microphone amplifier, whose output went to an RP2.1 A/D converter for sampling at 200 kHz. A fast Fourier transform (FFT) was performed on the resultant waveform. The magnitude of f1, f2, the 2f1–f2 distortion product and the noise floor of the frequency bins surrounding the 2f1–f2 component were measured from the FFT. The procedure was repeated for geometric mean frequencies ranging from 5.6 to 44.8 kHz (8 frequencies/octave) to adequately assess the neuroethologically functional range of mouse hearing. Wideband noise (3-30 kHz bandwidth) was applied to the contralateral ear at 55 dB SPL. The 55 dB level was chosen so as to be below the middle ear reflex in mice, and other mammals such as cats, guinea pigs and humans (Puel and Rebillard, 1990; Williams and Brown, 1995; Liberman et al., 1996; Sun and Kim, 1999; Kujawa and Liberman, 2001).
Distortion product (DP)-grams were acquired under two different stimulus conditions for each test animal: in quiet and with contralaterally applied wideband noise. Trials were alternated three or four times for both conditions (random order for each subject), with the recording session duration limited by the depth of anesthesia. Duration of the testing was approximately one hour per animal.
2.4.Data analyses and statistics
Results for each subject group were analyzed for individual frequencies, and averaged for each of these three frequency bands: F1 = 4-15 kHz, F2 = 15-30 kHz and F3 = 30-48 kHz. Contralateral suppression (CS) magnitude was defined and calculated as DP level with contralateral noise minus DP level in quiet, computed for each frequency (negative values indicate greater efferent feedback suppression). Since DPOAE responses are most reflective of the outer hair cell system at the F2 cochlear location, DPOAE and CS magnitudes are plotted as a function of F2. The two-way analysis of variance (ANOVA) across age groups and frequencies was used for statistical analyses, followed by Bonferroni post hoc pair-wise tests, with power corrected for multiple comparisons (Prism, GraphPad, La Jolla, CA). Unless otherwise specified, all error bars in the figures represent standard error of the mean (SEM).
3. RESULTS
3.1. Progression of age-related hearing loss in F1 hybrids
3.1.1 Outer hair cells – distortion product otoacoustic emissions
DPOAE level or amplitude is a nonlinear, physiological measure of the functionality of the cochlear outer hair cell system (Lonsbury-Martin et al. 1990, 1991, 2001; Parham et al. 1997, 1999, Sun and Kim 1999). In the present investigation, DPOAE levels were fairly stable as a function of age for F1 mice, as presented in Fig. 1A. Changes in the low frequencies were the greatest for the old age mice, whereas, DPOAE amplitude declines were minor in the middle and high frequency ranges.
Figure 1.
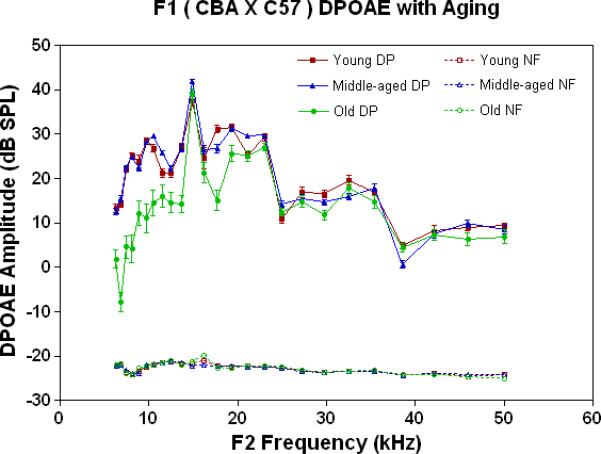
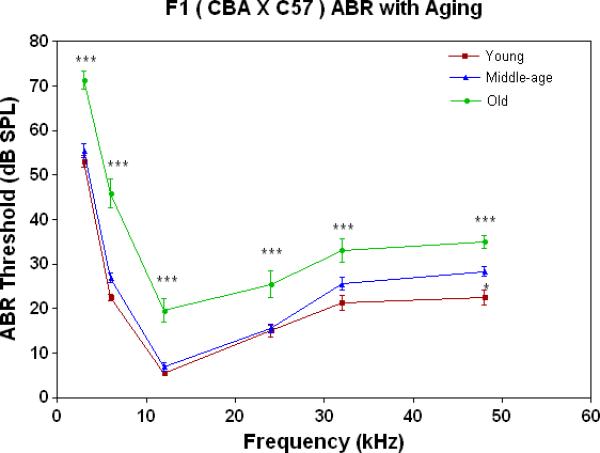
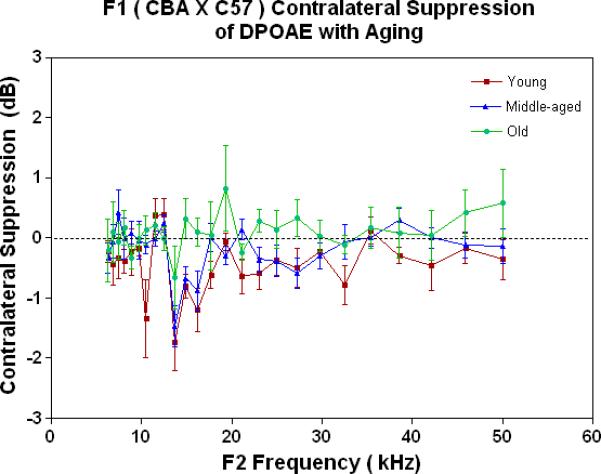
Peripheral hearing measures show surprising stability with age for the F1, whereas the auditory efferent feedback system showed modest responses. A) DPOAE amplitudes were robust and quite stable with age for the F1, except for declines in the lower frequencies in the oldest subject group. Noise floors for these data are given in Figs. 2A-2C, and are all below −15 dB SPL. The age main effect was statistically significant: F=336.8, df=2, p<0.0001. B) ABR thresholds for young adults and middle age mice were quite similar, except for an increase at the highest frequency tested in middle age for the F1. In contrast, the old F1 thresholds were significantly higher than the younger animals at all frequencies tested. The age main effect was statistically significant: F=149.2, df=2, p<0.0001. For the post-hoc Bonferroni pairwise comparisons: *p<0.05; ***p<0.001. C) The young adult F1 shows a modest amount of CS across the mouse frequency range of hearing. Declines begin to occur in middle age, and by old age, very little CS is observed along with a small amount of contralateral enhancement (+ CS). The age main effect was highly significant: F=18.6, df=2, p<0.0001, and the post-hoc pairwise comparisons were not.
3.1.2. Peripheral sensitivity – auditory brainstem response
ABR thresholds provide a quantitative measure of the overall sensitivity of the peripheral auditory system. As shown in Fig. 1B, thresholds are quite stable through middle age for the F1s, then rise about 10-20 dB in old age across the entire frequency range tested.
3.1.3. Feedback from the brain to the ear – medial olivocochlear bundle
The MOC is the major component of the auditory feedback system from the brainstem to the outer hair cells of the cochlea. If DPOAEs are measured in one ear, and when a noise is presented to the opposite ear, in a young adult mammal with normal hearing, the DPOAE levels will be suppressed (Veuillet 1991, Lieberman 1988). Previous studies have shown that a contralateral stimulus can result in a decrease of DPOAE magnitude by up to several dB (Moulin 1993, Liberman 1996, Williams 1997). This phenomenon is called contralateral suppression (CS). CS was measured in the present investigation for the F1s as a function of age, as given in Fig. 1C. The young adult mice show a small amount of CS (negative dB is a measure of CS strength), which starts to go away (move towards the 0 dB line on the graph) as the animals age. Note also, that the only significant amount of “contralateral enhancement” (+ responses above the 0 dB line) occurs in the old animals (green data).
3.2. Comparison of F1 hybrids to parent strains
3.2.1. Outer hair cells – distortion product otoacoustic emissions
The physiological integrity of the outer hair cell system for the three strains of mice in the present investigation is given in Fig. 2A. As young adults, all three strains have DPOAE levels well above the noise floor, indicating a significant response of the outer hair cells. The only significant differences among the strains at this young adult stage are the consistently lower DPOAE amplitudes at the middle and high frequencies for the C57 parent strain. A trend towards higher amplitudes of the F1s, seen at the high frequencies for the young adults (Fig. 2A), becomes more apparent across all frequencies tested for the middle age mice (Fig. 2B). As previous research has consistently shown, the middle age C57 displays a severe high frequency hearing loss by middle age (Fig. 2B-blue data), with very little DPOAE response. Remarkably, in old age, where the C57 is for the most part deaf, the F1 has significantly higher DPOAEs than either parent strain, including the CBAs, as shown in Figs. 2C-D.
Figure 2.
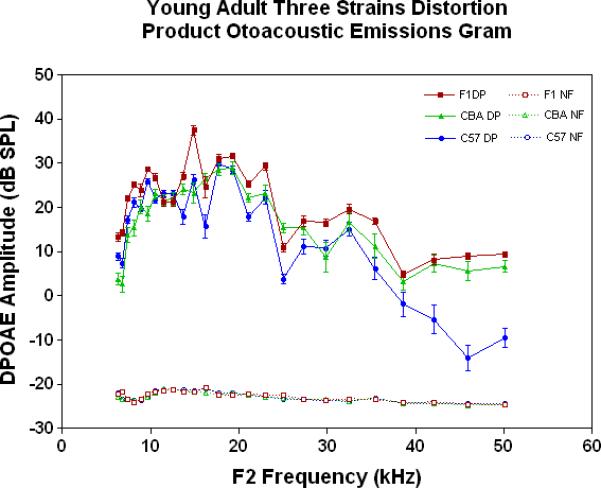
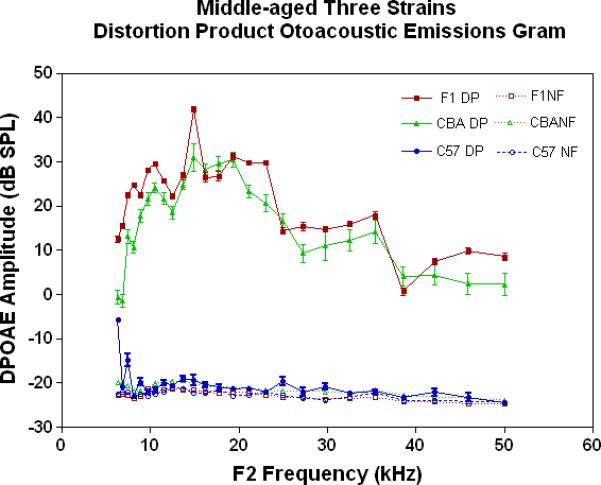
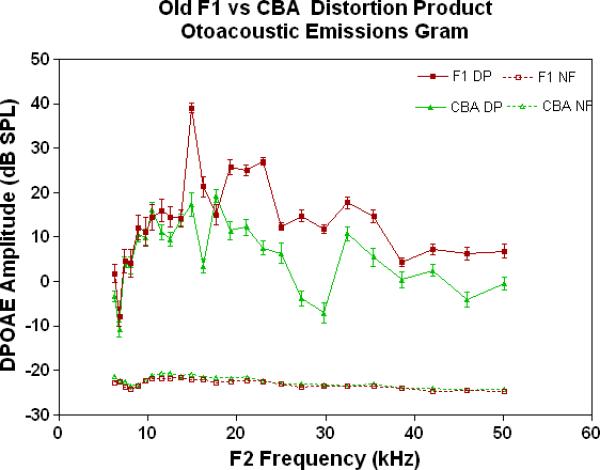
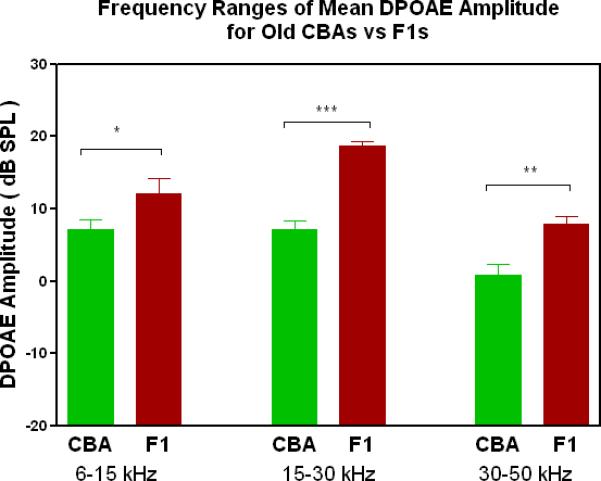
DPOAE levels declined with age most severely for the C57, least severely for the F1, and the CBA was intermediate and showed relatively good responses in old age. A) For young adults, all three strains were similar at low and middle frequencies, but the C57 started to manifest its rapid hearing loss at high frequencies. The strain main effect was statistically significant: F=123.6, df=2, p<0.0001. NF=noise floor. B) By middle age, the C57 outer hair cell system is quite impaired, whereas the F1 DPOAE levels are very similar to the young adults. The CBA levels are starting to drop. The strain main effect was statistically significant: F=7151, df=2, p<0.0001. C) The C57 is completely deaf in old age (data not shown), whereas the old F1 shows remarkably strong DPOAEs. The CBA shows some age-related hearing loss relative to the F1, particularly in the middle and high frequencies. The strain main effect was statistically significant: F=194.5, df=1, p<0.0001. D) The histogram highlights the healthier outer hair cell system of the F1 relative to the CBA in old age, in all three bandwidths of the mouse frequency range of hearing. For 6–15 kHz: t = 2.15, df = 26, *p<0.05; For 15–30 kHz, t = 6.92, df = 26, ***p< 0.0001; For 30–50 kHz, t = 3.35, df = 26, **p=0.0025.
3.2.2. Peripheral sensitivity – auditory brainstem response
All three strains have similarly good peripheral sensitivity as young adults, as shown by the ABR threshold data of Fig. 3A. Note, there is a trend at most frequencies for the F1 to be slightly more sensitive than the two parental strains, and for the C57 to begin to show a high-frequency hearing loss (threshold elevation for the blue data point at 48 kHz). In middle age, as given in Fig. 3B, the C57 is well on the way towards deafness, with sensitivity so poor that thresholds were only obtained at 6 kHz. At the other frequencies, the thresholds were above 80 dB, and therefore unattainable. At the middle and high frequencies (12-48 kHz) the F1 shows better peripheral sensitivity (lower ABR thresholds) than the CBA. For the old age groups, the C57 is deaf (thresholds not measurable), and the F1 has significantly lower ABR thresholds at all frequencies, except at the lowest frequency measured (3 kHz), relative to the CBA (See Fig. 3C).
Figure 3.
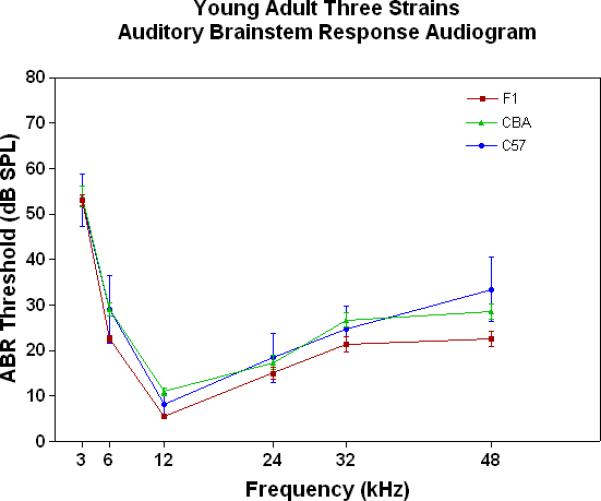
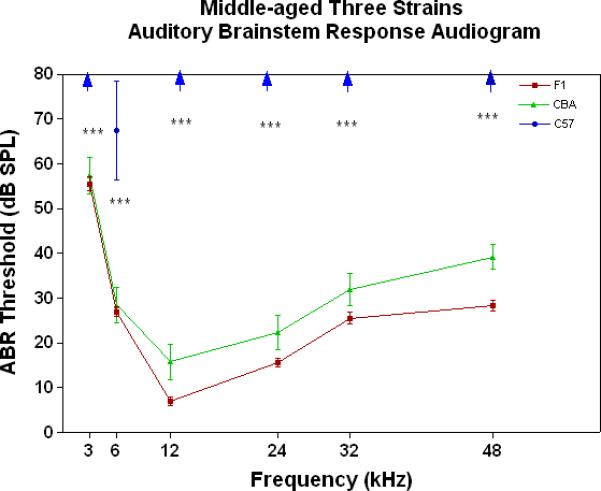
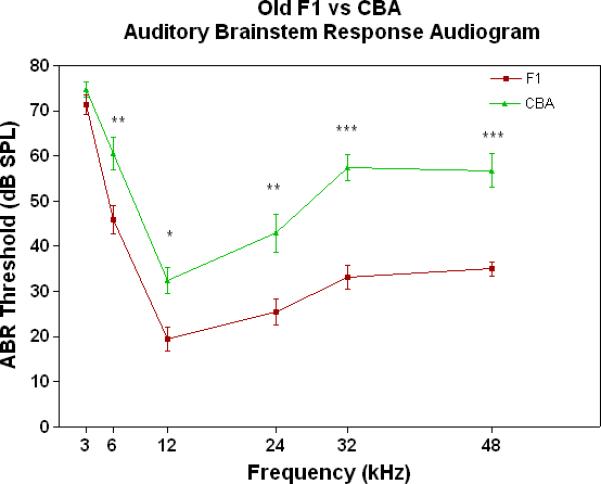
The F1 maintains noteworthy peripheral sensitivity into old age, whereas the C57 is well on its way to deafness by middle age. The CBA has relatively good hearing, but the ABR thresholds are not as sensitive as the F1, particularly in old age. A) All three strains have good hearing as young adults, although the CBA and C57 tend to be a little less sensitive than the F1 (ANOVA for the strain main effect: F=3.92, df=2, p=0.021). B) In middle age, the F1 and CBA have relatively good ABR thresholds, although the F1 tends to be more sensitive at middle and high frequencies. The C57 has thresholds above 80 dB SPL, except at 6 kHz. The strain main effect was statistically significant: F=544.2, df=2, p<0.0001. For the post-hoc Bonferroni pairwise comparisons, the C57 thresholds were significantly above the others: ***p<0.001. C) In old age, the F1 and CBA have moderate hearing losses, but the F1 is more sensitive at all frequencies, except 3 kHz. The age main effect was statistically significant: F=149.2, df=2, p<0.0001. For the post-hoc Bonferroni pairwise comparisons: *p<0.05; ***p<0.001.
3.2.3. Feedback from the brain to the ear – medial olivocochlear bundle
As young adults (Fig. 4A), the CBA (green data) shows better CS relative to the F1 and the C57, particularly at the low frequencies. There is a trend for the F1 (red data) to have slightly improved MOC function relative to the C57 (blue data) at the middle and high frequencies. As discovered previously, the C57 has very little MOC response by 2 months of age and thereafter (Varghese et al. 2005; Zhu et al. 2007). Fig. 4B shows that by middle age, as originally reported by Jacobsen et al. (2003), the CBA has lost a significant amount of the MOC response at low frequencies. In addition, the F1 is losing what little response it had as a young adult (Compare red data in Fig. 4A to Fig. 4B, and see Fig. 1C). However, at middle and high frequencies there is a tendency for the CBA (green) to show more CS than the F1 (red). In old age both strains have lost their CS, and show a bit of contralateral enhancement at many frequencies, as plotted in Fig. 4C.
Figure 4.
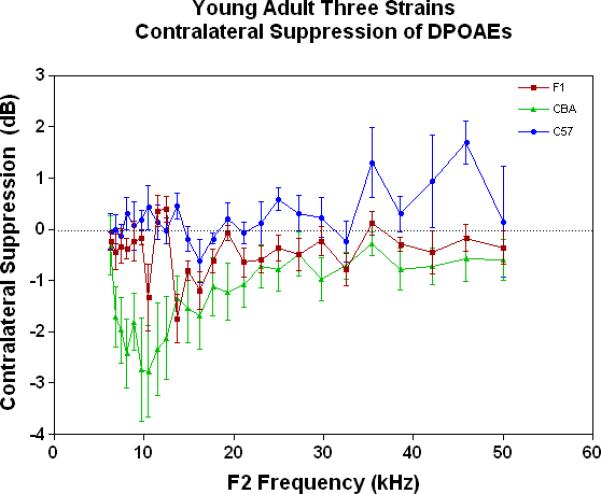
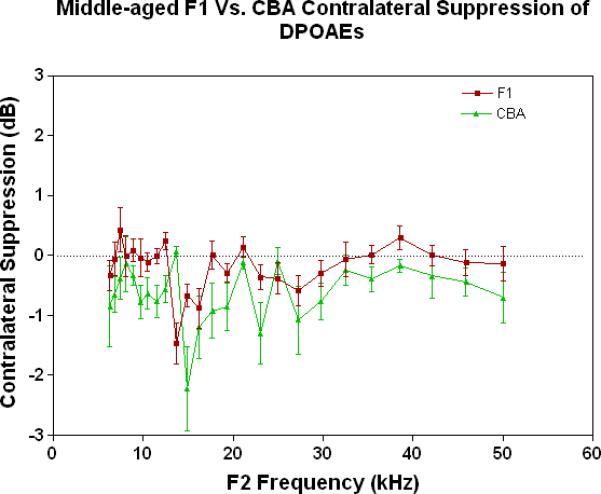
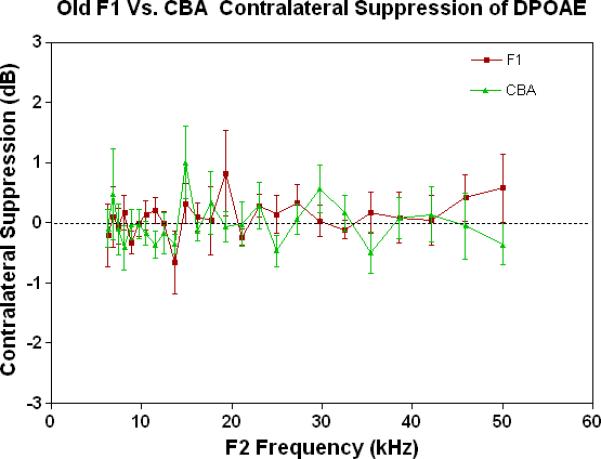
As young adults, the CBA has robust efferent feedback activation, particularly at low frequencies, the F1 has less response, and the C57 exhibits very little efferent activity. A) For young adults, the F1 is intermediate between the parental strains. CBA data re-plotted from Jacobson et al. (2003). The strain main effect was statistically significant: F=64.8, df=2, p<0.0001. B) The CBA continues to show more CS than the F1 in middle age, across the entire mouse hearing range. The strain main effect was statistically significant: F=27.7, df=1, p<0.0001. C) In old age, none of the strains show any significant CS efferent feedback system response.
4. DISCUSSION
4.1. Peripheral hearing sensitivity of F1 hybrids exceeds that of either parent strain
4.1.1. Outer Hair Cell System and Cochlear Sensitivity
Generally mammals, including humans, show declines in otoacoustic emissions amplitudes and elevations in ABR thresholds with age (human: Collet et al. 1990; Lonsbury-Martin et al. 1991; mouse: Jacobson et al. 2003; Guimaraes et al. 2004). These declines in cochlear sensitivity can be due to loss of hair cells or spiral ganglion neurons, or disruption of the endocochlear potential due to age-related damage or pathology of the lateral wall's stria vascularis. All three strains of the present investigation manifested a loss of hearing with age, but with a wide range of hearing loss rates and patterns, which likely reflect differences in the underlying cellular pathologies associated with presbycusis. For the F1, age-related ABR threshold elevations exceed the declines in DPOAE magnitudes. This suggests that the outer hair cells, which produce the DPOAEs, are more resistant to aging in the F1 than the inner hair cells, which are a primary determinant of the ABR thresholds.
It was not surprising that the age-related hearing loss of the F1 was not as severe as the C57 parental strain, since the F1, unlike the C57, has only 1 allele for the ahl gene, an autosomal recessive condition requiring two copies for phenotypic expression. Surprisingly though, the outer hair cell system functionality (DPOAEs) and overall peripheral sensitivity (ABRs) of the F1 mice exceeded that of the CBA parental strain at all ages, but most noticeably in the oldest age group. Specifically, the ABR results for F1 mice showed a significant increase in their thresholds between the middle and old-aged groups, but this loss in sensitivity was less than that displayed by the CBA (Jacobson et al., 2003; Guimaraes et al., 2004). This suggests that there are some, as yet unidentified genes, probably autosomal recessive, that contribute to the age-related hearing loss of CBA mice. Like the ahl gene, only one allele of these unknown contributors to presbycusis in the CBA would be passed on to the F1, preventing phenotypic expression in the F1.
4.2. Central auditory feedback is intermediate between the parent strains
4.2.1. Medial olivocochlear bundle
The MOC system neuronal cell bodies lie in the superior and ventral portions of medial areas of the superior olivary complex and innervate the outer hair cells via their myelinated fibers (Warr and Guinan 1979; Guinan et al. 1983; Guinan and Gifford 1988a-c; Maison et al. 2003). In addition, surgical transection of the MOC pathways at the base of the fourth ventricle has been shown to eliminate the effect of contralateral suppression mediated by the crossed component of the MOC (Puel and Rebillard 1990).
Previous investigations have reported a decline in MOC functionality with aging, starting in middle age for the CBA (Jacobson et al. 2003; Varghese et al. 2005). In contrast, using the same measurement paradigms, it was discovered that the C57 loses most of its MOC-CS response within a couple months of age (Frisina et al. 2007; Zhu et al. 2007). Unlike the superior peripheral auditory sensitivity displayed by the F1, the responsiveness of the F1 MOC system, as measured by CS in the present report, was intermediate between the two parental strains.
4.3. Is a functional auditory efferent feedback system necessary for optimal cochlear sensitivity?
4.3.1. Aging auditory system: efferent system declines usually precede peripheral pathology
The MOC auditory efferent feedback system, which upon activation suppresses or inhibits cochlear output and outer hair cell motility, i.e., the gain of the cochlear amplifier, has been implicated in several important aspects of auditory function and physiology (Liberman and Brown 1986; Liberman 1989; Kujawa and Liberman 2001). Previous reports present evidence that the MOC system is involved in increasing the signal-to-noise ratio for complex sound coding, including speech perception (Winslow 1987, Micheyl et al. 1995; Micheyl and Collet 1996), mediating auditory selective attention (Collet et al. 1990; Chery-Croze et al., 1993), and helping preserve cochlear function in the face of ototoxic insults such as loud noise (Rajan 1990).
The results of the present investigation revealed that the outer hair cell system and the overall sensitivity of the auditory system of the F1 was superior to both parental strains, and yet the MOC efferent feedback system was not as prominent in the F1 as in the CBA, at all ages. This is puzzling because the preponderance of evidence concerning relations between presbycusis and the efferent system are that declines in the efferent system precede declines in the outer hair cell system and age-related deficits in cochlear sensitivity. This degenerative sequence was true for both parental strains of the present investigation, as demonstrated by Jacobson et al. (2003) and Varghese et al. (2005) for the CBA, and by Frisina et al. (2007) and Zhu et al. (2007) for the C57. It is also true for humans, as discovered by Kim et al. (2002) who measured DPOAEs and CS for human subjects of different ages, and found significant declines in the MOC system in middle age and old human listeners, all of whom had audiograms within the normal range. In experimentally-altered instances of the progression of presbycusis, this has also been the case. For example, Thompson et al. (2006) found that the MOC efferent feedback system started to decline prior to a progressive peripheral hearing loss in young adult female CBA mice who were administered tamoxifen, an estrogen receptor blocker commonly employed clinically to prevent recurrence of breast or ovarian cancer.
4.3.2. Implications of the F1 hybrid's efferent system functionality
So what might explain the noteworthy sensitivity of the F1 cochlea well into old age, in light of its seemingly diminished auditory efferent feedback system from a relatively young age? Preservation of cochlear cells in old age, as a basis for the superior hearing of the old F1s, could result from a number of possible biological mechanisms that may be enhanced in the F1s that are aimed at preserving the function of peripheral sensory organs during aging. For instance, some recessive genes that make the parental strains more susceptible to age-related ototoxic processes or pathways, such as the age-linked buildup of reactive oxygen species or calcium excitotoxicity, might not be expressed in the F1s. Another possible explanation is that the methods employed in the present investigation, and most previous investigations of the aging mammalian auditory system are limited in regard to the measurement of the MOC auditory efferent feedback system. Specifically, the strength of the MOC system is dependent upon the particular parameters used to measure its response, including the F1/F2 ratio, the relative and absolute levels of F1 and F2, and whether a contralateral noise or ipsilateral response decay is used to measure the MOC activation (Liberman et al. 1996). It is not the case that one set of parameters is more optimal than another for all applications, but it is important to keep in mind that in most studies, including the present investigation, that only a small portion of the parameter space is investigated due to time constraints or other procedural limitations. To follow up thoroughly one of the provocative findings of the present report, that the efferent declines in the F1 does not precede significant declines in DPOAEs or ABRs, the full parameter space for measuring MOC activation should be employed. Specifically, the response areas for MOC activation should be measured as a function of age, varying the F1/F2 ratio and levels, over useful ranges indicated by previous reports (Halsey et al. 2005). Only then can the hypothesis that efferent declines necessarily precede aging deficits in cochlear sensitivity be more definitively accepted or rejected.
5. Summary and Conclusion
The maintenance of the peripheral auditory system with age in the F1 hybrid mice point to their similarity with humans who demonstrate good hearing in their advanced years, the individuals often referred to as having ‘golden ears’. So, it follows that F1 (CBA × C57) mouse might be an ideal model for humans with good audiometric sensitivity in old age, and provide an animal-model gateway for exploring age changes in the central auditory system relatively uncontaminated by a cochlear hearing loss. Investigation of the peripheral, central and efferent auditory systems in F1 might elucidate the interplay between biochemical, molecular and neurophysiological pathways in the auditory system that result in hearing preservation, despite advanced age.
Acknowledgements
Special thanks to John Housel, Enza Daugherty and Veronica Stefano for project assistance, and Dr. Robert Frisina, Sr., for helpful critiques. Work supported by NIH Grant P01 AG09524 from the National Institute on Aging, and NIH Grant P30 DC05409 from the National Institute on Deafness & Communication Disorders. There are no actual or potential conflicts of interest for this article.
Grant Support: NIH Grant P01 AG09524 from the National Institute on Aging, and NIH Grant P30 DC05409 from the National Institute on Deafness & Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Chery-Croze S, Moulin A, Collet L. Effect of contralateral sound stimulation on distortion product 2f1-f2 in humans: evidence of frequency specificity. Hear. Res. 1993;68:53–58. doi: 10.1016/0378-5955(93)90064-8. [DOI] [PubMed] [Google Scholar]
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micromechanical properties in human subjects. Hear Res. 1990a;43:251–261. doi: 10.1016/0378-5955(90)90232-e. [DOI] [PubMed] [Google Scholar]
- Collet L, Moulin A, Gartner M, Morgan A. Age-related changes in evoked otoacoustic emissions. Ann. Otol. Rhinol. Laryngol. 1990b;99:993–997. doi: 10.1177/000348949009901212. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Auditory temporal processing in elderly listeners. J. Am. Acad. Audiol. 1996;7:183–189. [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: Relations to possible neural sites. Hear. Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Newman SR, Zhu X. Auditory efferent activation in CBA mice exceeds that of C57s for varying levels of noise. J. Acoust. Soc. Am. 2007;121:EL-29–34. doi: 10.1121/1.2401226. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Walton JP. Neuroanatomy of the mouse central auditory system. In: Willott JP, editor. Handbook of Mouse Auditory Res.: From Behavior to Molecular Biology. CRC Press; New York: 2001a. pp. 243–277. [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JP, editor. Handbook of Mouse Auditory Res.: From Behavior to Molecular Biology. CRC Press; New York: 2001b. pp. 339–379. [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J. Speech Hear. Res. 1993;36:1272–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S-H, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear. Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hear. Res. 1988a;33:97–114. doi: 10.1016/0378-5955(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. II. Spontaneous rate. Hear. Res. 1988b;33:115–128. doi: 10.1016/0378-5955(88)90024-x. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III. Tuning curves and thresholds at CF. Hear. Res. 1988c;37:29–46. doi: 10.1016/0378-5955(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr., Warr WB, Norris BE. Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J. Comp. Neurol. 1983;221:358–370. doi: 10.1002/cne.902210310. [DOI] [PubMed] [Google Scholar]
- Halsey K, Skjonsberg S, Ulfendahl M, Dolan DF. Efferent-mediated adaptation of the DPOAE as a predictor of aminoglycoside toxicity. Hear. Res. 2005;201:99–108. doi: 10.1016/j.heares.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse. Audiol. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Kim SH, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion otoacoustic emissions declines with age: a comparison of findings in CBA mice with human listeners. Laryngoscope. 2003;113:1707–1713. doi: 10.1097/00005537-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Age-related loss of distortion product otoacoustic emissions in four mouse strains. Hear. Res. 1999;138:91–105. doi: 10.1016/s0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Kim S, Frisina DR, Frisina RD. Effects of Age on Contralateral Suppression of Distortion-Product Otoacoustic Emissions in Human Listeners with Normal Hearing. Audiol. Neuro-otol. 2002;7:348–357. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Effects of olivocochlear feedback on distortion product otoacoustic emissions in guinea pig. J. Assoc. Res. Otolaryngol. 2001;2:268–78. doi: 10.1007/s101620010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Rapid assessment of sound-evoked olivocochlear feedback: suppression of compound action potentials by contralateral sound. Hear. Res. 1989;38:47–56. doi: 10.1016/0378-5955(89)90127-5. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear. Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Puria S, Guinan JJ., Jr. The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1–f2 distortion product otoacoustic emission. J. Acoust. Soc. Am. 1996;99:3572–3584. doi: 10.1121/1.414956. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Cutler WM, Martin GK. Evidence for the influence of aging on distortion product otoacoustic emissions in humans. J. Acoust. Soc. Am. 1991;89:1749–1759. doi: 10.1121/1.401009. [DOI] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in the mouse: Immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J. Comp. Neurol. 2003;455:406–416. doi: 10.1002/cne.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Collet L. Involvement of the olivocochlear bundle in the detection of tones in noise. Journal of the Acoustical Society of America. 1996;99:1604–1610. doi: 10.1121/1.414734. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Morlet T, Giraud AL, Collet L, Morgon A. Contralateral suppression of evoked otoacoustic emissions and detection of a multi-tone complex in noise. Acta Otolaryngol. 1995;14:6992–7007. doi: 10.3109/00016489509139286. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Frisina RD. Clinical Characterization of Age-Related Hearing Loss and Its Neural and Molecular Bases. In: Schacht J, Popper A, Fay R, editors. Auditory Trauma, Protection and Treatment. Springer-Verlag; New York: 2008. pp. 145–194. Ch. 6. [Google Scholar]
- Parham K. Distortion product otoacoustic emissions in the C57BL/6J mouse model of age-related hearing loss. Hear. Res. 1997;112:216–234. doi: 10.1016/s0378-5955(97)00124-x. [DOI] [PubMed] [Google Scholar]
- Parham K, Sun X-M, Kim DO. Distortion product otoacoustic emissions in the CBA/J mouse model of presbycusis. Hear. Res. 1999;134:29–38. doi: 10.1016/s0378-5955(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Benson NJ, Hamstra SJ. Effect of age on detection of gaps in speech and nonspeech markers varying in duration and spectral symmetry. J. Acoust. Soc. Am. 2006;119:1143–1155. doi: 10.1121/1.2149837. [DOI] [PubMed] [Google Scholar]
- Puel JL, Rebillard G. Effect of contralateral sound stimulation on the distortion product 2F1-F2: Evidence that the medial efferent system is involved. J. Acoust. Soc. Am. 1990;84:1630–1635. doi: 10.1121/1.399410. [DOI] [PubMed] [Google Scholar]
- Rajan R. Electrical stimulation of the inferior colliculus at low rates protects the cochlea from auditory desensitization. Brain Res. 1990;506:192–204. doi: 10.1016/0006-8993(90)91251-b. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Cochlear potentials in gerbils: Does the aging cochlea need a jump start? In: Verrillo RT, editor. Sensory Research: Multimodal Perspectives. Erlbaum Assoc.; Hillsdale, NJ: 1993. pp. 91–103. [Google Scholar]
- Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear. Res. 1996;102:125–132. doi: 10.1016/s0378-5955(96)00154-2. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura H, Schulte BA. Effects of furosemide applied chronically to the round window: A model of metabolic presbycusis. J. Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na,K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear. Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-k. [DOI] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear. Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Frisina RD, Walton JP. Age reduces response latency of mouse inferior colliculus neurons to AM sounds. J. Acoust. Soc. Am. 2004;116:469–477. doi: 10.1121/1.1760796. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J. Acoust. Soc. Am. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Snell KB, Mapes FM, Hickman ED, Frisina DR. Word recognition in competing babble and the effects of age, temporal processing, and absolute sensitivity. J. Acoust. Soc. Am. 2002;112:720–727. doi: 10.1121/1.1487841. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57B1/6 mice throughout their life spans. J. Acoust. Soc. Am. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Sun XM, Kim DO. Adaptation of 2f1-f2 distortion product otoacoustic emission in young-adult and old CBA and C57 mice. J. Acoust. Soc. Am. 1999;105:3399–3409. doi: 10.1121/1.424668. [DOI] [PubMed] [Google Scholar]
- Thompson SK, Zhu X, Frisina RD. Estrogen Blockade Reduces Auditory Feedback in CBA Mice. Otolaryngol.- Head & Neck Surg. 2006;135:100–105. doi: 10.1016/j.otohns.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Varghese GI, Zhu X, Frisina RD. Age-related declines in contralateral suppression of distortion product otoacoustic emissions utilizing pure tones in CBA/CaJ mice. Hear Res. 2005;209:60–67. doi: 10.1016/j.heares.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JE, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J. Comp. Physiol. A. 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O'Neill WE. Age-related alteration in neural processing of silent gaps in the central nucleus of the inferior colliculus in the CBA mouse model of presbycusis. J. Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. J. Neurophysiol. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ., Jr. Efferent innervation of the organ of Corti: Two separate systems. Brain Res. 1979;173:152–155. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the Auditory System: Anatomy, Physiology, and Psychophysics. Singular Pub. Group; San Diego: 1991. [Google Scholar]
- Willott JF, Erway LC. Genetics of age-related hearing loss in mice. IV. Cochlear pathology and hearing loss in 25 BXD recombinant inbred mouse strains. Hear. Res. 1998;119:27–36. doi: 10.1016/s0378-5955(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tone in noise. J. Neurophysiol. 1987;57:1002–1021. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Zhu X, Vasilyeva ON, Kim S-H, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent system declines precede age-related hearing loss: Contralateral suppression of otoacoustic emissions in mice. J. Comp. Neurol. 2007;503:593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]


