Abstract
Humans are exposed to different mixtures of PCBs depending on the route of exposure. In this study we investigated the potential contribution of inhalation to the overall human exposure to PCBs in an urban area. For this purpose, the mechanistically based, non-steady state bioaccumulation model ACC-HUMAN was applied to predict the PCB body burden in an adult living in the Midwestern United States who eats a typical North American diet and inhales air contaminated with PCBs. Dietary exposure was estimated using measured data for eighteen PCB congeners in different food groups (fish, meat and egg, dairy products). Two scenarios for inhalation exposure were evaluated: one using air concentrations measured in Chicago, and a second using air measurements in a remote area on Lake Michigan, Sleeping Bear Dunes. The model predicted that exposure via inhalation increases the accumulated mass of PCBs in the body by up to thirty percent for lower chlorinated congeners, while diet is by far the dominant source of exposure for those PCB congeners that accumulate most in humans.
Keywords: PCB, human exposure, inhalation, modeling
Introduction
The commercial production of polychlorinated biphenyls (PCBs) started in 1929 and increased until 1976, when production and sales were banned in the Unites States. PCBs are still being emitted into the atmosphere from primary sources ( vaporization from PCB containing products) and secondary processes including water-air exchange (Hornbuckle et al., 1995) and emission from sludge drying beds (Yi et al., 2008). They are transported to remote areas through long range atmospheric transport followed by deposition (Brunciak et al., 2001; Mandalakis and Stephanou, 2004). It has been shown that PCB concentrations in the atmosphere are correlated with population density, indicating that urban areas are important sources of PCBs (Sun et al., 2007). The urban cities surrounding the Great Lakes, especially Chicago, have significantly higher gas-phase PCB concentrations than non-urban areas (Hafner and Hites, 2003; Sun et al., 2007; Wethington and Hornbuckle, 2005). Furthermore, PCB concentrations in indoor air can be over an order of magnitude higher than in outdoor air (Harrad et al., 2006), and indoor air has been shown to be a greater source of PCBs to the atmosphere than emissions from soil (Jamshidi et al., 2007).
The physical and chemical properties of the different PCB congeners, which determine their relative presence in different environmental compartments, span over a wide range (e.g., 4.46 - 8.18 for log KOW (Hawker and Connell, 1988) and 6.65 – 11.96 for the log octanol-air partition coefficient (KOA) (Zhang et al., 1999). All 209 PCB congeners have KOA-KOW combinations that give them a high potential for bioaccumulation in humans if they are persistent in the environment and not readily metabolized (Czub and McLachlan, 2004a). PCB bioaccumulation in humans is known to occur via partitioning of PCBs from air and water into terrestrial plants and aquatic autotrophs and invertebrates of the lowest trophic levels, followed by transfer through the food webs (Schwarzenbach et al., 2003). Although it has long been assumed to be insignificant compared to dietary exposure, little is known about the relative importance of PCB inhalation. In studying PCB congener patterns in human serum, indications were found of exposure to PCBs of atmospheric origin (DeCaprio et al., 2005). Recently it was estimated that inhalation of PCBs can make an important contribution to the accumulation of PCBs in humans (Harrad et al., 2006). This is more likely to be the case in populations where fish consumption is low and airborne PCB concentrations are high and for occupational exposure (ATSDR, 2000).
The aim of this study was to investigate the relevance of PCB accumulation from air for populations in urban areas with an industrial history of PCB distribution or use. The contribution of inhalation to the external and internal exposure was quantified using the human module in the bioaccumulation model ACC-HUMAN (Czub and McLachlan, 2004b). The inputs to the model were concentrations of individual PCB congeners in the diet and in the air, together with the physical chemical properties of the congeners. To evaluate the influence of exposure to urban air, two different scenarios were compared, one using PCB concentrations in air from Chicago and a second using data from a remote site on Lake Michigan (Sleeping Bear Dunes). Additionally, the model was explored using indoor exposure levels. The results of the model are compared to PCB congener concentrations measured in five blood bank samples collected from Chicago men.
Methods
Bioaccumulation Model
The mechanistically based, non-steady state bioaccumulation model ACC-HUMAN was employed to estimate the concentrations of PCBs in humans (Czub and McLachlan, 2004b). The model is subdivided into an agricultural and a marine system, each represented by one food chain. The top predator linking both systems is the human. The model calculates chemical uptake and accumulation for humans and the organisms along the food chains that are key vectors of human exposure. A chemical mass balance is conducted for each organism, including humans of both sexes. The uptake pathways considered are diet and inhalation, while the elimination pathways treated are metabolism, percutaneous excretion, urination, egestion, birth, nursing and exhalation.
For this study, the human mass balance module was extracted from ACC-HUMAN and run as a stand-alone model. The model inputs were a historical scenario for the PCB concentration in air, a historical scenario for the PCB concentrations in the major food groups contributing to dietary exposure, namely fish, meat (including poultry and egg), and dairy products, and the physical chemical properties of the given PCB congener. Using these inputs, the model calculated the time course of the lipid normalized PCB concentration in tissue over the lifetime of a man born in 1950. Changes in mother-child transfer of contaminants, growth rate, body lipid fraction, dietary habits, and respiration rate as a function of age were considered. The parameterization suggested in Czub and McLachlan, 2004b was adopted, with the only difference that the specific ingestion rates for different food items were taken from the Nutrition Canada Survey (Bureau of Nutritional Science, 1977) as summarized in Dabeka et al. (1993) and normalized to the item specific average lipid content (Jake Ryan, Health Canada, Bureau Chemical Safety, Canada, personal communication). Dietary habits were defined on the basis of dietary intake data for an average male Canadian of the age group 20-39 years (Dabeka et al., 1993) and the daily lipid ingestion rate was set to 60 g for a 25 year old man. The relative fractions of the three food groups in the diet considered were assumed to be constant throughout the human lifetime, with 52% of the ingested lipids originating from dairy products, 47% from meat (including poultry and eggs), and 1% from fish. The daily lipid ingestion rate was assumed to vary with age in the same way as defined in ref. (Czub and McLachlan, 2004b). The model endpoint was the lipid normalized concentration in the man at the age of 56.
Model Input
Physical chemical properties
Data for eighteen PCB congeners were available in both food and air (see Table 1); these were chosen for the study. The physical chemical properties of the PCB congeners required as model input were KOW and the air-water partition coefficient (KAW) at 25°C, the respective heats of phase transfer (ΔUOW and ΔUAW) with units of (J mol−1), and the first order rate constant for metabolism kM (h−1). The KOW values were taken from Hawker and Connell (Hawker and Connell, 1988) and the KAW values were calculated from the Henry’s Law constants reported in Dunnivant et al. (1992). The heats of phase transfer were calculated from regressions of ΔU values versus molar mass presented in Li et al. (2003), whereby ΔUOW was calculated as the sum of ΔUAW and the heat of phase transfer between octanol and air, ΔUOA. The first order rate constants for metabolism were selected from Brown (1994). These rate constants were determined for adults. No rate constants were available for children, so these rate constants were applied for the whole lifetime. Since the model endpoint was a 56 year old man, this assumption was of little consequence. Log KOW, log KAW and kM are shown in Table 1.
Table1.
Log KAW, log KOW and first order metabolic rate constants used in ACC-HUMAN (Dunnivant et al., 1992; Hawker and Connell, 1988; Brown, 1994), and PCB concentration in air and diet used as model input. PCB numbering according to Ballschmiter et al. (1993).
| Food (pg/g l.w.) | Air (pg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Log KAW 25 °C |
Log KOW 25 °C |
kM (yr−1) | Dairy products |
Meat and Egg |
Fish | Chicago | Sleeping Bear Dunes |
|
| PCB 1 | −0.191 | 4.46 | 200 | 7.51 | ||||
| PCB 2 | −1.93 | 4.69 | 60 | 4.1 | ||||
| PCB 3 | −1.95 | 4.69 | 80 | 5.91 | ||||
| PCB 4 | −1.87 | 4.65 | 200 | 41.6 | 1.95 | |||
| PCB 8 | −1.91 | 5.67 | 80 | 53.9 | 3.29 | |||
| PCB 11 | −1.93 | 5.28 | 10 | 24.1 | ||||
| PCB 15 | −2.04 | 5.3 | 0.5 | 13.8 | 5.69 | |||
| PCB 16 | −1.99 | 5.16 | 12 | 25.8 | 1.64 | |||
| PCB 17 | −1.82 | 5.25 | 40 | 26.1 | ||||
| PCB 18 | −1.88 | 5.24 | 20 | 55.8 | 3.69 | |||
| PCB 28 | −1.93 | 5.67 | 0.48 | 283 | 519 | 4710 | 49.6 | 3.74 |
| PCB 33 | −2.01 | 5.6 | 5.2 | 136 | 232 | 879 | 28 | 3.38 |
| PCB 37 | −2.21 | 5.83 | 0.8 | 110 | 75.8 | 284 | 3.43 | 0.969 |
| PCB 49 | −1.84 | 5.85 | 5.8 | 134 | 131 | 3250 | 10.2 | 1.45 |
| PCB 52 | −1.88 | 5.62 | 6.5 | 184 | 277 | 6700 | 41 | 3.44 |
| PCB 60 | −2.20 | 6.11 | 1 | 214 | 194 | 2420 | 1.08 | 0.587 |
| PCB 66 | −2.08 | 6.2 | 0.8 | 330 | 201 | 6260 | 5.76 | 0.891 |
| PCB 74 | −2.06 | 6.2 | 0.21 | 393 | 170 | 404 | 15.1 | 0.594 |
| PCB 87 | −2.12 | 6.29 | 3.6 | 196 | 148 | 4020 | 3.02 | 0.747 |
| PCB 99 | −1.99 | 6.39 | 0.2 | 403 | 277 | 9420 | 14.2 | 0.662 |
| PCB 110 | −2.10 | 6.48 | 4.2 | 778 | 609 | 21200 | 6.84 | 1.13 |
| PCB 118 | −2.29 | 6.74 | 0.11 | 776 | 438 | 16600 | 7.98 | 0.587 |
| PCB 138 | −2.27 | 6.83 | 0.08 | 509 | 705 | 24500 | 6.92 | 0.534 |
| PCB 153 | −2.17 | 6.92 | 0.046 | 603 | 768 | 30100 | 6.68 | 1.06 |
| PCB 156 | −2.44 | 7.18 | 0.01 | 51.5 | 66.5 | 2070 | 0.457 | 0.00492 |
| PCB 170 | −2.45 | 7.27 | 0.016 | 88.3 | 161 | 3760 | 0.785 | 0.0125 |
| PCB 180 | −2.36 | 7.62 | 0.003 | 19.5 | 58.0 | 2620 | 0.675 | 0.0224 |
| PCB 201 | −2.27 | 7.36 | 0.003 | 222 | 437 | 11300 | 0.968 | 0.153 |
| PCB 206 | −2.44 | 8.09 | 0.013 | 23.2 | 27.7 | 932 | 0.486 | |
| PCB 209 | −2.34 | 8.18 | 0.02 | 4.30 | 10.1 | 514 | 0.068 | |
PCB concentrations in air
The urban air PCB exposure scenario (gaseous phase) was based on average concentrations measured in 184 air samples collected at 37 different outdoor locations in the city of Chicago between November 2006 and November 2007 (Hu et al., 2008). For the sampling, high-volume air samplers mounted on the rear of two mobile medical clinic vans were used (Hu et al., 2008). The quality of the PCB sampling and analysis was determined using field blanks, solvent blanks, side-by-side replicate sampling, surrogate standard recoveries and performance standards. The range of concentrations measured fell within the ranges measured in Chicago by the Integrated Atmospheric Deposition Network (IADN) (Sun et al., 2006).
For the remote environment air exposure scenario, data on gaseous PCB air concentrations from the IADN station at Sleeping Bear Dunes were used, a remote site on the north east shore of Lake Michigan (Ronald Hites, University of Indiana, U.S., personal communication). The data from both the urban and remote environments were for gaseous PCB concentrations only, which accounts for almost all of the airborne PCBs at these sites. Some of the data points represented more than one PCB congener due to the co-elution of two or more congeners. In such cases, the data point was assigned the PCB congener in the coeluting group with the highest content in different Arochlor mixtures as reported by Frame (1996).
The mean air concentrations of the PCB congeners from the two locations are shown in Table 1. The prediction of the human lifetime exposure to PCBs requires that the historical air concentrations be taken into consideration. The historical time trends of PCB concentrations were estimated using concentrations in herring gull eggs collected at Chantry Island, Lake Huron, between 1974 and 2004 in the framework of the Great Lakes Herring Gull Monitoring Program (Bishop et al., 1992; Jermyn-Gee et al., 2005; Pekarik et al., 1998; Petti et al., 1994; Chip Weseloh, Canadian Wildlife Service , Canada, personal communication). Of all of the sampling stations involved in the monitoring program, Chantry Island was considered to be the one that best reflected background concentrations and was most suitable for the estimation of generalized historical time trends. For the period from 1930 to 1974, the trends were reconstructed using PCB emission estimates for Canada (Breivik et al., 2002). The resulting normalized scenario was then fitted to the measured air concentrations, yielding contamination scenarios for the individual PCB congeners (see Table S1 in Supporting Information).
For modeling inhalation exposure, indoor air exposure was also taken into consideration. No measurements of PCB concentrations in indoor air in Chicago were available; hence it was assumed that the concentrations in indoor air were 10 times higher than in outdoor air, based on the work of Harrad et al. (2006) for the English midlands. Furthermore, it was assumed that 90 % of the air inhaled was indoor air and the congener distribution was the same indoors as outdoors..
PCB concentrations in the diet
The daily PCB intake with diet was calculated on the basis of the ingestion rates (see above) and the PCB concentrations in the different food items measured in market basket samples collected between 1992 and 1996 in six Canadian cities (Newsome et al., 1998). A congener specific dataset of comparable quality could not be found for the United States. Since the market food in Canada and the northern United States originate from similar sources, the Canadian data were judged to be a good approximation of PCB levels in Chicago food. The following data rich food items were used: cheddar cheese, cottage cheese, processed cheese and butter, steak, roast, and ground beef, fresh and cured pork, veal, lamb, cold cuts, luncheon meat, organ meat, sausages, poultry and egg, fish (marine, freshwater, and canned), and shellfish. The PCB concentrations were reported on a fresh weight basis and were lipid normalized. The food items were then grouped into 3 food groups (dairy products, meat and eggs, and fish) and the mean lipid-normalized concentration in the food group was calculated for each PCB congener (see Table 1). These concentrations were assumed to represent the year 1994 (i.e. the mid-point of the years 1992-1996) and were extrapolated over time using the same time trend employed to the historical air contamination scenario (see Table S1).
Both the internal and the external exposure are assessed using the model. The internal exposure refers to the lipid normalized concentration of the PCB in the human (assumed to be the same in all tissues). This is compared with the lifetime cumulative external exposure via inhalation, which is the total mass of PCB inhaled during a lifetime, corrected for the proportion of total inhaled air (7500 ml/min) that reaches the gas exchange region of the lung (5250 ml/min) of 70 % in an adult (West, J.B. 2008).
Model Evaluation
To evaluate the model, the predicted lipid normalized PCB concentrations in a 56 year old man were compared with measured lipid normalized data from five blood plasma samples that were purchased from a Chicago blood bank in 2006. The lipids were measured gravimetrically. The anonymous donors were all African-American men born between 1939 and 1955. The plasma was extracted and purified using the method of Hovander et al. (2000) and analyzed on the same instrument (GC-MS/MS) as was used for the air samples reported in Hu et al. (2008), were it is possible to analyze 170 PCB congeners. Two samples from each donor were analyzed as duplicates. The samples were analyzed in parallel with Standard Reference Material plasma (SRM 1589a, National Institutes of Standards and Testing (NIST)). analysis of SRM 1589a was in good agreement with the results generated by NIST. The concentrations of the detected PCB in the Chicago men ranged from 120 ng/g lipids to430 ng/g lipids (see Figure S1 in the supporting information). The mean recovery of the surrogate standard PCB 166 was 64% (standard deviation 7.6%).
Results and Discussion
Model Evaluation
The model evaluation revealed reasonable agreement between the predicted and the measured concentrations in human tissue for most of the higher chlorinated PCB congeners, but considerable discrepancies for the lower chlorinated, more volatile congeners (see Figure 1). Analysis of the results indicated that the cause of the poor agreement varied between the congeners (see the Appendix for a detailed discussion). The individual variability in the concentrations of the persistent PCB congeners (e.g. congeners 138, 153, 180, 187) would have been influenced by the variability in body lipid mass. The model’s consistent underestimation of the concentrations of PCB 74 and PCB 99 was likely in part attributable to an overestimation of the metabolic rate constants of these congeners. For PCB 52 and PCB 209 on the other hand, a comparison with PCB congener patterns in the literature suggested that the poor agreement in Figure 1 may be due to a positive bias in the measured concentrations in blood, or that contamination occurred during the sampling procedure since the blank samples did not contain any of these two congeners. The model error that could explain a consistent underprediction would be the failure to include an important exposure pathway, but this could not explain the large difference in agreement between the different congeners. However, although the model evaluation raises interesting issues, the tendency of the model to underpredict the concentrations is unlikely to affect the results and conclusions of the modeling work presented in the following (see Appendix).
Figure 1.
Ratio between measured and predicted lipid normalized concentrations of PCB congener in serum of 5 male residents of Chicago.
Comparison of Internal Exposure to PCBs in Urban and Remote Environments
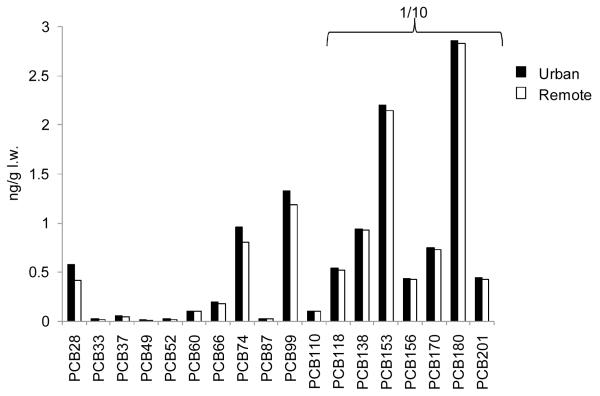
The predicted lipid normalized tissue concentrations of 18 PCB congeners in a 56 year old man after a lifetime of exposure to the contaminants both via diet and inhalation of outdoor air from either Chicago or the remote area of Sleeping Bear Dunes are shown in Figure 2. Both the predicted PCB concentrations and the PCB congener pattern are similar for the two scenarios. The model predicts that the elevated air concentrations in Chicago compared with Sleeping Bear Dunes increase the accumulated mass of PCBs in the body by 1 to 30 % depending on the congener number.
Figure 2.
Predicted PCB concentrations in a 56 year old man exposed to PCBs via the diet and via inhalation of air from an urban (Chicago) and a remote area (Sleeping Bear Dunes). The dietary exposure was the same in both simulations and represented background exposure for this region. The concentrations of PCB 118 to PCB 201 have been divided by ten. l.w. = lipid weight.
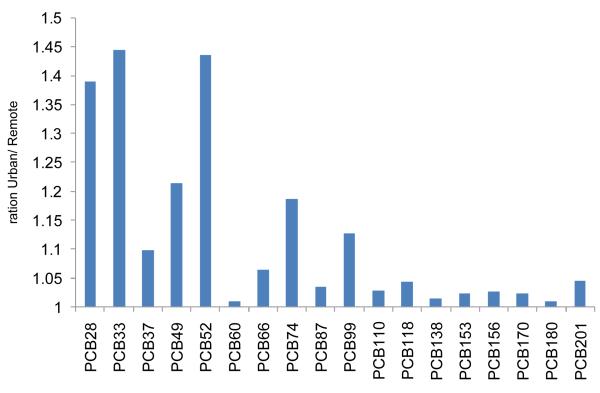
To better illustrate the difference between the two groups, the predicted tissue concentration of each PCB congener in the urban exposure scenario was divided by the predicted tissue concentration of the respective congener in the remote exposure scenario (Figure 3). The impact of the urban air exposure on body tissue concentrations was greatest for several of the lower chlorinated PCBs (PCB 28, PCB 33 and PCB 52). Of the 18 congeners modeled, these are the ones with the highest concentrations in urban air, while they have among the lowest concentrations in the diet (Table 1). The higher chlorinated congeners, being more lipophilic, bioaccumulate to a higher degree from air and water into biota. Furthermore, many are resistant to metabolism and are thus effectively transferred up the food chain. These factors combined give higher concentrations in food compared to the lower chlorinated congeners, which are less lipophilic, generally more subject to metabolism, and more volatile.
Figure 3.
Predicted PCB concentrations in a 56 year old man living in an urban environment divided by the corresponding concentrations for a person living in a remote environment.
Figure 4 shows the predicted lipid normalized concentrations of the 10 lower chlorinated congeners in a 56 year old man. The predicted concentrations are so low (0.06-5 pg/g lipid weight) that they probably are under the detection limit of current analytical methods. These calculations were based on exposure to air only, as no data were available on concentrations of these congeners in food. The lower lipophilicity of these congeners and their greater susceptibility to metabolism suggests that food will make a comparatively less important contribution to human exposure. The rapid metabolism of most of these congeners (see Table 1), is a major factor contributing to the low predicted concentrations. For instance, if PCB 4 was not metabolized in humans, the model predicts that its concentration would be a factor of 1700 higher.
Figure 4.
Predicted PCB concentrations (ng/g l.w.) in a 56 year old man for whom the only source of exposure is inhalation of air from either an urban or remote environment. The concentrations of PCB 15 have been divided by ten. l.w. = lipid weight.
External Exposure to PCBs
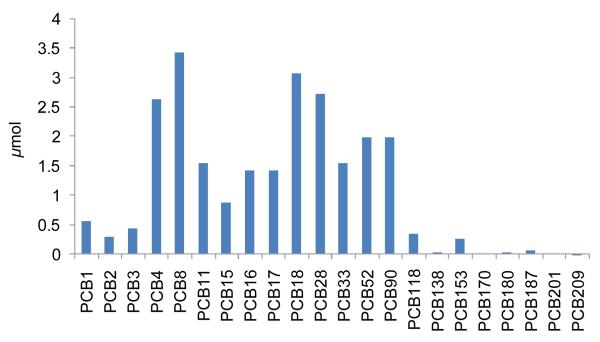
Even if the lower chlorinated PCB congeners are not retained in the human body, exposure is still occurring. The predicted cumulative external exposure over the entire lifetime of a 56 year old man to PCBs via inhalation of Chicago air is illustrated in Figure 5. The model predicts this external exposure to be in the range of 0.044-3.4 μmoles for each of the 17 congeners ranging from the volatile PCB1 to the less involatile PCB153. For the 56 year old man simulated, the lifetime external exposure is up to five orders of magnitude greater than the internal exposure (i.e. body burden) for the low chlorinated PCBs. For PCB 180, on the other hand, the ratio is only 1.
Figure 5.
Predicted cumulative lifetime external exposure to PCBs via inhalation (56 year old man living in Chicago).
The major factor determining the ratio between external and internal exposure is the metabolic rate of the PCB congener. This is especially apparent for PCB 15, which show a comparatively high internal exposure in Figure 4 compared to the relative external exposure illustrated in Figure 5. The metabolic rate of PCB 15 is 100-400 times lower than those of the neighboring congeners (Table 1).
The high rate of metabolism of many of the lower chlorinated congeners implies that the body will be exposed to metabolites of these compounds. This exposure is not reflected in the analysis of native PCBs in tissue. Some of these metabolites may be carcinogenic or genotoxic since they can bind to proteins. For example PCB 3 is metabolized to an 3,4-quinone which probably is an ultimate carcinogen (Ludewig et al., 2008). Hence, exposure to lower chlorinated PCBs via contaminated urban air may be of toxicological relevance.
Discussion
There are other locations where exposure to airborne PCBs is even greater than in the Chicago urban environment studied here. In England, average concentrations of ΣPCB of 18,000 pg/m3 and 30,000 have been reported for indoor air in offices and public microenvironments, respectively (Harrad et al., 2006). In Switzerland, the average ΣPCB concentration in indoor air from 160 buildings in which joint sealants containing PCBs had been employed was 790,000 pg/m3 (Kohler et al., 2005). This compares with 8000 pg/m3 for urban and 700 pg/m3 for remote indoor air in this study (i.e. ten times the ambient air concentrations). In such environments the contribution of inhalation to both internal and external exposure to PCBs would be greater than predicted here.
Using the English data mentioned above, Harrad et al. (2006) concluded that inhalation from indoor air contributes up to 63% of the overall exposure of English adults to PCBs. However, in their assessment they considered only external exposure to the ΣPCB. By using a congener specific approach, we have shown here that the contribution of inhalation to external and internal exposure varies greatly between congeners. Furthermore, this work has shown that there are great discrepancies between internal and external exposure via inhalation as a result of metabolism. Inhalation is expected to be of greatest relevance for PCB congeners that are readily metabolized and that produce toxic metabolites.
Supplementary Material
Figure S1. PCB concentrations in five different blood plasma samples purchased from a Chicago blood bank in 2006. The anonymous donors were all African-American men born between 1939 and 1955.
Table S1. Contamination scenario in Chicago outdoor air and dairy products for PCB 153 between 1930 and 2006.
Acknowledgement
This work was funded as part of the Iowa Superfund Basic Research Program, NIEHS ES013661. One of us (PST) was supported by NIH P30 ES05605. We wish to thank Chip Weseloh of the Canadian Wildlife Service for providing unpublished data from the herring gull monitoring program. We also want to thank Ronald Hites for providing IADN data from Sleeping Bear Dunes, Jake Ryan and finally Emma Undeman for help and support.
Appendix
Model Evaluation
Five blood samples from men aged 51-67 were purchased from a Chicago blood bank and analyzed for PCBs. Thirteen of the PCB congeners present in the food were found in at least one of the five samples. The measured concentration of each congener was normalized to the concentration of that congener predicted by the model (see Figure 1). The model predicted lower concentrations for all PCBs in almost all cases. The agreement was reasonable for the higher chlorinated, most frequently detected congeners with the exception of PCB 209. The agreement was poorest for the lower chlorinated PCBs. For PCB 28, PCB 74 and PCB 99 the model underestimated the measured concentrations by 7 to 16 times, and for PCB 52 and PCB 110 the difference was a factor 500 and 50, respectively.
There are a number of possible explanations for the disagreement between the predicted and measured concentrations. The most likely are model error, that the levels in the diet that served as model input were incorrect for the individuals whose blood was analyzed (too low), that the metabolic rates employed were too high, that the body lipid mass of the 56 year old man in the model was higher than that of the individuals sampled, or that the lipid normalized PCB concentrations measured in the plasma were erroneous (too high).
Of these explanations, only the body lipid mass would necessarily influence the concentrations of all of the congeners in the same manner. This variable, which cannot be verified, may explain some of the interpersonal variability in the measured concentrations of the higher chlorinated congeners seen in Figure 1, e.g. the consistently higher levels in persons 4 and 5 compared with persons 1 and 3.
Model error could also influence the concentration of all congeners in a similar manner. The model error most likely to explain a systematic underprediction is the failure to account for a significant exposure pathway. For instance, it is conceivable that dust ingestion, which is suspected to be a relevant exposure pathway for PCBs (Rudel et al., 2008) and other contaminants (Harrad et al., 2006) but which could not be evaluated for PCBs in Chicago due to a lack of relevant data, contributes significantly to exposure in highly contaminated environments. This exposure pathway would be expected to more strongly contribute to exposure of the less volatile PCB congeners. However, it could not account for the large differences in agreement between the different congeners in Figure 1. This would require that dust has a congener pattern (e.g. ratio of PCB 52/74) that is vastly different than in other environmental matrices.
A second possible source of model error is that the assumption of partitioning equilibrium of the PCBs between the lipids of the organism is inappropriate. One could hypothesize that the lipid normalized concentrations of PCBs in blood are much higher than the lipid normalized concentrations in other tissues. However, analysis of blood and adipose tissue from the same individuals has shown that the lipid normalized concentrations are similar (Brown and Lawton, 1984). Hence model error is not considered to be a major cause of the discrepancies in Figure 1.
Differences in dietary exposure are another possible explanation for the indications of systematic interpersonal variability of the higher chlorinated congeners. It is possible that some of the men sampled had dietary habits that resulted in a greater exposure. However, it is unlikely that the dietary data could explain the very large systematic underprediction of the lower chlorinated congeners. Other studies of PCBs in food have reported lower concentrations of the lower chlorinated congeners with respect to PCB153 (Schlummer et al., 1998). Employing these data would result in an even greater systematic underprediction of the lower chlorinated congeners.
Large discrepancies in model performance between congeners, e.g. the much higher discrepancies for PCBs 28 through 110 compared to PCBs 118 through 206 in Figure 1, are likely to be largely due to errors in either the metabolism rates or the measured concentrations. A closer examination of the measured data offers further insight into the possible importance of these two sources of error.
The concentrations in the blood from the Chicago men were 4-9 ng/g l.w. for PCB 28, 15-20 ng/g l.w. for PCB 52, 8-13 ng/g l.w. for PCB 74, 16-22 ng/g l.w. for PCB 99 and 20-140 ng/g l.w. for PCB 153. For PCB 153, one of the 4 congeners quantified in all 5 samples, the concentrations were in the range of what has been found in populations in Europe, Canada and Russia, as reviewed by Hofvander (2006). Previous studies have reported similar concentrations for PCB 28 but five times lower concentrations for PCB 52 (Cerna et al., 2008; Turrio-Baldassarri et al., 2008). Inoue et al. (2006) reported ten time lower concentrations for PCB 74 and PCB 99. However, due to the wide range of PCB concentrations in a given population and the possibilities of systematic errors between studies, e.g. in the lipid determination, it is difficult to evaluate the quality of the data based on the comparison of concentrations.
Comparing the PCB congener patterns can be a more useful approach, as the variability in congener patterns is generally much smaller than the variability in absolute concentrations. In Table A1 the PCB concentrations measured in the five men from Chicago and the predicted concentrations are divided by PCB 153 and compared with corresponding ratios in the literature. The ratios were calculated from the average concentrations in all cases. The results for the different congeners can be summarized as follows:
The PCB153-normalized concentration ratio for PCB 52, one of the 2 congeners that showed poorest agreement between the model and the Chicago measurements, is 23 and 130 times higher for the Chicago data than for the other two datasets reporting this congener, while the ratio for the model and these two other datasets agree quite well. This raises the possibilities of an error in the Chicago measurements.
For PCB 110, the second congener showing very poor agreement, the PCB153-normalized concentration ratio was 20 times higher in the Chicago measurements than in the model. PCB 110 was detected in just one of the Chicago samples and is close to the corresponding ratio in the literature, reported in only one study. A possible explanation for the underpredicted concentrations by the model could be an error in the metabolic rate; i.e. PCB 110 is more persistent.
The PCB153-normalized concentration ratio for PCB 28 ratio for the Chicago data is a factor 3 and 30 higher than the ratios in the other two studies reporting this congener, while the model predicts a ratio that lies in between the ratios for these two other studies. This suggests that error in the blood analysis is a more likely explanation for the discrepancies in Figure 2 than the metabolism rates, but the latter may be a contributing factor.
Two studies have reported PCB 74 and 99 concentrations in serum. The ratios of these congeners to PCB 153 are similar in this study and in the Chicago measurements, whereas the model predicts ratios that are a factor of 3 to 6 lower. This suggests that in this case an overestimation of the metabolism rates may be the major cause of the discrepancies in Figure 1.
For PCBs 118, 138, 180, 187, and 206 there is good agreement between the ratios for all studies reporting measurements and the model,
The PCB153-normalized concentration ratio for PCB 209 is 4 times higher in Chicago men than in the one other study reporting this congener, while the model predictions agree well with this other study. This suggests that the discrepancies between the model and the Chicago data are more likely attributable to an overestimate of the measured concentration. This congener was only quantifiable in 3 of the 5 samples.
In summary, there is evidence suggesting that some of the poor agreement between the predicted and measured concentrations of the lower chlorinated congeners can be attributed to overestimated concentrations in the Chicago men (e.g. PCB 52 and PCB 209), while for other congeners an overestimation of the metabolism rate constants is a contributing factor (e.g. PCB 74 and PCB 99). However, the tendency of the model to underpredict the concentrations does not affect the conclusions drawn in this paper. The consequences would be limited to an overestimation of the contribution of inhalation in Figure 3 as a result of an underestimation of dietary exposure, and an underestimation of the expected tissue levels of volatile PCBs arising from inhalation alone (Figure 4) due to an overestimation of metabolism.
Table A1.
Concentrations of PCB congeners normalized to PCB 153 calculated from literature data, from the measured blood plasma concentrations of the Chicago men and the simulation results with the human module of ACC-HUMAN.
| Inoue et al. 2006 | Cerna et al. 2007 |
Turrido-Baldassarri et al. 2008 |
Goncharov et al.2008 | Chicago men |
ACC- HUMAN |
|
|---|---|---|---|---|---|---|
| PCB 28 | 0.031 | 0.0038 | 0.096 | 0.026 | ||
| PCB 52 | 0.011 | 0.0019 | 0.25 | 0.0015 | ||
| PCB 74 | 0.12 | 0.28 | 0.15 | 0.044 | ||
| PCB 99 | 0.16 | 0.38 | 0.28 | 0.061 | ||
| PCB 110 | 0.16 | 0.098 | 0.0048 | |||
| PCB 118 | 0.24 | 0.034 | 0.079 | 0.44 | 0.17 | 0.25 |
| PCB 138 | 0.54 | 0.45 | 0.38 | 0.81 | 0.47 | 0.43 |
| PCB 153 | 1 | 1 | 1 | 1 | 1 | 1 |
| PCB 180 | 0.53 | 0.93 | 1.6 | 0.66 | 0.80 | 1.3 |
| PCB 187 | 0.27 | 0.22 | 0.21 | 0.26 | ||
| PCB 206 | 0.030 | 0.071 | 0.084 | |||
| PCB 209 | 0.022 | 0.10 | 0.024 |
References
- ATSDR 2000 http://www.atsdr.cdc.gov/toxprofiles/tp17.pdf.
- Ballschmiter K,, Mennel A, Buyten J. Long chain alkyl-polysiloxanes as non-polar stationary phases in capillary gas chromatography. Fresenius’ J. Anal. Chem. 1993;346:396–402. [Google Scholar]
- Bishop CA, Weseloh DV, Burgess NB, Norstrom RJ, Turle R, Logan KA. An atlas of contaminants in eggs of colonial fish-eating birds of the Great Lakes (1970-1988) 1992. p. 400. Accounts by location. CWS, Ontario Region, Technical Report.
- Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners - a mass balance approach. 2. Emissions. Sci. Total Environ. 2002;290:199–224. doi: 10.1016/s0048-9697(01)01076-2. [DOI] [PubMed] [Google Scholar]
- Brown JF, Jr., Lawton RW. Polychlorinated biphenyl (PCB) partitioning between adipose tissue and serum. Contam. Toxicol. 1984;33:277–280. doi: 10.1007/BF01625543. [DOI] [PubMed] [Google Scholar]
- Brown JF., Jr. Determination of PCB Metabolic, Excretion, and Accumulation Rates for Use as Indicators of Biological Response and Relative Risk. Environ. Sci. Technol. 1994;28:2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- Brunciak PA, Dachs J, Franz TP, Gigliotti CL, Nelson ED, Turpin BJ, Eisenreich SJ. Polychlorinated biphenyls and particulate organic/elemental carbon in the atmosphere of Chesapeake Bay, USA. Atmos. Environ. 2001;35:5663–5677. [Google Scholar]
- Bureau of Nutritional Science . Nutrition Canada Food Consumption Patterns Report. Bureau of Nutritional Science, Health protection Branch, Health and Welfare Canada; Ottawa: 1977. pp. 1–26. [Google Scholar]
- Cerna M, Maly M, Grabic R, Batariova A, Smid J, Benes B. Serum concentrations of indicator PCB congeners in the Czech adult population. Chemosphere. 2008;72:1124–1131. doi: 10.1016/j.chemosphere.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Czub G, McLachlan MS. Bioaccumulation Potential of Persistent Organic Chemicals in Humans. Environ. Sci. Technol. 2004a;38:2406–2412. doi: 10.1021/es034871v. [DOI] [PubMed] [Google Scholar]
- Czub G, McLachlan MS. A food chain model to predict the levels of lipophilic organic contaminants in humans. Environ. Toxicol. Chem. 2004b;23:2356–2366. doi: 10.1897/03-317. [DOI] [PubMed] [Google Scholar]
- Dabeka RW, McKenzie AD, Laroix GMA, Cleroux C, Bowe S, Graham RA, Conacher HBS. Survey of Arsenic in Total Diet Food Composites and Estimation of the Dietary Intake of Arsenic by Canadian Adults and Children. J. AOAC Int. 1993;76:14–25. [PubMed] [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ. Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Dunnivant FM, Elzerman AW, Jurs PC, Hasan MN. Quantitative structure-property relationships for aqueous solubilities and Henry’s law constants of polychlorinated biphenyls. Environ. Sci. Technol. 1992;26:1567–1573. [Google Scholar]
- Frame GM, Wagner RE, Carnahan JC, Brown JC, Jr., May RJ, Smullen LA, Bedard DL. Comprehensive, quantitative congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ. Res. 2008;106:226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner WD, Hites RA. Potential Sources of Pesticides, PCBs, and PAHs to the Atmosphere of the Great Lakes. Environ. Sci. Technol. 2003;37:3764–3773. doi: 10.1021/es034021f. [DOI] [PubMed] [Google Scholar]
- Harrad S, Hazrati S, Ibarra C. Concentrations of Polychlorinated Biphenyls in Indoor Air and Polybrominated Diphenyl Ethers in Indoor Air and Dust in Birmingham, United Kingdom: Implications for Human Exposure: Environ. Sci. Technol. 2006;40:4633–4638. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- Hawker DW, Connell DW. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environ. Sci. Technol. 1998;22:382–387. [Google Scholar]
- Hofvander L. Polychlorianted biphenyls and their metabolites in human blood - method development, identification and quantification. Department of Environmental Chemistry, Stockholm University; Sweden: 2006. [Google Scholar]
- Hornbuckle KC, Sweet CW, Pearson RF, Swackhamer DL, Eisenreich SJ. Assessing Annual Water-Air Fluxes of Polychlorinated Biphenyls in Lake Michigan. Environ. Sci. Technol. 1995;29:869–877. doi: 10.1021/es00004a006. [DOI] [PubMed] [Google Scholar]
- Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J. Anal. Toxicol. 2000;24:696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC. Discovery of non-Aroclor PCB (3, 3′-dichlorobiphenyl) in Chicago air. Environ Sci Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T, Takasuga T, Senthilkumar K, Yamashita F, Koizumi A. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. Environ. Health Perspect. 2006;114:1179–1185. doi: 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi A, Hunter S, Hazrati S, Harrad S. Concentrations and chiral signatures of polychlorinated biphenyls in outdoor and indoor air and soil in a major U.K. conurbation. Environ. Sci. Technol. 2007;41:2153–2158. doi: 10.1021/es062218c. [DOI] [PubMed] [Google Scholar]
- Jermyn-Gee K, Pekarik C, Havelka T, Barrett G, Weseloh DV. An atlas of contaminannts in eggs of colonial fish-eating birds of the Great Lakes (1998-2001) 2005. p. 341. Accounts by location. CWS, Ontario Region, Technical Report.
- Kohler M, Tremp J, Zennegg M, Seiler C, Minder-Kohler S, Beck M, Lienemann P, Wegmann L, Schmid P. Joint Sealants: An Overlooked Diffuse Source of Polychlorinated Biphenyls in Buildings. Environ. Sci. Technol . 2005;39:1967–1973. doi: 10.1021/es048632z. [DOI] [PubMed] [Google Scholar]
- Li N, Wania F, Lei YD, Daly GL. A Comprehensive and Critical Compilation, Evaluation, and Selection of Physical-Chemical Property Data for Selected Polychlorinated Biphenyls. J. Phys. Chem. Ref. Data. 2003;32:1545–1590. [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic activation of PCBs to carcinogens in vivo. A review. Environ. Toxicol. Pharmacol. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandalakis M, Stephanou EG. Wet Deposition of Polychlorinated Biphenyls in the Eastern Mediterranean. Environ. Sci. Technol. 2004;38:3011–3018. doi: 10.1021/es030078q. [DOI] [PubMed] [Google Scholar]
- Newsome WH, Davies DJ, Sun WF. Residues of polychlorinated biphenyls (PCB) in fatty foods of the Canadian diet. Food Addit. Contam. 1998;15:19–29. doi: 10.1080/02652039809374596. [DOI] [PubMed] [Google Scholar]
- Pekarik C, Weseloh DV, Barrett MS, Bishop CA, Pettis KE. An atlas of contaminanants in eggs of colonial fish-eating birds of the Great Lakes (1993-1997) 1998. p. 245. Accounts by location. CWS, Ontario Region, Technical Report.
- Petti KE, Bishop CA, Weseloh DV, Norstrom RJ. An atlas of contaminanants in eggs of colonial fish-eating birds of the Great Lakes (1989-1992) 1994. p. 319. Accounts by location. CWS, Ontario Region, Technical Report.
- Rudel RA, Seryak LM, Brody JG. PCB-containing wood floor finish is a likely source of elevated PCBs in residents’ blood, household air and dust: a case study of exposure. Environ. Health. 2008;7:2. doi: 10.1186/1476-069X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlummer M, Moser GA, McLachlan MS. Digestive tract absorption of PCDD/Fs, PCBs, and HCB in humans: mass balances and mechanistic considerations. Toxicol. Appl. Pharmacol. 1998;152:128–137. doi: 10.1006/taap.1998.8487. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Chemistry. Second Edition John Wiley & Sons; New Jersey: 2003. [Google Scholar]
- Sun P, Basu I, Hites RA. Temporal trends of polychlorinated biphenyls in precipitation and air at Chicago. Environ. Sci. Technol. 2006;40:1178–1183. doi: 10.1021/es051725b. [DOI] [PubMed] [Google Scholar]
- Sun P, Basu I, Blanchard P, Brice KA, Hites RA. Temporal and Spatial Trends of Atmospheric Polychlorinated Biphenyl Concentrations near the Great Lakes. Environ. Sci. Technol. 2007;41:1131–1136. doi: 10.1021/es061116j. [DOI] [PubMed] [Google Scholar]
- Turrio-Baldassarri L, Abate V, Battistelli CL, Carasi S, Casella M, Iacovella N, Indelicato A, La Rocca C, Scarcella C, Alivernini S. PCDD/F and PCB in human serum of differently exposed population groups of an Italian city. Chemosphere. 2008;73:S228–S234. doi: 10.1016/j.chemosphere.2008.01.081. [DOI] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology: The Essentials. 8th Edition Lippincott Williams & Wilkins; 2008. p. 14. [Google Scholar]
- Wethington DM, III, Hornbuckle KC. Milwaukee, WI, as a Source of Atmospheric PCBs to Lake Michigan. Environ. Sci. Technol. 2005;39:57–63. doi: 10.1021/es048902d. [DOI] [PubMed] [Google Scholar]
- Yi SM, Pagilla S. Reddy, Seo YC, Mills WJ, Holsen TM. Emissions of polychlorinated biphenyls (PCBs) from sludge drying beds to the atmosphere in Chicago. Chemosphere. 2008;71:1028–1034. doi: 10.1016/j.chemosphere.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Zhang X, Schramm KW, Henkelmann B, Klimm C, Kaune A, Kettrup A, Lu P. A Method to Estimate the Octanol-Air Partition Coefficient of Semivolatile Organic Compounds. Anal. Chem. 1999;71:3834–3838. doi: 10.1021/ac981103r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PCB concentrations in five different blood plasma samples purchased from a Chicago blood bank in 2006. The anonymous donors were all African-American men born between 1939 and 1955.
Table S1. Contamination scenario in Chicago outdoor air and dairy products for PCB 153 between 1930 and 2006.