Abstract
Natural Killer (NK) cells destroy (lyse) tumor cells, virally infected cells and antibody-coated cells. Previous studies indicated that exposure to the environmental contaminant tributyltin (TBT) decreases the lytic function of NK cells and activates mitogen activated protein kinases (MAPK), including p44/42 (Aluoch and Whalen, 2005). If activation of p44/42 is required for TBT-induced decreases of lytic function, then activation of p44/42 to similar extents by pharmacological agents such as Phorbol 12-myristate 13-acetate (PMA) should mimic to some extent changes induced in NK cells with TBT exposures. NK cells were exposed to PMA concentrations between 0.25 and 10 nM for 10 min, 1 h, and 6 h before determining the lytic function (51Cr release assay) and phosphorylation state of MAPKs (Western blot). A 1 h exposure of NK cells to 5 nM PMA resulted in a loss of lytic function of 47%. Western blot analysis showed that a 1 h exposure to 5 nM PMA caused a 6 fold increase in phospho-p44/42 levels. Previous studies showed a 5 fold increase in phospho-p44/42 in response to a 1 h exposure to 300 nM TBT. Exposure to 300 nM TBT caused about a 40% decrease in lytic function. This study supports the hypothesis that p44/42 activation (as seen with TBT exposures) can cause a loss of NK-cell lytic function.
Keywords: MAPK, p44/42, p38, PMA, Anisomycin, NK cells
INTRODUCTION
Natural Killer (NK) cells are a subset of lymphocytes that are capable of killing tumor cells, virally infected cells and antibody-coated cells. NK cells are defined by the absence of T cell receptor/CD3 complex and by the presence of CD56 and/or CD16 on the cell surface. NK cells are capable of killing the above target cells without prior sensitization, putting them in the forefront of lymphocyte defense against tumor cells and virally infected cells (Lotzova, 1993, Vivier et al., 2004). The earliest and possibly predominant defense against tumor cells has been attributed to NK cells (Lotzova, 1993; O’Shea and Ortaldo, 1992; Trinchieri, 1989). They are responsible for limiting the spread of blood borne metastases, as well as limiting the development of primary tumors (Kiessling and Haller, 1978; Hanna, 1980). NK cells also play a central role in immune defense against viral infection as evidenced by greatly increased incidence of viral infection seen in individuals where the NK subset of lymphocytes is completely absent (Fleisher et al, 1982; Biron et al, 1989).
Tributyltin (TBT) is a very significant environmental contaminant (Kimbrough, 1976; Laughlin and Linden, 1985; Tanabe et al., 1998; Loganathan et al., 2000). TBT was mainly in used in wood preservation, marine antifouling paints, disinfection of circulating industrial cooling waters, and slime control in paper mills (Kimbrough, 1976; Roper, 1992; Yamada et al., 1993). It has been found in various household products such as siliconized-paper baking parchments and shower curtains (Yamada et al., 1993). TBT has been detected in human food, such as fish (Kannan et al., 1995a,b,c). Due to the wide spread industrial and household applications, it is not a surprise that TBT is found in human blood (Kannan et al., 1999; Whalen et al., 1999).
Previous studies indicated that exposure to TBT decreases the lytic function of NK cells (Dudimah et al., 2007; Whalen et al., 1999), decreases their expression of cytolytic proteins (granzyme B and perforin) (Thomas, et al., 2004; Thomas et al., 2005), and activates mitogen activated protein kinases (MAPK), including p44/42 (Aluoch and Whalen, 2005; Aluoch et al., 2006; Aluoch et al., 2007). TBT-induced loss of NK function could leave exposed individuals with an increased risk of viral infection and /or tumor formation. We have recently shown that activation of p44/42 was not the only factor involved in the mechanism of TBT-induced loss of lytic function (using p44/42 pathway inhibitors) (Abraha and Whalen, 2009). However, it is still necessary to determine what role the activation of p44/42 play in the loss of NK lytic function. If activation of p44/42 is required for any of these TBT-induced alterations of NK cell function then pharmacological agents such as Phorbol 12-myristate 13-acetate (PMA) that selectively activate p44/42 should mimic (to some extent) changes induced in NK cells with TBT exposures.
The current study examined the effect of varying PMA concentrations on the activation state of MAPKs. Experiments verifying the extent and selectivity of PMA activation of p44/42 in NK cells were carried out. Additionally, concentrations of PMA that activate p44/42 to an extent similar to that seen with TBT were examined for their ability to induce alterations of cytotoxic function and cytolytic protein expression in NK cells that were similar to those with TBT .
MATERIALS AND METHODS
Isolation of NK cells
Peripheral blood from healthy adults (male and female) volunteer donors was used for this study. Buffy coats (source leukocytes) obtained from either the American Red Cross (Portland, OR) or Key Biologics, LLC (Memphis, TN) were used to prepare NK cells. Highly purified NK cells were obtained using a rosetting procedure. Buffy coats were mixed with 1 mL of RosetteSep human NK cell enrichment antibody cocktail (StemCell Technologies, Vancouver, British Columbia, Canada) per 45 mL of buffy coat. The mixture was incubated for 20 min at room temperature (∼ 25º C). Following the incubation, 5–8 mL of the mixture was layered onto 4 mL of Ficoll-Hypaque (1.077 g/mL) (MP Biomedicals, Irvine, CA) and centrifuged at 1200 g for 30–45 min. The cell layer was collected and washed twice with phosphate buffered saline (PBS) pH 7.2 and stored in complete media (RPMI-1640 supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 µg streptomycin/mL (Mediatech, Inc. Herndon, VA)) at 1 million cells/mL. The resulting cell preparation was >95% CD16+, 0% CD3+ by fluorescence microscopy (Whalen et al., 2002).
Chemical preparation
PMA was dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO) to give 10 mM stock solution. A 10 mM stock of anisomycin was also prepared in DMSO. Desired concentrations of the compound were prepared by dilution of the stock into complete media containing 0.5% gelatin in place of the 10% bovine serum. Gelatin replaced BSA to avoid binding of the hydrophobic compounds to serum albumin, which could interfere with their delivery of the cells. The final concentration of DMSO did not exceed 0.01%.
Cell Treatments
Purified NK cells were separated by centrifugation from complete media (defined above) and transferred to complete media containing 0.5% gelatin in place of the 10% bovine serum. As mentioned above, this was done in order to avoid binding of the hydrophobic compounds to serum albumin, which could interfere with their delivery to the cells. NK cells (at a concentration of 1.5 million cells/ mL) were then exposed to the compound or control for varying lengths of time (10 min, 1 h, 6 h, or 24 h). Following the exposure the cells were assayed for tumor-destroying function or lysed for Western Blot analysis. Additionally, NK cells were exposed to compounds for 1 h; following the 1 h exposure period, the compound-containing or control media was removed and replaced with fresh compound-free media and the cells were incubated in compound-free media for 24 h before assaying for lytic function. The concentration ranges examined for the compound were: 0.25 nM to 10 nM for PMA and 0.5 µM to 10 µM for anisomycin.
Cell Viability
Cell viability was determined by trypan blue exclusion. Cell numbers and viability were assessed at the beginning and end of each exposure period. Viability was determined at each concentration for each of the exposure periods. The viability of treated cells was compared to that of control cells at each length of exposure (Whalen et al., 2003).
Cytotoxicity assay
The ability of NK cells to lyse tumor cells was measured using a 51Cr release assay (Whalen et al., 1999; Whalen, 1997). The target cell in all cytotoxicity assays was the NK-susceptible K562 (human chronic myelogenous leukemia) cell line. K562 cells were incubated with 51Cr (Perkin-Elmer Life Sciences, Boston, MA) in 0.2–0.5 ml of BCS for 1–1.5 h at 37 °C in air/CO2 (19:1). Following this incubation the target cells were washed twice with gelatin media. NK (effector) cells (1.2×105/100 µL for 12:1 ratio with target cells) were added to the wells of round-bottom microwell plates. The effectors were diluted to 6:1 ratio (0.6×105/100 µL) and 3:1 ratio (0.3×105/100 µL); each ratio was tested in triplicate. Target cells were added (1×104/100µL) to each well of the microwell plate and the plate was centrifuged at 300g for 3.5 min and incubated for 2 h at 37 °C (air/CO2, 19:1). After incubation a 0.1 ml aliquot of the supernatant was collected and counted for radioactivity for 60 sec in a Packard COBRA gamma radiation counter (Packard Instrument Co., Meriden, CT). Target lysis was calculated as follows: 100x[(test c.p.m - spontaneous c.p.m.)/maximum c.p.m.- spontaneous c.p.m.)]. Maximum release was produced by adding 100 µL of 10% Triton X-100.
Cell lysates
NK cells at a concentration of 6 million/mL were exposed to compounds (PMA and anisomycin) as described above. Following the treatments, the cells were centrifuged and the cell pellets were lysed using 133 µL of lysis buffer (Active motif, Carlsbad, CA) per 2 million cells. The cell lysates were stored frozen at −80 °C up to the point when they were run on SDS-PAGE. Control and treated cells for a given experimental setup (described above) were from an individual donor (Aluoch and Whalen, 2005).
Western Blot
Cell lysates were run on 10% SDS-PAGE (sodium dodecylsulfate polyacrylamide gel electrophoresis) membrane. The PVDF was immunoblotted with anti-phospho p38 (Thr180 and Tyr182), anti-p38, anti-phospho-p44/42 (Thr202/Tyr204), anti-p44/42, anti-phospho-SAPK/JNK (Thr183 and Tyr185), anti-SAPK/JNK, and anti-β-actin antibodies (Cell signaling Technologies, Beverly, MA). Antibodies were visualized using ECL chemiluminescent detection sytem (Amersham, Piscataway, NJ) and Kodak Image Station (Kodak, Rochester, NY). The density of each protein band was determined by densitometric analysis using the Kodak Image Station analysis software. The settings on the image station were optimized to detect the largest possible signal range and prevent saturation of the system. Differences in protein expression are determined relative to an internal control. This determination provides relative quantitation by evaluating whether a given treatment changed expression of phospho-p44/42, p44/42, phospho-p38, p38, phospho-SAPK/JNK, or SAPK/JNK, relative to untreated cells. β-actin levels were determined for each condition to verify that equal amounts of protein were loaded. In addition, the density of each protein band was normalized to β-actin to correct for small differences in loading among the lanes.
RNA isolation and real-time quantitative RT-PCR
Human NK cells were isolated from whole blood as described in the section under NK cell isolation, treated with the compounds of interest for the desired lengths of time before proceeding with total RNA extraction. Total RNA was extracted using an RNeasy mini kit (Qiagen; Valencia, CA) and its concentration determined by reading the optical density at 260 nm. Possible genomic DNA contamination was removed by on-column DNase digestion using the RNase–free DNase set. Two million NK cells per treatment condition were used. Samples were diluted in RNase-free water to 15 ng/uL and stored in micro-centrifuge tubes at −80 ºC. Real-time quantitative RT-PCR (qRT-PCR) was performed with an iCycler iQ System. Primers were designed using Primer Express 2.0 software (ABI). Primers were designed in such a way that they included at least one intron-exon junction so as to increase the specificity for the target genes and to minimize the amplification of closely related genes. Genes of interest were compared to closely related genes using BLAST and primers were designed based on divergent regions of the target gene sequence compared to those of the highly related genes. The QuantiTect SYBR green RT-PCR kit (Qiagen) and gene-specific PCR primers were used in a 20 µL reaction following protocols recommended by the manufacturer. To ensure that there is no significant difference in the concentration of total RNA in each sample, 18s RNA was analysed by qRT-PCR.
Generally, the PCR mixture (20 µL) contained 30 ng of total RNA and 0.2 µM each primer, 10 nM fluorescein calibration dye, 0.2 µL RT mix and 10 µL of 2× master mix. . Reverse transcription was performed for 20 min at 50 °C, followed by amplification for 35 cycles (15 s at 94 °C, 60 s at 60 °C) after an initial activation at 95 °C for 15 min. Melting curve analysis was carried out to determine the specificity of amplification. Analysis of results was done using the comparative Ct method. This involves comparing the Ct values of the samples of interest with a control or calibrator such as a non-treated sample. The comparative Ct method is also known as the delta Ct method, where [delta]Ct = [delta]Ct,sample - [delta]Ct,reference where [delta]Ct,sample is the Ct value for any treated sample and [delta]Ct, reference is the Ct value for the calibrator or untreated sample. The mRNA levels were represented in arbitrary units.
Statistical Analysis
Statistical analysis of the data was carried out utilizing ANOVA and Student's t test. Data were initially compared within a given experimental setup by ANOVA. A significant ANOVA was followed by pair wise analysis of control versus exposed data using Student’s t test.
RESULTS
Effects of 1 h exposures to PMA on NK lytic function
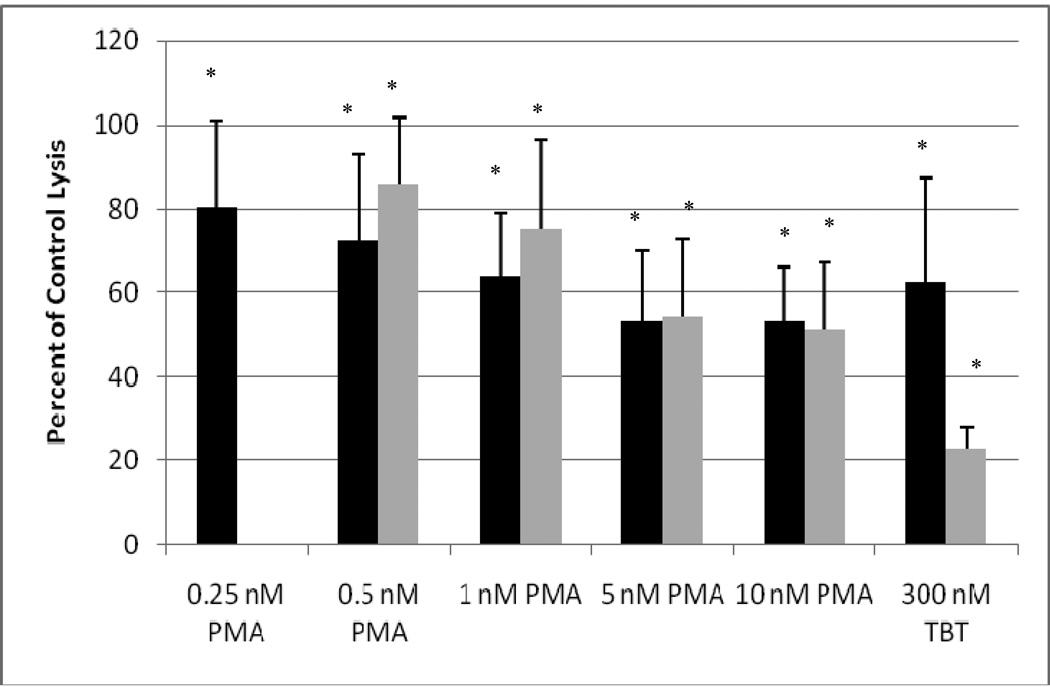
In order to combine results from separate experiments (using cells from different donors) the tumor lysis caused by treated cells was normalized to that of control cells in a given experiment. The concentrations of PMA used in the 1 h exposure ranged from 0.25–10 nM. NK cells exposed to concentrations of 0.5 and 0.25 nM PMA showed a 15% decrease in lytic function (Figure 1, black bars) (p<0.05). Exposure to 1 nM PMA for 1 h resulted in an approximately 36% loss of lytic function (Figure 1, black bars) (p<0.05). Concentrations of 10 nM and 5 nM resulted in a 47% reduction of cytotoxic function (p<0.05).
Figure 1.
Effects of exposures to PMA on the ability of NK cells to lyse tumor cells. NK cells were exposed to 0.25–10 nM PMA or 300 nM TBT for 1 h (black bars) or exposed to 0.5–10 nM PMA or 300 nM TBT for 1 h followed by a 24 h period in PMA-free media prior to assaying for NK lytic function (gray bars): To combine results from separate experiments (using cells from different donors) the levels of lysis were normalized as the percentage of the lytic function of the control cells in a given experiment. Values are mean±S.D. from four separate experiments using different donors (triplicate determinations for each experiment, n = 12). An asterisk indicates a significant decrease as compared to control (p<0.05).
Effects of 1 h exposures to PMA followed by 24 h in PMA-free media on NK lytic function
NK cells were exposed to PMA (0.5–10 nM) for 1 h. Following this exposure the PMA was removed the cells were incubated in PMA-free media for 24 h prior to assaying for lytic function. Previous studies have shown that a 1 h exposure to TBT results in a progressive loss of lytic function 24 h after removal of the compound (Dudimah et al. 2007; Whalen et al., 2002). A 1 h exposure of NK cells to concentrations of 2.5, 5, and 10 nM PMA resulted in a loss of lytic function of approximately 46% 24 h after the removal of the PMA (p<0.004) (Figure 1, gray bars). The 1 nM concentration resulted in a 25% loss of cytotoxic function whiles the 0.5 nM concentration led to a loss of lytic function by about 14% (p< 0.004) compared to the control (Figure 1, gray bars).
Effects of 10 min, 1 h, and 6 h exposures to PMA on the phosphorylation state of p44/42 in NK cells
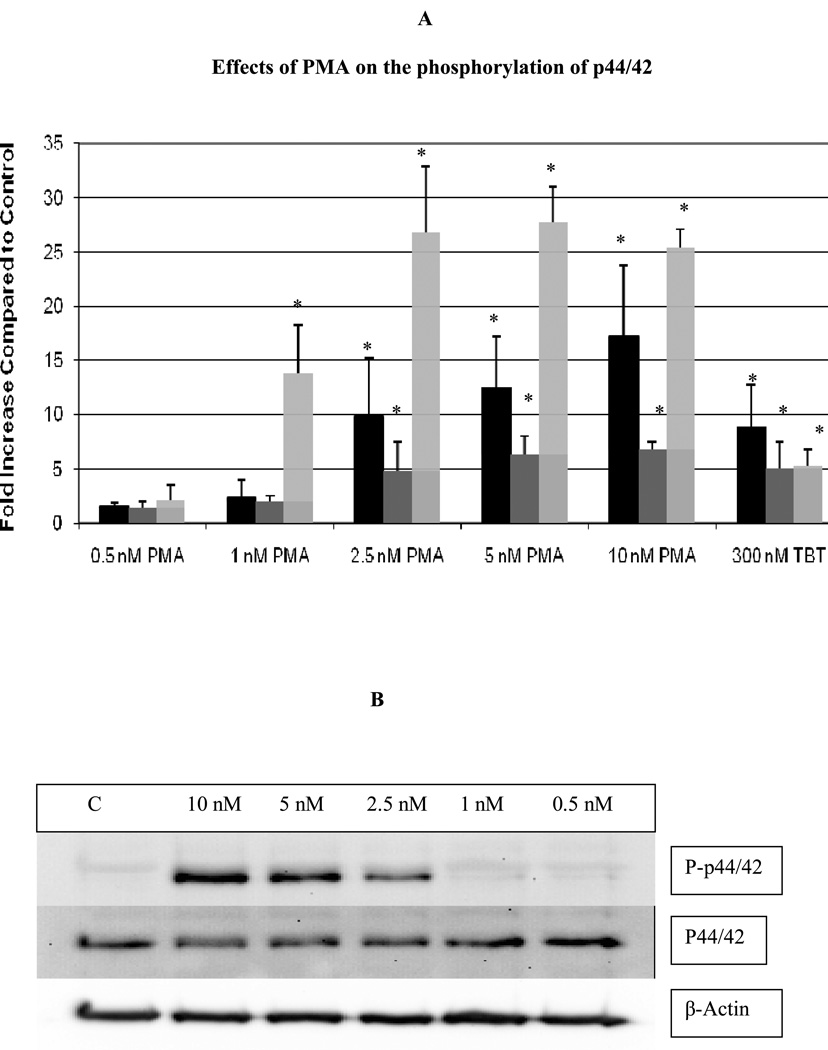
Exposure of human NK cells to concentrations of PMA ranging between 0.5 and 10 nM for 10 min resulted in observable increases in phosphorylation of p44/42. However, only concentrations of 2.5, 5, and 10 nM resulted in a statistically significant increases in phospho-p44/42 of 9.9±5.2, 12.4±4.8, and 17.2±6.6, respectively (p<0.05) (Figure 2A, black bars). There were no significant changes in the levels of total p44/42. Figure 2B shows results from a representative experiment for the 10 min exposure to PMA.
Figure 2.
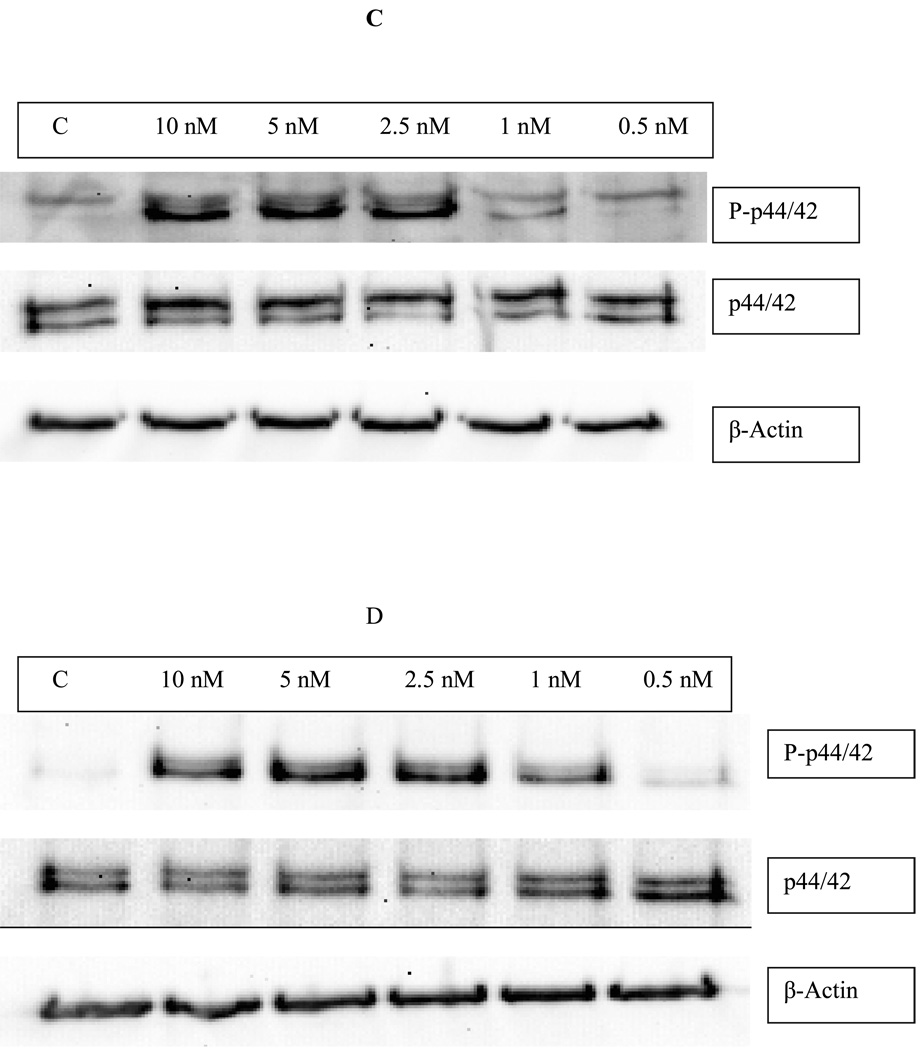
Effects of exposures of human NK cells to PMA on the phosphorylation state of p44/42. A) Fold increase in phospho-p44/42 as compared to control cells following 10 min (black bar), 1 hour (dark gray bar), and 6 hour (light gray bar) exposures to varying concentrations of PMA or to 300 nM TBT. Values are mean ±S.D. from at least three separate experiments using different donors (triplicate determinations for each experiment, n ≥ 9). An asterisk indicates a significant increase as compared to control (p<0.05). B) Representative western blot of the effect of 10 min exposures of NK cells to PMA on the phosphorylation state of p44/42. C) Representative western blot of the effect of 1 h exposures of NK cells to PMA on the phosphorylation state of p44/42. D) Representative western blot of the effect of 6 h exposures of NK cells to PMA on the phosphorylation state of p44/42.
A 1 h exposure of NK cells to PMA also caused significant increases in the phosphorylation of p44/42 MAPK (p<0.05). 5 nM PMA increased phospho-p44/42 by 6.3±1.7 fold, while 10 nM PMA caused 6.8±0.7 fold increase in phosphorylation of p44/42 (Figure 2A, dark gray bars). Again, total p44/42 was unaffected by PMA. Figure 2C shows results from a representative 1 h experiment.
Following a 6 h exposure to PMA, there were significant increases in phosphorylation of p44/42 at 1, 2.5, 5, and 10 nM PMA (p<0.05). Concentrations of 2.5,5, and 10 nM PMA all caused over 25 fold increases in phospho-p44/42 levels, whereas the 1 nM concentration resulted in a 13.9 fold increase in phospho-p44/42 (Figure 2A, light gray bars). There was no change in the level of total p44/42. Figure 2D shows results from a representative experiment at 6 h.
Effects of 10 min, 1 h, and 6 h exposures to PMA on the phosphorylation states of p38 and JNK
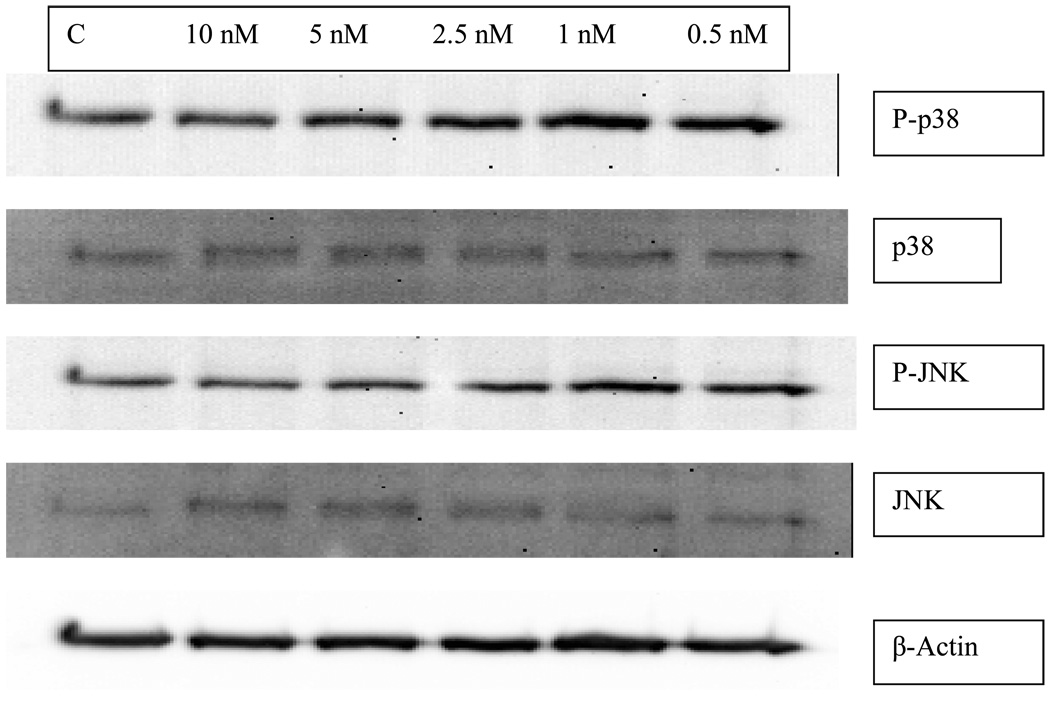
Exposure of human NK cells to various concentrations of PMA for 10 min did not result in any observable change in the phosphorylation state of p38 or JNK compared to the controls (Figure 3). Exposure of NK cells to PMA for 10 min did not affect the total levels of the above mentioned MAPKs either (Figure 3).
Figure 3.
The effect of 10 min exposures of human NK cells to PMA on the phosphorylation states of p38 and JNK. The exposures did not cause any significant changes in the levels of total p38, phospho-p38, total JNK, or phospho-JNK.
Neither 1 h nor 6 h exposures of human NK cells to PMA caused any significant changes in the phosphorylation of either p38 or JNK, or in their total levels (data not shown).
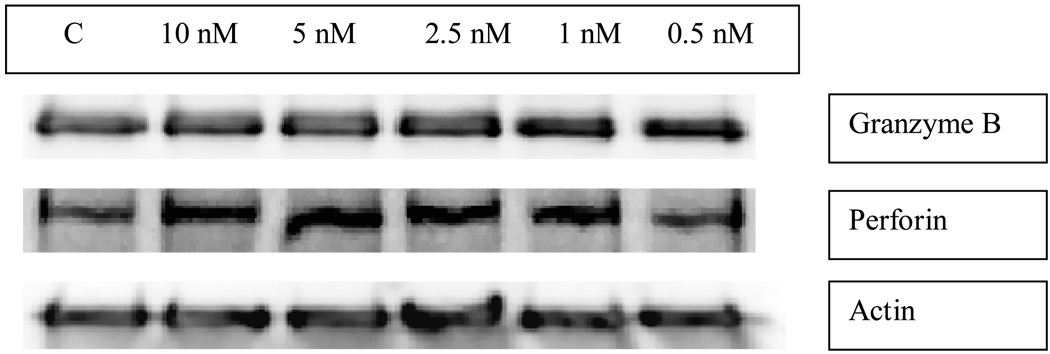
Effects of 24 h exposures to PMA on the levels of granzyme B and perforin in NK cells
NK cells exposed to concentrations of PMA ranging from 0.5–10 nM for 1, 6, or 24 h showed no decrease in the levels of granzyme B or perforin as compared to control cells. Figure 4 shows a representative experiment. In studies examining the effects of a 24 h exposure to TBT on granzyme and perforin protein levels, granzyme B levels were decreased by 51±27% and perforin levels were decreased by 47±24% (Thomas et al., 2004).
Figure 4.
The effect of 24 h exposures of human NK cells to PMA on the expression of the cytolytic proteins, granzyme B and perforin.
Effects of Anisomycin on the activation state of MAPKs, lytic function, and expression of cytolytic proteins in NK cells
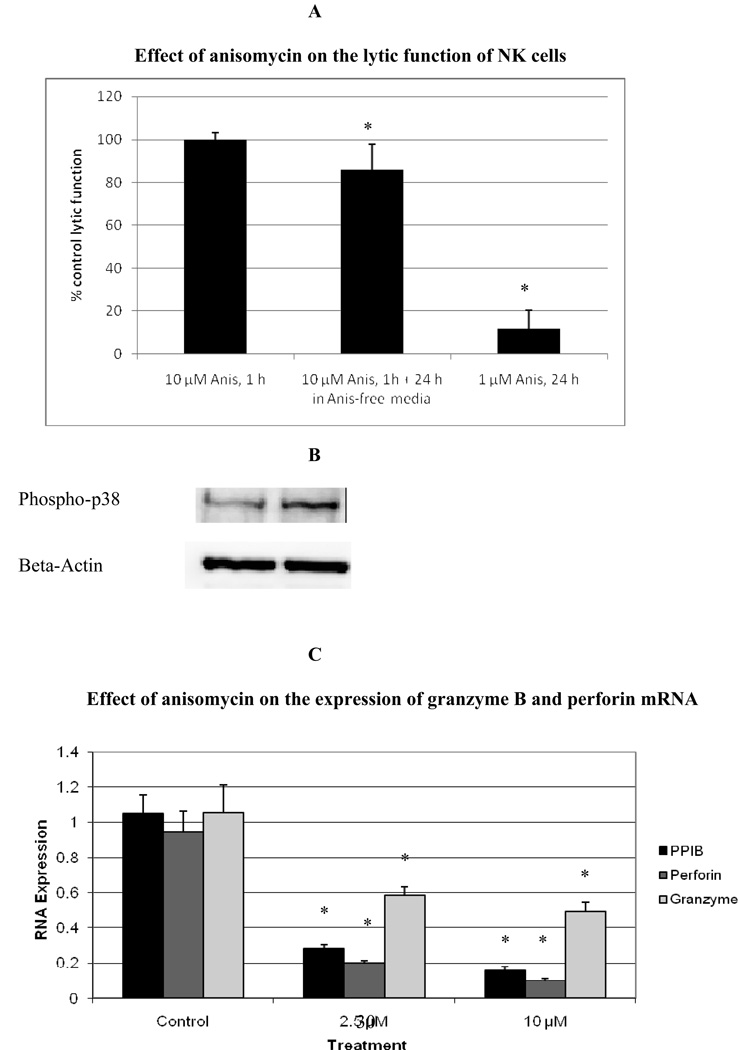
Previous studies with TBT have shown that there was also a significant activation of p38 MAPK when NK cells were exposed to TBT (Aluoch and Whalen, 2005; Aluoch et al., 2006). This activation was approximately 2 fold when NK cells were exposed to 300 nM TBT for 10 min and maintained out to 6 h. Thus, in order to investigate the potential role of p38 activation in TBT-induced loss of function, NK cells were exposed to anisomycin, which is among other things an activator of p38 (Shifrin and Anderson, 1999). When NK cells were exposed to anisomycin for 1 h, only the 10 µM concentration caused a significant activation of p38 of about 1.5 fold (p<0.05), it did not significantly activate other MAPKs. Figure 5B is a representative experiment showing increased phosphorylation of p38 with exposure to 10 µM anisomycin for 1 h. The same treatment caused no significant decrease in NK lytic function when lytic function was tested immediately after the 1 h exposure to anisomycin (Figure 5A). However, if the anisomycin was removed after the 1 h exposure and the cells were incubated for 24 h in anisomycin-free media before testing for lytic function, there was a small decrease in lytic function of 14±12% (p<0.0001) (Figure 5A). However, if NK cells were treated with 1 µM anisomycin for 24 h they lost 88±11% of their lytic function (p<0.0001) (Figure 5A). Since anisomycin is known to interfere with protein synthesis, its effects on granzyme and perforin expression were examined at the level of mRNA levels. RT-PCR results showed a remarkable reduction in the levels of granzyme B mRNA of anisomycin treated cells compared to control cells (Figure 5C). Both the 2.5 µM and 10 µM treatments resulted in significantly lowered levels of mRNA expression (P<0.02 in both cases). The exposure of human NK cells to anisomycin for 24 h also resulted in a significant reduction in the expression of perforin mRNA (Figure 5C). Additionally, the mRNA levels of peptidylprolyl isomerase B (PPIB) were also very significantly decreased with anisomycin exposure.
Figure 5.
Effect of exposures of human NK cells to anisomycin on: A) lytic function of NK cells. Values are mean ±S.D. from at least three separate experiments using different donors (minimum of triplicate determinations for each experiment, n ≥ 9 for 1 h exposure followed by 24 h period in anisomycin-free media n=21). An asterisk indicates a significant decrease as compared to control. B) phospho-p38 levels, representative experiment. C) the expression of granzyme B, perforin and PPIB mRNA. Values are mean±S.D. of four different samples from different donors. A significant difference in the expression of mRNA in treated cells compared to their respective controls is indicated by an asterisk. The viability of anisomycin treated cells was identical to that of control cells.
DISCUSSION
The main goal of this study was to determine what role the activation of p44/42 plays in the TBT-induced loss of NK lytic function. We have previously shown that TBT exposures that cause a loss of NK lytic function were able to activate p44/42 (Aluoch and Whalen , 2005, Aluoch et al., 2006, Aluoch et al., 2007). We have also shown that the activation of p44/42 is not solely responsible for the loss of NK cell function as specific inhibitors of p44/42 activation cannot block the negative effects of TBT (Abraha and Whalen, 2009). This is likely due to the fact that TBT causes additional changes in NK cells, such increases in cytosolic calcium ion concentrations (Lane et al., 2009). However, in order to understand the mechanism(s) by which TBT interferes with NK cell function, it is important to define the role played by p44/42 activation as it is an important consequence of TBT exposure in NK cells. By selectively activating p44/42 with Phorbol 12-myristate 13-acetate (PMA) and monitoring NK cell function, we will begin to determine the contribution that p44/42 activation makes to the TBT-induced loss of lytic function.
The results indicated that exposure of human NK cells to PMA concentrations of −0.25–10 nM for 1 h caused a significant loss in their ability to lyse tumor cells. Additionally when NK cells were exposed to PMA for 1 h followed by 24 h in compound-free media they showed a persistent loss of lytic function. These results are similar to what we have previously seen when NK cells were exposed to TBT for 1 h or for 1 h followed by a 24 h period in TBT-free media (Figure 1, Dudimah et al., 2007; Whalen et al., 2002).
The activation state of p44/42 was also determined at the same concentrations of PMA where loss of lytic function was seen. We have shown previously shown that TBT exposures for 1 h caused significant activation of p44/42 (Aluoch and Whalen, 2005. Aluoch et al., 2006). Very significant activation of the MAPK, p44/42, was seen within 10 min of exposure of human NK cells to PMA concentrations of 2.5, 5, and 10 nM (9.9, 12.4, and 17.2 fold, respectively) with neither p38 nor JNK being activated. An increase in phospho-p44/42 was also seen in NK cells exposed to these same concentrations of PMA for 1 h, however the fold increase at 1 h is somewhat lower that seen at 10 min (4.8, 6.3, and 6.8 fold increases, respectively). These results suggest that activation of p44/42 may result in loss of NK lytic function. The fact that the level of phospho-p44/42 observed after 1 h is lower than that observed after 10 min, could be as a result of phosphatase activity occurring between the 10 minute and 1 h time period. However, it appears that this phosphatase activity may fluctuate, since a 6 h exposure of NK cells to PMA concentrations of 1, 2.5, 5 and 10 nM caused much greater increases in phospho-p44/42 levels than were seen at 1 h (13.8, 26.8, 27.7, and 25.3 fold, respectively).
As mentioned earlier, previous studies have shown that exposure of NK cells to TBT decreases their lytic function (Dudimah et. al., 2007) and activates the MAPK, p44/42 (Aluoch and Whalen, 2005; Aluoch et. al., 2006; Aluoch et al., 2007). Here we show that an activation of p44/42 of roughly 6–7 fold by 5 or 10 nM PMA (1 h ) causes an approximately 47% loss of lytic function (Figure 1). When NK cells were exposed to 300 nM TBT for 1 h there was an approximately 5 fold activation of p44/42 and an approximately 40–50% loss of lytic function (Figure 1) (Dudimah et al., 2007; Aluoch and Whalen 2005). As mentioned above PMA is selectively activating p44/42 in NK cells, thus it appears that the activation of p44/42 by either PMA or TBT of 5–7 fold may be causing an approximately 50% loss of NK lytic function. This allows us to make an estimate of the contribution of p44/42 activation to the overall loss of lytic function seen with TBT exposure.
When we examined whether activation of p44/42 by PMA could decrease expression of the cytolytic proteins found in NK cells, granzyme B and perforin, we saw no PMA-induced change in their levels of expression. Thus, the decreases in granzyme B and perforin expression seen with exposures to TBT (Thomas et al., 2004; and Thomas et al., 2005) cannot be explained by TBT-induced activation of p44/42, in contrast to the TBT-induced effects on lytic function. This indicates that other TBT-induced changes, such as p38 activation (Aluoch and Whalen, 2005; Aluoch et al.,2006) or increases in cytosolic calcium ion (Lane et al., 2009), may be responsible for the decreases seen in these proteins.
Because we have previously also seen significant increases (approximately 2 fold) in the activation of the MAPK, p38, with TBT exposures (Aluoch and Whalen, 2005, Aluoch et al., 2006), we attempted to address the possible contributions that activation of p38 has in the TBT-induced loss of NK function using the p38 selective activator, anisomycin (Shifrin and Anderson, 1999). However, in addition to potentially activating p38, anisomycin is also able to block translation by inhibiting the peptidyl transferase reaction (Middlebrook and Leatherman, 1989). Thus, interpreting the results of anisomycin treatment of NK cells was complicated by the fact that longer term effects such as those seen at 24 h of exposure could be attributed to the translational effects rather than p38-activating effects of anisomycin. However we were able to determine that anisomycin could cause a very small activation of p38 about 1.5 fold at 1 h of exposure. We also found that this exposure could cause a small but significant loss of lytic function when the cells were tested for lytic function following a 24 h period in anisomycin-free media. This result indicated that anisomycin may be activating a process, such as transcriptional changes due to p38 activation, that does not immediately affect NK lytic function but takes several hours to occur. We went on to examine whether anisomycin treatment of NK cells could cause a decrease in the levels of the NK cytolytic proteins, Granzyme B and perforin, as these two proteins are decreased by TBT exposure. We found that anisomycin did cause a decrease in both granzyme B and perforin mRNA levels. Granzyme B has been shown to have a binding site for the transcription regulator, AP-1 (which can be activated by p38), in its promoter region (Hanson et al., 1993 )
In summary the results of the current study indicate that activation of p44/42 by TBT could be a contributing factor to the loss of lytic function seen with TBT exposures as a selective activation of p44/42 by PMA is able to cause losses of function similar to those seen when p44/42 was activated by TBT. Additionally, they indicate that the decreases in lytic proteins seen with TBT exposures could not be attributed to TBT-induced activation of p44/42. However, as TBT is also known to activate other MAPKs in NK cells it may be that the effects on protein expression were mediated through activation of p38, which also occurs but to a lesser extent. Experiments using anisomycin to activate p38 were complicated in their interpretation by the fact that anisomycin has other cellular effect beyond activation of p38.
Table 1.
Quantitative RT-PCR (TaqMan) Primers
| Gene | Primer Sequence | Amplicon Size (bp) |
|---|---|---|
| 18S RNA | Forward 542F TCGAGGCCCTGTAATTGGAA | 71 |
| Reverse 602R CCCTCCAATGGATCCTCGTT | ||
| Granzyme | Forward 68F TGCAACCAATCCTGCTTCTG | 67 |
| Reverse 134R CCGATGATCTCCCCTGCAT | ||
| Perforin | Forward 1638F CTCCTTGGCACCTGTGATCAG | 69 |
| Reverse 1706R GCCATGATTCAGGTTGCATCT | ||
| Peptidylprolyl isomerase B (PPIB) | Forward 247F GCTGCCGGGACCTTCTG | 101 |
| Reverse 347R CCCGGCCTACATCTTCATCTC | ||
ACKNOWLEDGEMENTS
This research was supported by Grant 2S06GM-08092-34 from the National Institutes of Health.
Contributor Information
Fred D. Dudimah, Department of Biological Sciences, Tennessee State University, Nashville, TN 37209
Denisha Griffey, Department of Chemistry, Tennessee State University, Nashville, TN 37209.
Xiaofei Wang, Department of Biological Sciences, Tennessee State University, Nashville, TN 37209.
Margaret M. Whalen, Department of Chemistry, Tennessee State University, Nashville, TN 37209
REFERENCES
- Abraha A, Whalen MM. The role of p44/42 activation in tributyltin-induced inhibition of human natural killer cells: effects of MEK inhibitors. J. Appl. Toxicol. 2009;29:165–173. doi: 10.1002/jat.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluoch AO, Whalen MM. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–277. doi: 10.1016/j.tox.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. doi: 10.1016/j.tox.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Pattern of MAP kinases p44/42 and JNK activation by non-lethal doses of tributyltin in human natural killer cells. Arch. Toxicol. 2007;81:271–277. doi: 10.1007/s00204-006-0155-4. [DOI] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpes virus in an adolescent without natural killer cells. New Engl. J. Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of Tributyltin (TBT) on the ATP levels in human natural killer cells: Relationship to TBT- induced decreases in NK function. J. Appl. Toxicol. 2007;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Fleisher G, Koven N, Kamiya H, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein-Bar virus infections. J. Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- Hanna N. Expression of metastatic potential of tumor cells in young nude mice is correlated with low levels of natural-killer cell mediated cytotoxicity. Int. J. Cancer. 1980;26:675–690. doi: 10.1002/ijc.2910260521. [DOI] [PubMed] [Google Scholar]
- Hanson RD, Grisolano JL, Lay TJ. Consensus AP-1 and CRE motifs upstream from the human cytotoxic serine protease B (CSP-B/CGL-1) gene synergizes to activate transcription. Blood. 1993;82:2749–2757. [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain foodstuffs. Bull. Environ. Contam. Toxicol. 1995a;55:510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R, Williams RJ. Butyltin residues in fish from Australia, Papua New Guinea and the Solomon Islands. Int. J. Environ. Anal. Chem. 1995b;61:263–273. [Google Scholar]
- Kannan K, Tanabe S, Iwata H, Tatsukawa R. Butyltins in muscle and liver of fish collected from certain Asian and Oceanian countries. Environ. Pollut. 1995c;90:279–290. doi: 10.1016/0269-7491(95)00028-p. [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environ. Sci. Technol. 1999;33:1776–1779. [Google Scholar]
- Kiessling R, Haller O. Natural killer cells in the mouse, an alternative surveillance mechanism? Contemp. Top. Immunobiol. 1978;8:171–201. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD. Toxicity and health effects of selected organotins compounds: a review. Environ. Health Perspect. 1976;14:51–56. doi: 10.1289/ehp.761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R, Ghazi SO, Whalen MM. Increases in cytosolic calcium ion levels in human natural killer cells in response to butyltin exposure. Archives of Environmental Contamination and Toxicology. 2009 doi: 10.1007/s00244-009-9313-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RB, Linden O. Fate and effects of organotin compounds. Ambio. 1985;14:88–94. [Google Scholar]
- Loganathan BG, Kannan K, Owen DA, Sajwan KS. Butyltin compounds in freshwater ecosystems. In: Lipnick RL, Hermens J, Jones JK, Muir D, editors. Persistent, Bioaccumulative, and Toxic Chemicals. I Fate and Exposure Am. Chem. Soc. Pub. London: Oxford Univ. Press; 2000. [Google Scholar]
- Lotzova E. Definition and function of natural killer cells. Natural. Immun. 1993;12:177–193. [PubMed] [Google Scholar]
- Middlebrook JL, Leatherman DL. Binding of T-2 toxin to eukaryotic cell ribosomes. Biochem. Pharmacol. 1989;38:3103–3110. doi: 10.1016/0006-2952(89)90021-x. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Ortaldo JR. The biology of natural killer cells: Insight into the molecular basis of function. In: Lewis CE, McGee JOD, editors. The Natural Killer Cell. Oxford: IRL Press; 1992. pp. 1–40. [Google Scholar]
- Roper WL. Toxicological profile for tin. U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry, USA. 1992 [Google Scholar]
- Shifrin VI, Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Prudente M, Mizuno T, Hasegawa J, Iwata H, Miyazaki N. Butyltin contamination in marine mammals from north Pacific and Asian coastal waters. Environ. Sci. Technol. 1998;32:193–198. [Google Scholar]
- Thomas LD, Shah H, Green SA, Bankhurst AD, Whalen MM. Tributyltin exposure causes decreased granzyme B and perforin levels in human natural killer cells. Toxicology. 2004;200:221–233. doi: 10.1016/j.tox.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Thomas LD, Shah H, Bankhurst AD, Whalen MM. Effects of interleukins 2 and 12 on the levels of granzyme B and perforin and their mRNAs in tributyltin-exposed human natural killer cells. Arch. Toxicol. 2005;79:711–720. doi: 10.1007/s00204-005-0002-z. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Whalen MM. Inhibition of human natural killer cell function in vitro by glucose concentrations seen in poorly controlled diabetes. Cell. Physiol. Biochem. 1997;7:53–60. [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ. Res. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Williams TB, Green SA, Loganathan BG. Interleukins 2 and 12 produce recovery of cytotoxic function in tributyltin-exposed human natural killer cells. Environ. Res. 2002;88:189–209. doi: 10.1006/enrs.2002.4332. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Yamashita N, Saito T. Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chemico-Biological Int. 2003;145:311–319. doi: 10.1016/s0009-2797(03)00027-9. [DOI] [PubMed] [Google Scholar]
- Yamada S, Fuji Y, Mikami E, Kawamura N, Hayakawa J. Small-scale survey of organotin compounds in household commodities. J. AOAC Int. 1993;76:436–441. [Google Scholar]