Abstract
The cannabis plant and products produced from it, such as marijuana and hashish, have been used for centuries for their psychoactive properties. The mechanism for how Δ9 -tetrahydrocannabinol (THC), the active constituent of cannabis, elicits these neurological effects remained elusive until relatively recently, when specific G-protein coupled receptors were discovered that appeared to mediate cellular actions of THC. Shortly after discovery of these specific receptors, endogenous ligands (endocannabinoids) were identified. Since that time, an extensive number of papers have been published on the endocannabinoid signaling system, a widespread neuromodulatory mechanism that influences neurotransmission throughout the nervous system. This paper summarizes presentations given at the 12th International Neurotoxicology Association meeting that described the potential role of endocannabinoids in the expression of neurotoxicity. Dr. Raphael Mechoulam first gave an overview of the discovery of exogenous and endogenous cannabinoids and their potential for neuroprotection in a variety of conditions. Dr. Larry Parsons then described studies suggesting that endocannabinoid signaling may play a selective role in drug reinforcement. Dr. Carey Pope presented information on the role that endocannabinoid signaling may have in the expression of cholinergic toxicity following anticholinesterase exposures. Together, these presentations highlighted the diverse types of neurological insults that may be modulated by endocannabinoids and drugs/toxicants which might influence endocannabinoid signaling pathways.
Keywords: neuromodulation, drugs of abuse, insecticides, mechanisms, head trauma, hepatic encephalopathy
1. The “Latecomers”. (R.M.)
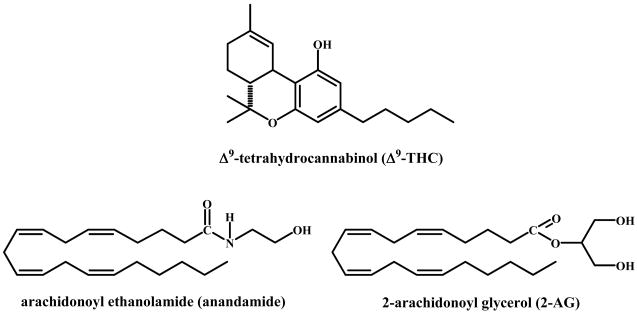
The plant cannabinoids and the biochemical endogenous cannabinoid system are relative latecomers to chemistry and biology. While cannabis preparations were for centuries – and still are - the most widely used illicit drugs in many parts of the world, their chemistry and biological actions were not well known until the 1960’s. This is in sharp contrast with our knowledge on morphine and cocaine, the two other major illicit drugs, which were already isolated during the 19th century. Again, while the major neurotransmitter systems, the cholinergic, adrenergic, dopaminergic systems, were discovered in the 1930’s, the endocannabinoid system was uncovered only in the late 1980’s and early 1990’s. The reasons were both technical and conceptual. While morphine and cocaine are alkaloids, which can be easily crystallized as salts, the active cannabis principle, Δ9 -tetrahydrocannabinol (THC, Figure 1), is an oily substance in a mixture of many dozens of chemically related compounds.
Figure 1.
Chemical structures of 9-tetrahydrocannabinol, anandamide and 2-arachidonoyl glycerol.
Its isolation as a pure substance in 1964 became possible only when good chromatographic methods became available [1]. Over the next 20 years hundreds of publications on its chemistry, biochemistry, pharmacology and clinical effects appeared, but its mechanism of action remained unknown. It was generally believed (but never substantiated), that THC, being highly liposoluble, somehow acts on membranes. With the discovery that the actions of THC are stereospecific [2], it became reasonable to expect that its action is also specific. Indeed, in the late 1980’s and early 1990’s two cannabinoid receptors (known today as CB1 and CB2) were discovered [3, 4] and shortly thereafter the two major endogenous agonists, anandamide (AEA) [5] and 2-arachidonoyl glycerol (2-AG) [6] were isolated (Figure 1). Thousands of publications have described their formation, metabolism and actions. The endocannabinoid system has been found to be involved in almost all physiological systems that have been looked into [7].
The basic function of the endocannabinoid system may be protective in nature. Endocannabinoids participate in a variety of processes including thermoregulation, food intake, immune function, perception (hearing, color, vision, taste), cognition (long-term potentiation, short-term memory) and motor function (locomotor activity, proprioception, muscle tone). The endocannabinoids have also been reported to affect a large number of pathological conditions and can be viewed as part of a general protective network, working in conjunction with the immune system and with various other physiological systems.
In the CNS, eCB signaling is mediated mostly by the CB1 receptor, a putative membrane eCB transporter, and hydrolytic enzymes involved in both synthesis (diacylglycerol lipase, DAGL, and N-acylphosphatidyl-ethanolamine-specific phospholipase D, NAPE-PLD) and inactivation (fatty acid amide hydrolase, FAAH and monoacylglycerol lipase, MAGL) of eCBs. The eCBs are synthesized “on demand” from arachidonic acid of membrane phospholipids [8]. Postsynaptic neuron depolarization leads to eCB release, after which the endocannabinoids diffuse across the synapse and activate CB1 receptors on the pre-synaptic terminal [9–13]. Endocannabinoid synthesis can also be stimulated in a receptor-mediated fashion by activation of the Gq protein-coupled metabotropic glutamate (MGlu) receptors and the muscarinic M1 and M3 receptors [14–16].
Although a specific transporter protein has not yet been isolated or cloned, termination of eCB signaling appears to require cellular reuptake via a carrier-mediated transport process [17–21]. [3H]Anandamide uptake is temperature-dependent and saturable, and the AEA analog (N- 4-hydroxy-phenyl arachidonylamide, AM 404) inhibits its reuptake [18]. AM 404 and other inhibitors of eCB transport increase brain levels of AEA and 2-AG [22] and enhance the electrophysiological effects of both endogenous and exogenous cannabinoids [21,23,24].
Following cellular reuptake, eCBs are enzymatically degraded. FAAH is distributed throughout the brain and appears to be primarily responsible for AEA hydrolysis [25–29]. While 2-AG can also be hydrolyzed by FAAH [30,31], MAGL appears to be the primary enzyme involved in its degradation [32]. Both FAAH and MAGL are intracellular enzymes and inhibition of both has been shown to increase intracellular eCB levels [27,33,34].
Endocannabinoid signaling is neuromodulatory through inhibiting the release of a number of neurotransmitters [35–41]. The calcium-dependent synthesis and release of endocannabinoids to activate presynaptic receptors take place throughout the nervous system to modulate neurotransmitter release and hence could have an important role in protecting various systems from excessive stimulation. From our own work (in collaboration with many colleagues as indicated in the references cited), examples of the protective action of endocannabinoids in the nervous system will be presented. Many others have been reported.
Neuroprotection
Traumatic brain injury (TBI) in mice led to an increase in blood brain barrier permeability, brain water content, lesion volume and hippocampal cell death. 2-AG levels in the brain were also enhanced, which we assumed to be a protective reaction. Indeed, treatment with 2-AG following TBI reduced the brain trauma effects and improved behavioral function. 2-AG inhibits mRNA expression of proinflammatory cytokines and NF-κB inhibition is observed. 2-AG also possesses antioxidant properties. The beneficial effects were abolished when 2-AG was co-administrated with the CB1 antagonist SR141716A (Rimonabant) as well as in CB1−/− knockout mice, suggesting that, at least in part, the beneficial effects of AEA are produced via activation of the CB1 receptor [42,43]. In contrast, during cerebral ischemia/reperfusion injury, while activation of the CB2 receptor was found to be protective, the greatest degree of neuroprotection was obtained by combining a CB1 inhibitor with a CB2 agonist [44].
Hepatic encephalopathy
Experimental encephalopathy in mice, which is caused by thioacetamide-induced acute liver failure, is an animal model for hepatic encephalopathy, a neuropsychiatric syndrome. The CB2 receptor is involved in the pathogenesis of this syndrome. This contention was supported by the observations that (as in brain trauma) in encephalopathic mice there was a significant increase in brain levels of 2-AG and that systemic administration of 2-AG led to improvement of the neurological score, cognitive function, and activity. These actions are mediated in part by AMP-activated protein kinase. The effect of 2-AG on the neurological score could be fully eliminated by a CB2 antagonist. Again, as in cerebral ischemia/reperfusion injury, the best results were obtained by combining an inhibitor of CB1 activation with an exogenous CB2 agonist [45–47]. Surprisingly, cannabidiol which does not bind to the endocannabinoid receptors also ameliorates cognitive and motor impairment in mice with bile duct ligation, another model of hepatic encephalopathy [48].
2. Endocannabinoid signaling and drugs of abuse. (L.H.P.)
Endocannabinoids such as AEA and 2-AG participate in long-term synaptic plasticity in several neural circuits that mediate the motivational effects of abused drugs. Converging evidence from human and animal studies points to an important modulatory influence of cannabinoid CB1 receptors in the behavioral response to addictive drugs. For example, genetic deletion of CB1 receptors attenuates the reinforcing effects of ethanol, opiates and nicotine as measured by the conditioned place-preference paradigm [49–51]. CB1 receptor knockout mice also display reduced ethanol and opiate self-administration [52–57]. Similarly the pharmacologic blockade of CB1 receptors with SR141716A attenuates nicotine-induced conditioned place-preference [58,59] and reduces the self-administration of ethanol [54,60–63], heroin [64–69] and nicotine [70]. In contrast, administration of CB1 receptor agonists increases the self-administration of ethanol [62,71] and heroin [68]. Collectively these findings indicate that CB1 receptors exert a facilitory influence on ethanol, opiate and nicotine conditioning and self-administration.
In contrast to ethanol, nicotine and opiates, the influence of central cannabinoid receptors in the mediation or modulation of psychostimulant reinforcement is less clearly defined. CB1 receptors have been implicated in cocaine-induced motor sensitization [71] and several physiologic effects produced by cocaine [73–75]. However, CB1 receptor knockout mice appear to self-administer cocaine as avidly as wild type mice [49,52] and several reports indicate that cocaine self-administration by rats is unaltered by treatments with the CB1 antagonist SR141716A [69,76–80] though a recent report demonstrates that the reinforcing effects of cocaine self-administration are reduced by a structurally similar CB1 antagonist AM251 [80]. Thus, the relative involvement of CB1 receptors in modulating the behavioral effects of cocaine is somewhat less clear than the involvement of these receptors in the behavioral effects of other abused substances such as ethanol, opiates and nicotine.
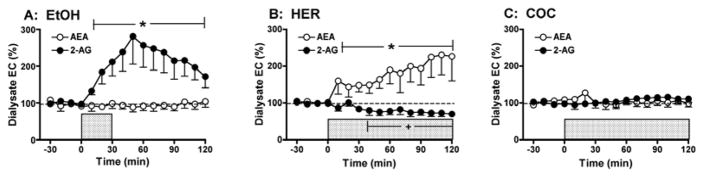
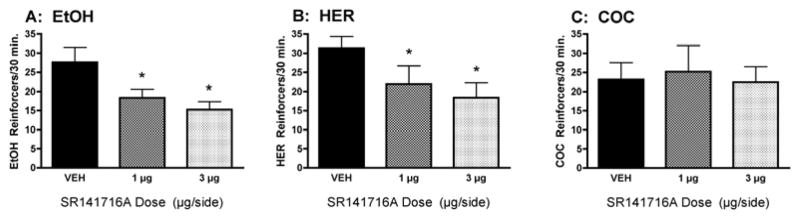
Although these findings implicate CB1-mediated mechanisms in the motivation for drug consumption, there has been little evidence directly demonstrating that intake of abused drugs alters eCB formation in the brain. To address this issue we have developed an in vivo microdialysis method for monitoring eCB levels in awake freely-moving rodents [81,82] and have used this approach to explore the effect of volitional drug self-administration on extracellular eCB levels in the rodent brain. We have found that limited-access to ethanol, heroin and cocaine self-administration results in dose-dependent and drug-specific alterations in brain eCB levels [82]. For example, initial evaluations in the nucleus accumbens (a brain region critically involved in mediating the rewarding properties of most abused substances) revealed that ethanol increases 2-AG levels without altering AEA levels, heroin increases AEA and decreases 2-AG, while cocaine does not alter either eCB (Figure 2). To test the functional significance of these drug-related alterations in brain eCB levels we evaluated drug self-administration behavior following either systemic or intra-accumbens CB1 antagonist (SR141716A) administration (Figure 3). Consistent with the drug-specific alterations in eCB levels we found that intraperitoneal SR141716A dose-dependently reduced ethanol, and heroin self-administration but did not alter cocaine intake. Moreover, intra-accumbens SR141716A significantly reduced ethanol and heroin self-administration, whereas cocaine self-administration was unaltered by these same antagonist doses [64,82]. Additional studies have revealed that drug intake produces distinct effects on interstitial eCB levels in other brain regions involved in mediating drug reward. For example, ethanol self-administration increases both 2-AG and AEA levels in the ventral tegmental area but does not alter levels of either eCB in the prefrontal cortex. Consistent with this profile we find that ethanol intake is reduced by SR141716A administration into the ventral tegmental area but not prefrontal cortex [83].
Figure 2.
Selective effects of ethanol, heroin, and cocaine self-administration on microdialysate AEA and 2-AG levels collected from the NAc. In each panel the shaded bar reflects the period of drug self-administration. Panel A: Dialysate 2-AG levels were significantly increased by voluntary oral EtOH self-administration (10% w/v; 0.39 ± 0.03 g/kg total EtOH intake during 30 min. self-administration session; n = 9). There was no significant effect of EtOH intake on AEA levels measured from these same dialysate samples. Panel B: Dialysate AEA levels were significantly increased by voluntary heroin self-administration (20 μg/infusion; 443 ± 62 μg/kg total intake during 120 min self-administration session; n = 7). In contrast, dialysate 2-AG levels were subtly, but significantly decreased during the latter portions of the self-administration session. Panel C: Dialysate AEA and 2-AG levels were not altered by cocaine self-administration (0.25 mg/infusion; 18 ± 0.9 mg/kg total intake during 120 min self-administration; n = 8). Data are modified from [82].
Figure 3.
Localized infusion of the CB1 receptor antagonist SR141716A (1 and 3 μg/0.5 μl/side) into the rat nucleus accumbens significantly reduces the operant self-administration of EtOH (Panel A: 10% w/v; n = 11) and heroin (Panel B: 20 μg/infusion; n = 10), but not cocaine (Panel C: 0.25 mg/infusion; n = 7). Thus, intra-NAc CB1 antagonist administration reduced the self-administration of the two drugs whose intake significantly increases interstitial NAc EC levels (e.g. EtOH and heroin, see Fig. 2) but did not alter the self-administration of the drug that did not significantly alter interstitial NAc EC levels (e.g. cocaine). Data are modified from [69, 82].
We have conducted several experiments to characterize the neurochemical mechanisms underlying the eCB modulation of drug reward. Substantial evidence indicates that the rewarding effects of most abused drugs are mediated in part by increased dopamine levels in the nucleus accumbens, and we have found that CB1 receptor antagonism attenuates both ethanol- and nicotine-induced increases in nucleus accumbens dopamine levels. However, heroin-induced increases in accumbens dopamine are not altered by CB1 receptor blockade and these receptors appear to influence opiate reward by modulating drug-induced reductions in ventral pallidal GABA release [71,84].
Collectively our data indicate that the intake of various abused substances leads to increases in brain eCB levels, an effect that may serve to modulate the motivation for continued drug intake. However, the specific mechanisms through which this occurs likely vary among different classes of abused substances. Ongoing experiments are characterizing the effects of long-term drug exposure on brain eCB signaling, and data gathered so far indicate that chronic ethanol exposure results in a potentiation of ethanol-induced increases in nucleus accumbens 2-AG [85]. As described below, we have also gathered evidence that long-term alcohol exposure leads to disrupted eCB signaling in the amygdala and our data indicate that this contributes to the etiology of excessive alcohol intake associated with alcohol dependence.
eCB Function and Alcohol Dependence
The development of alcohol use disorders [86] is thought to follow a transition from social use motivated by the hedonic and anxiolytic aspects of alcohol intoxication to dependence on alcohol motivated by increasing withdrawal symptoms and an evolving desire to drink during abstinence [87,88]. In this latter stage of alcohol dependence, abstinence from drinking is often accompanied by negative emotional symptoms, such as increased anxiety and depression, and the alleviation of these negative emotional states is hypothesized to be a major driving force for continued alcohol consumption [86,87]. Thus there is believed to be a shift in the motivational mechanisms for alcohol use from positive to negative reinforcement in course of dependence induction, and it is likely this shift results from enduring adaptive changes in CNS function induced by excessive alcohol consumption [88,90–93].
Endocannabinoids are present in stress-responsive neural circuits and a growing body of evidence indicates that eCB tone provides negative feedback to attenuate stress responses and re-establish homeostasis [94,95]. Disrupted eCB signaling is associated with an inability to adapt to stress and has been implicated in affective disorders such as anxiety and depression. In light of evidence that brain eCB signaling is altered by acute alcohol consumption, we theorized that long-term, high dose alcohol exposure may induce adaptations in eCB signaling that contribute to the maladaptive stress responses, anxiety and depression associated with acute and prolonged withdrawal in human alcoholics and alcohol-dependent rodents.
We have found that alcohol dependence in rats is associated with dysregulated eCB function in the central nucleus of the amygdala (CeA), a brain region critically involved in mediating stress responses and anxiety-like behavior. Long-term alcohol consumption results in diminished cannabinoid-1 (CB1) receptor mRNA expression, less efficient CB1 receptor G-protein coupling and reduced baseline extracellular 2-AG levels in the CeA even during ongoing alcohol consumption. These deficits in extracellular eCB levels are exacerbated during alcohol withdrawal and renewed alcohol consumption after a period of abstinence restores extracellular eCBs to pre-withdrawal levels. Thus, eCB signaling is compromised in the CeA of alcohol-dependent rats, and withdrawal-associated deficits in amygdalar eCB levels are ameliorated by resumption of alcohol intake.
Because alcohol-dependent rats display exaggerated emotional behavior in response to mild stress [96,97] we hypothesized that deficits in amygdalar eCB signaling contribute to dependence-associated increases in anxiety-like behavior. In support of this hypothesis we found that increased anxiety-like behavior in alcohol-dependent rats observed after 7 days of alcohol abstinence is reversed by systemic doses of the eCB clearance inhibitor AM404 that are behaviorally inactive in non-dependent rats. Similarly we found that AM404 reverses dependence-related deficits in social interaction behavior observed after 2 days of alcohol abstinence. Together these findings suggest that bolstering eCB tone through inhibition of eCB clearance reduces the anxiety-like behavior associated with alcohol dependence and protracted withdrawal.
As previously mentioned, a growing body of both clinical and preclinical literature correlates dependence-related increases in anxiety with excessive alcohol consumption, and pharmacological manipulations known to attenuate dependence-associated anxiety have been shown to also selectively reduce alcohol consumption in dependent subjects [98,99]. Based on these observations we hypothesized that deficient eCB signaling contributes to excessive alcohol intake by dependent rats. Consistent with findings by others, we have found that alcohol experienced but non-dependent rats display an initial burst of alcohol consumption in the first 10 minutes of a 30 minute drinking session, with minimal consumption during the final 20 minutes of the session. In contrast, alcohol-dependent rats continue to drink steadily during the entire 30 minute session and typically consume 2 – 3 times more alcohol than non-dependent rats. We have found that systemic pretreatment with the eCB clearance inhibitor AM404 dose-dependently attenuates alcohol consumption by dependent rats at doses that do not alter alcohol self-administration by non-dependent controls. Interestingly, AM404 pretreatment did not induce a general suppression of alcohol consumption in dependent rats, but rather produced a pattern of intake that resembled intake by non-dependent rats (e.g. reduced consumption following an initial “loading up” period of intake). Similar alterations in alcohol consumption by alcohol-dependent rats were observed following intra-CeA infusions of the CB1 receptor agonists WIN 55,212-2 or 2-AG. There were no significant alterations in alcohol consumption by non-dependent rats following intra-CeA CB1 agonist administration, and infusions of these drugs into the closely related basolateral nucleus of the amygdala did not alter alcohol consumption in either dependent or non-dependent rats.
Collectively these findings suggest that chronic alcohol exposure induces deficits in amygdalar eCB signaling that contribute to dependence-related anxiety and withdrawal-induced excessive alcohol consumption. Accordingly, treatments that enhance eCB tone may be a viable approach for the alleviation of affective dysregulation and excessive alcohol consumption associated with alcoholism.
3. Endocannabinoid signaling and anticholinesterase toxicity. (C.P.)
As noted above, the primary psychotropic compound in cannabis (i.e., Δ9 -tetrahydrocannabinol, THC) alters neurological functions primarily through interaction with a specific G protein–coupled receptor, the cannabinoid 1 (CB1) receptor. The density of the CB1 receptor is generally high with respect to other neurotransmitter receptors, with regions such as the caudate nucleus, globus pallidus, CA3 and dentate formation in the hippocampus, olfactory bulb, and piriform cortex expressing abundant levels [100–102]. Based on the dense and widespread distribution of eCB signaling, we hypothesized that eCB signaling plays a role in the ultimate expression of various types of neurotoxicity. Neurotoxicants that primarily act by altering synaptic neurotransmitter levels, e.g., organophosphorus insecticides (OPs), may be particularly sensitive to the neuromodulatory actions of eCBs.
The mechanism of toxicity of OPs is initiated by inhibition of acetylcholinesterase [103]. Cholinergic neurons in the peripheral and central nervous systems release acetylcholine (ACh) upon depolarization, which in turn activates muscarinic or nicotinic receptors on postsynaptic neurons, muscle cells or autonomic end-organs. Acetylcholinesterase, the enzyme which degrades ACh, is an extremely efficient enzyme (turnover rate of 4 × 105 molecules of acetylcholine/min, [104]. Thus, ACh is normally degraded rapidly and has only a transient opportunity to activate cholinergic receptors. Extensive acetylcholinesterase inhibition following exposure to an OP prevents this efficient breakdown of acetylcholine, leading to ACh accumulation and persistent/prolonged stimulation of cholinergic receptors throughout the nervous system.
Many of the classical signs of cholinergic toxicity associated with OP poisoning are related to changes in cholinergic transmission in the peripheral nervous system. The most debilitating responses to severe anticholinesterase exposure involve the CNS, however [105]. Lethality from OP intoxication is typically due to depression of brainstem respiratory control centers, compounded by peripheral effects, e.g., excessive airway secretions and diaphragm/intercostal muscle dysfunction. Whole body tremors can result from extensive acetylcholinsterase inhibition in the CNS, with activation of muscarinic receptors in the basal ganglia being of prominent importance [105–107]. With severe intoxications, CNS stimulation can lead to seizures that can in turn lead to irreversible neuropathology [109–114]. A number of epidemiological studies have reported subtle, long-term neuropsychological sequelae associated with past anticholinesterase intoxication [115–119]. Extrapyramidal motor effects (e.g., mask face, cogwheel rigidity, choreoathetosis, rigid posture with resting tremor) persisting for months following severe anticholinesterase intoxication have been reported [120–123]. Thus, central actions of organophosphorus anticholinesterases can be critical in both acute toxic responses and long-term neurologic sequelae following acute intoxication.
While disruption of cholinergic neurotransmission is a hallmark of OP poisoning, substantial evidence indicates non-cholinergic signaling can contribute to the ultimate expression of toxicity. Shih and coworkers [109] reported that seizures elicited by the nerve agent soman were initially sensitive, but later resistant, to the prototype anticholinergic antidote, atropine. Activation of glutamatergic signaling in limbic regions appeared particularly important in OP–induced seizures [124,125]. NMDA receptor antagonists blocked the seizures elicited by a number of OP insecticides [113,114]. In contrast, Cassel and Fosbraey [126] reported that higher GABA levels in striatum were correlated with the severity of toxicity. Seizure intensity and epileptiform bursting were also correlated with higher striatal GABA levels following soman exposure [127]. Furthermore, Jacobsson et al., [127] reported that striatal release of dopamine was also highly correlated with the severity of seizures elicited by soman. Bourne and coworkers [128] reported that the dopamine D1-like receptor antagonist SCH23390 completely blocked soman-induced seizures. The activation of non-cholinergic signaling pathways therefore appears to contribute to some CNS-mediated signs of OP toxicity.
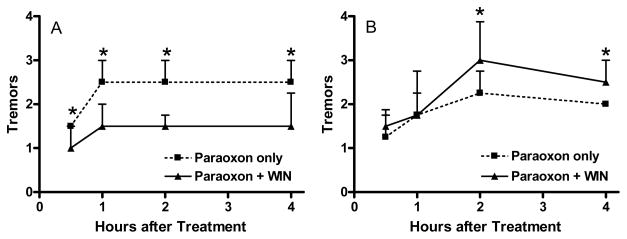
As eCBs can modulate the release of various neurotransmitters in selected pathways throughout the nervous system, eCB signaling may play a role in the expression of anticholinesterase toxicity elicited through changes in cholinergic and/or non-cholinergic systems. We have conducted a series of studies to evaluate the possible role of eCB signaling in OP toxicity. We first evaluated the effects of the direct cannabinoid receptor agonist WIN 55212-2 (WIN) on the acute toxicity of paraoxon [129]. Adult Sprague Dawley rats were treated with either vehicle or paraoxon (0.4 mg/kg, sc) and then immediately given either vehicle or WIN (1.5 mg/kg, ip). WIN decreased the severity of cholinergic signs elicited by paraoxon, Figure 4A). We also studied the effects of repeated WIN exposures on acute paraoxon toxicity. Rats were given WIN daily (1.5 mg/kg/day) for seven days and then challenged with paraoxon two hours after the final WIN treatment. Interestingly, repeated WIN dosing increased the severity of cholinergic signs elicited by paraoxon (0.4 mg/kg, sc; Figure 4B). Hippocampal CB1 ([3H]CP 55940) binding was significantly reduced in rats treated repeatedly with WIN. Together, these data suggested that acute and repeated exposures to a cannabinoid receptor agonist could have significant but potentially opposing effects on acute OP toxicity. We next compared the effects of WIN with other cannabinomimetics (URB597, an inhibitor of FAAH; URB602, an inhibitor of MAGL; AM404, an eCB clearance inhibitor) on the acute toxicity of DFP (1.5 mg/kg, sc) [130]. All four cannabinomimetics decreased involuntary movements elicited by DFP. Together, these data suggested that direct or indirect cannabinoid receptor activation can modulate cholinergic toxicity following OP exposure.
Figure 4.
Effects of A) acute WIN 55212-2 and B) repeated WIN 55212-2 exposures on acute paraoxon toxicity. Rats were treated with paraoxon (0.4 mg/kg, sc) either just before a single dose of WIN (1.5 mg/kg, ip) or 2 hours after the seventh daily dose of WIN. Functional signs of toxicity (involuntary movements, tremors) were scored (1 = normal; 2 = slight tremors in neck and head; 3 = moderate tremors involving more caudal aspects of body; 4 = severe whole body tremors) by a blinded observer from 0.5–4 hours after paraoxon challenge. Data represent median ± interquartile range. No signs of toxicity were noted in rats receiving vehicles or WIN only (data not shown). An asterisk indicates a significant difference between groups. Data are modified from [129].
Our laboratory has been interested in the differential expression of acute toxicity elicited by the insecticides parathion (O,O′-diethyl-p-nitrophenyl-phosphorothioate) and chlorpyrifos (O,O′-diethyl-3,5,6-trichloropyridinyl-phosphorothioate) for a number of years. We have repeatedly noted that rats treated with high dosages of parathion exhibit moderate to severe cholinergic signs of toxicity, while rats treated with dosages of chlorpyrifos that elicit similar degrees of cholinesterase inhibition show markedly less signs of toxicity [131–133]. Chlorpyrifos and parathion have markedly different in vivo potencies, with parathion being much more potent based on both biochemical and functional toxicity endpoints [131,134,135]. A primary basis for this difference in in vivo potency is the more effective detoxification of chlorpyrifos oxon [136,137]. As noted above, however, equi-inhibitory dosages of chlorpyrifos and parathion (i.e., dosages that elicited similar degrees of acetylcholinesterase inhibition) lead to very different toxic responses, with parathion treated rats showing much more extensive cholinergic signs. Thus, the differential expression of toxicity noted following parathion and chlorpyrifos exposure is not due to OP-differences in in vivo anticholinesterase potency.
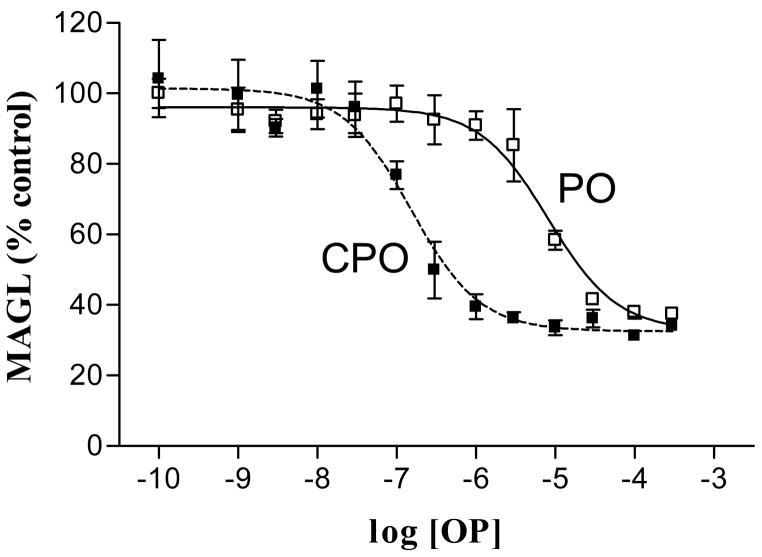
AChE is the macromolecular target for OPs in eliciting acute toxicity, but interaction with other macromolecules may have toxicological relevance [138,139]. A number of eCB signaling-related proteins are directly targeted by some OPs [129,130,140–142). Indeed, direct comparisons in a number of studies suggest that chlorpyrifos oxon is markedly more potent than paraoxon at interacting with eCB-related macromolecules [141,142]. Figure 5 shows the comparative in vitro effects of chlorpyrifos oxon and paraoxon on rat hippocampal MAGL activity. As noted in this figure, MAGL was >50-fold more sensitive to inhibition by chlorpyrifos oxon than paraoxon (IC50 = 0.15 and 8.1 μM, respectively). We thus proposed that following in vivo exposures, chlorpyrifos more effectively activates eCB signaling to decrease cholinergic and/or non-cholinergic neurotransmitter release and block the expression of cholinergic toxicity.
Figure 5.
Comparative in vitro inhibition of rat hippocampal monoacylglycerol lipase activity by chlorpyrifos oxon and paraoxon. Rat hippocampus was homogenized on ice in 0.32 M sucrose, pH 8.0 with a Polytron at 27,000 rpm. Tissues were centrifuged at 100,000 × g for 60 minutes to obtain a soluble fraction. Aliquots of the soluble fraction were preincubated for 30 minutes with vehicle or one of a range of concentrations of either paraoxon (PO) or chlorpyrifos oxon (CPO) prior to evaluating residual monoacylglycerol lipase (MAGL) activity with 2-oleoyl [3H]glycerol as the substrate (10 μM final concentration).
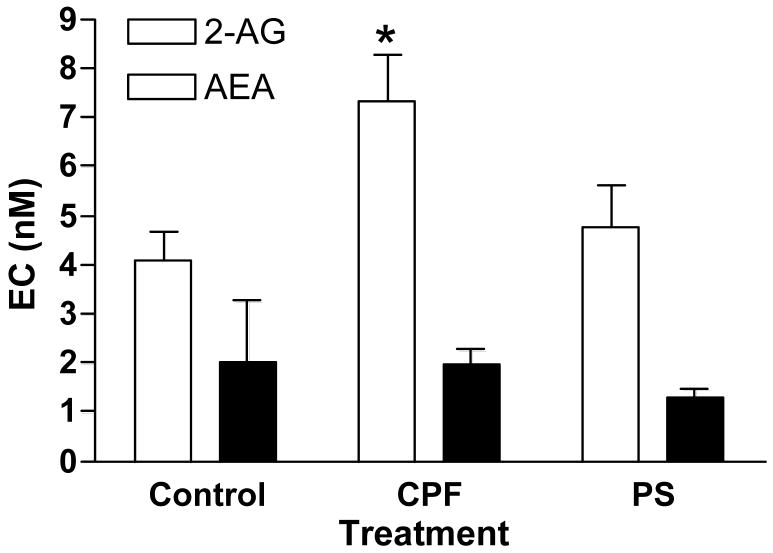
As both FAAH and MAGL (i.e., enzymes that degrade eCBs) appear more sensitive to inhibition by chlorpyrifos oxon than paraoxon, we hypothesized that chlorpyrifos exposure would lead to a greater elevation in eCB levels. We therefore evaluated the comparative effects of chlorpyrifos and parathion on extracellular eCB levels in rat hippocampus using the method developed by Parsons’ group (see above). Figure 6 shows that four days after exposure, hippocampal extracellular AEA levels were not significantly affected by either chlorpyrifos or parathion, while 2-AG levels were significantly elevated in the chlorpyrifos-treated rats only. The selective modulation of 2-AG and AEA levels by chlorpyrifos could have a number of implications. First, 2-AG may be the most relevant eCB in modulating presynaptic neurotransmitter release [143,144]. While AEA is a partial agonist at CB1 receptors, 2-AG is a full agonist [145,146]. Anandamide (but not 2-AG) can also activate the TRPV1 receptor, a nonselective cation channel that modulates intracellular calcium levels [147,148]. Moreover, TRPV1 is coupled to the regulation of 2-AG synthesis in some pathways [149]. More extensive in vivo MAGL inhibition following chlorpyrifos exposure could thus lead to relatively higher extracellular 2-AG levels, and in turn more effective activation of eCB signaling.
Figure 6.
Effects of chlorpyrifos and parathion on extracellular hippocampal endocannabinoid levels. Cannula were placed in dorsal hippocampus three days prior to treatment. Rats (n=4/treatment group) were given either vehicle (peanut oil), chlorpyrifos (280 mg/kg, sc) or parathion (27 mg/kg, sc). Dialysis probes were inserted four days later and were perfused with buffer containing 30% hydroxypropyl β-cyclodextrin to increase eCB recovery [82]. After pre-perfusion, five 10-min samples were collected, each separated by 50 min. Dialysate levels of AEA and 2-AG were determined by HPLC-MS, average values from replicates over time calculated, and mean ± SE values by treatment reported [82].
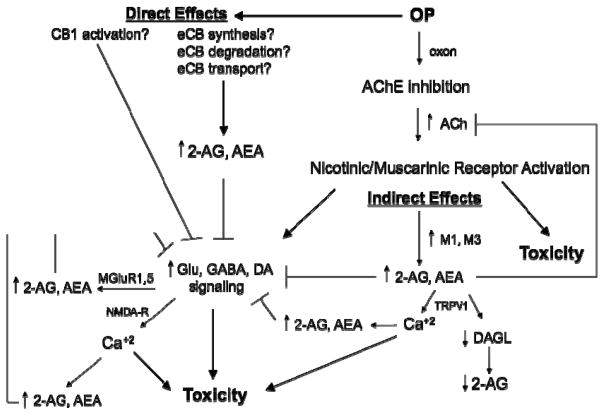
Figure 7 shows how OPs and eCBs may interact in the expression of OP toxicity. Cholinergic transmission can activate eCB signaling and eCB signaling can in turn modulate cholinergic activity and downstream signaling following acetylcholinesterase inhibition. Moreover, some OPs may directly modulate discreet components of the eCB pathway. These sites of coordinated regulation of cholinergic and eCB signaling pathways, and their selective modulation by some OPs, could be important in the expression of both acute and prolonged neurological consequences following anticholinesterase intoxication.
Figure 7.
Possible interactions between cholinergic and eCB signaling in the toxicity of organophosphorus anticholinesterases. OPs may enhance eCB signaling either indirectly through acetylcholinesterase inhibition or directly by binding to components of the eCB pathway. Extensive acetylcholinesterase inhibition will increase synaptic acetylcholine levels and prolong cholinergic receptor activation to elicit cholinergic signs of toxicity. Depolarization of postsynaptic neurons can lead to release of eCBs and modulation of neurotransmitter release. Prolonged cholinergic receptor activation can stimulate downstream non-cholinergic neurons. Glutamatergic neuron activation through NMDA receptors can increase intracellular Ca++ levels and cellular toxicity, or through metabotropic MGluR receptors can lead to eCB release that may attenuate further recruitment of downstream signaling and toxicity. Postsynaptic muscarinic M1/M3 receptor activation leads to receptor-mediated eCB release to activate CB1 receptors and inhibit ACh release at the presynaptic cholinergic terminal. Upon release, AEA can activate CB1 receptors (extracellularly) to inhibit neurotransmitter release and TRPV1 receptors (intracellularly) to increase Ca++ influx, while 2-AG acts at the CB1 receptor but not at the TRPV1 site. In the direct pathway, some OPs may bind to molecular components of eCB signaling to alter CB1 receptor activation, eCB synthesis, eCB degradation or eCB uptake and modulate cholinergic and/or non-cholinergic neurotransmitter release. Direct FAAH inhibition by some OPs can increase intracellular AEA, and in some neurons, this may lead to increased TRPV1 activation, elevated intracellular Ca++ and decreased 2-AG signaling. Reduced 2-AG signaling could in turn reduce eCB mediated inhibition of neurotransmitter release, enhancing cholinergic and non-cholinergic signaling. Direct inhibition of MAGL by some OPs may in turn selectively increase 2-AG signaling.
Overall Summary
The eCB system has neuroprotective properties. Here we briefly summarize data showing that 2-AG, a major eCB, ameliorates the effects of brain trauma, presumably via the CB1 receptor. However, work by another group shows that in cerebral ischemia/reperfusion injury the protective effect is due mainly to activation of the CB2 receptor. The protective eCB effect in a model of hepatic encephalopathy is also associated with activation of the CB2 receptor. Surprisingly, cannabidiol (which does not bind to eCB receptors) also ameliorates cognitive and motor impairment in another model of hepatic encephalopathy. Neuroprotection through activation of the eCB system is obviously a complicated phenomenon.
Endocannabinoids appear to facilitate self-administration of a number of drugs of abuse. The blockade of CB1 receptors decreases self-administration of heroin and other drugs. In some cases, self-administration of these same drugs alters brain regional eCB levels. Drug-induced increases in brain eCBs appear to selectively modulate the motivation for continued drug intake and thus the long-term neurotoxic consequences of these chemicals.
Endocannabinoid signaling may play a role in the expression of neurotoxicity elicited by a number of xenobiotics. As eCB signaling decreases neurotransmitter secretion at a variety of synapses, neurotoxicity elicted by xenobiotics that act primarily through altering neurotransmitter levels may be particularly sensitive to modulation by eCBs. Indeed, neurotoxicity following exposure to organophosphorus insecticides (which act by inhibiting acetylcholinesterase and elevating synaptic acetylcholine levels) can be modulated by drugs that affect eCB signaling. Moreover, eCB signaling may be directly affected by some OPs.
Together, these findings illustrate the widespread potential for eCB signaling to contribute to a number of neurological conditions. Understanding the interactions between neurological insults and eCB signaling may ultimately lead to therapeutic advances in a number of settings.
Acknowledgments
The authors recognize the support of the organizers of the 12th Meeting of the International Neurotoxicology Association, the United States Environmental Protection Agency, the Oklahoma State University Board of Regents, and the Oklahoma State University Center for Veterinary Health Sciences. Research was supported in part by grants R01 ES009119 (C.P.), DA-9789 (R.M.), R01 AA014619 (L.H.P.), P60 AA006420 (L.H.P.), and by the US-Israel Binational Science Foundation.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Amer Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 2.Mechoulam R, Feigenbaum JJ, Lander N, Segal M, Jarbe TUC, Hiltunen AJ, Consroe P. Enantiomeric cannabinoids: stereospecificity of psychotropic activity. Experientia. 1988;44:762–764. doi: 10.1007/BF01959156. [DOI] [PubMed] [Google Scholar]
- 3.Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- 4.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 7.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII, Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–84. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 9.Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 10.Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res. 2001;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- 11.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–92. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 13.Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 14.Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signaling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 18.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 19.Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- 20.Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci USA. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci USA. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lago E, Petrosino S, Valenti M, Morera E, Ortega-Gutierrez S, Fernandez-Ruiz J, Di Marzo V. Effect of repeated systemic administration of selective inhibitors of endocannabinoid inactivation on rat brain endocannabinoid levels. Biochem Pharmacol. 2005;70:446–452. doi: 10.1016/j.bcp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulie P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- 24.Hajos N, Kathuria S, Dinh T, Piomelli D, Freund TF. Endocannabinoid transport tightly controls 2-arachidonoyl glycerol actions in the hippocampus: effects of low temperature and the transport inhibitor AM404. Eur J Neurosci. 2004;19:2991–2996. doi: 10.1111/j.0953-816X.2004.03433.x. [DOI] [PubMed] [Google Scholar]
- 25.Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 26.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 27.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 29.Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 30.Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 31.Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. Substrate specificity and stereoselectivity of rat brain microsomal anandamide amidohydrolase. J Med Chem. 1999;42:896–902. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- 32.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lago E, Ligresti A, Ortar G, Morera E, Cabranes A, Pryce G, Bifulco M, Baker D, Fernandez-Ruiz J, Di Marzo V. In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake. Eur J Pharmacol. 2004;484:249–257. doi: 10.1016/j.ejphar.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 35.Gifford AN, Ashby CR., Jr Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exp Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- 36.Gessa GL, Mascia MS, Casu MA, Carta G. Inhibition of hippocampal acetylcholine release by cannabinoids: reversal by SR 141716A. Eur J Pharmacol. 1997;327:R1–2. doi: 10.1016/s0014-2999(97)89683-5. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- 38.Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AS, Walker JM. Effects of a cannabinoid on spontaneous and evoked neuronal activity in the substantia nigra pars reticulate. Eur J Pharmacol. 1995;279:179–185. doi: 10.1016/0014-2999(95)00151-a. [DOI] [PubMed] [Google Scholar]
- 40.Cadogan AK, Alexander SP, Boyd EA, Kendall DA. Influence of cannabinoids on electrically evoked dopamine release and cyclic AMP generation in the rat striatum. J Neurochem. 1997;69:1131–1137. doi: 10.1046/j.1471-4159.1997.69031131.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 43.Mechoulam R, Shohami E. Endocannabinoids and traumatic brain injury. Mol Neurobiol. 2007;36:68–74. doi: 10.1007/s12035-007-8008-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–60. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magen I, Avraham Y, Berry E, Mechoulam R. Endocannabinoids in liver disease and hepatic encephalopathy. Curr Pharmaceut Design. 2008;14:2362–2369. doi: 10.2174/138161208785740063. [DOI] [PubMed] [Google Scholar]
- 46.Avraham Y, Israeli E, Gabbay E, Okun A, Zolotarev O, Silberman I, Ganzburg V, Dagon Y, Magen I, Varobia L, Pappo O, Mechoulam R, Ilan Y, Berry EM. Endocannabinoids affect neurological and cognitive function in thioacetamide – induced hepatic encephalopathy. Neurobiol Disease. 2006;21:237–245. doi: 10.1016/j.nbd.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Dagon Y, Avraham Y, Ilan Y, Mechoulam R, Berry EM. Cannabinoids ameliorate cerebral disfunction following liver failure via AMP-activated protein kinase. FASEB J. 2007;21:2431–2441. doi: 10.1096/fj.06-7705com. [DOI] [PubMed] [Google Scholar]
- 48.Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairment in mice with bile duct ligation. J Hepatology. 2009;51:528–534. doi: 10.1016/j.jhep.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 50.Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 51.Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- 52.Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 53.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang SJ. Cannabinoid CB1 receptor-mediated inhibition of glutamate release from rat hippocampal synaptosomes. Eur J Pharmacol. 2003;469:47–55. doi: 10.1016/s0014-2999(03)01734-5. [DOI] [PubMed] [Google Scholar]
- 55.Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Lallemand F, de Witte P. Ethanol induces higher BEC in CB1 cannabinoid receptor knockout mice while decreasing ethanol preference. Alcohol Alcohol. 2005;40:54–62. doi: 10.1093/alcalc/agh115. [DOI] [PubMed] [Google Scholar]
- 57.Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 58.Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- 59.Forget B, Hamon M, Thiebot MH. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berl) 2005;181:722–734. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 60.Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 61.Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 62.Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 63.Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- 64.Caille S, Parsons LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–3149. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- 65.De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- 66.Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- 67.Navarro M, Carrera MR, Del Arco I, Trigo JM, Koob GF, Rodriguez de Fonseca F. Cannabinoid receptor antagonist reduces heroin self-administration only in dependent rats. Eur J Pharmacol. 2004;501:235–237. doi: 10.1016/j.ejphar.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 68.Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- 69.Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 70.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 71.Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- 72.Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alonso R, Voutsinos B, Fournier M, Labie C, Steinberg R, Souilhac J, Le Fur G, Soubrie P. Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience. 1999;91:607–620. doi: 10.1016/s0306-4522(98)00675-7. [DOI] [PubMed] [Google Scholar]
- 74.Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centonze D, Rossi S, De Chiara V, Prosperetti C, Battista N, Bernardi G, Mercuri NB, Usiello A, Maccarrone M. Chronic cocaine sensitizes striatal GABAergic synapses to the stimulation of cannabinoid CB1 receptors. Eur J Neurosci. 2007;25:1631–1640. doi: 10.1111/j.1460-9568.2007.05433.x. [DOI] [PubMed] [Google Scholar]
- 76.Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104:141–146. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 77.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 78.Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Filip M, Golda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegalinski E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- 80.Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng XQ, Gardner EL. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–45. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- 81.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 82.Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alvarez-Jaimes L, Polis I, Parsons LH. Regional influence of CB1 receptor signaling on ethanol self-administration by rats. The Open Neuropsychopharmacology. 2009 doi: 10.2174/1876523800902020077. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caille S, Parsons LH. Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. Eur J Neurosci. 2004;20:593–596. doi: 10.1111/j.1460-9568.2004.03497.x. [DOI] [PubMed] [Google Scholar]
- 85.Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06301.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 87.McKinley RA, Moorhead HH. Alcoholism. Prog Neurol Psychiatry. 1967;22:459–468. doi: 10.1016/b978-1-4831-9662-6.50030-5. [DOI] [PubMed] [Google Scholar]
- 88.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 89.Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- 90.Heyne A, May T, Goll P, Wolffgramm J. Persisting consequences of drug intake: towards a memory of addiction. J Neural Transm. 2000;107:613–638. doi: 10.1007/s007020070065. [DOI] [PubMed] [Google Scholar]
- 91.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 92.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell L/E, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur J Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baldwin HA, Wall TL, Schuckit MA, Koob GF. Differential effects of ethanol on punished responding in the P and NP rats. Alcohol Clin Exp Res. 1991;15:700–4. doi: 10.1111/j.1530-0277.1991.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 97.Barnes NM, Costall B, Kelly ME, Onaivi ES, Naylor RJ. Ketotifen and its analogues reduce aversive responding in the rodent. Pharmacol Biochem Behav. 1990;37:785–93. doi: 10.1016/0091-3057(90)90564-x. [DOI] [PubMed] [Google Scholar]
- 98.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 99.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain: An immunohistochemical study. Peptides. 2000;21:1735–42. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 102.Egertová M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol. 2000;422:159–71. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 103.Pope C, Karanth S, Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol. 2005;19:433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 104.Kronman C, Ordentlich A, Barak D, Velan B, Shafferman A. The ”back door” hypothesis for product clearance in acetylcholinesterase challenged by site-directed mutagenesis. J Biol Chem. 1994;269:27819–27822. [PubMed] [Google Scholar]
- 105.Pope CN. Central nervous system effects and neurotoxicity. In: Gupta RC, editor. Toxicology of Organophosphate and Carbamate Compounds. Elsevier; New York, NY: 2006. pp. 271–291. [Google Scholar]
- 106.Espinola EB, Oliveira MG, Carlini EA. Differences in central and peripheral responses to oxotremorine in young and aged rats. Pharmacol Biochem Behav. 1999;62:419–423. doi: 10.1016/s0091-3057(98)00192-0. [DOI] [PubMed] [Google Scholar]
- 107.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:1692–7. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miwa H, Nishi K, Fuwa T, Mizuno Y. Differential expression of c-FOS following administration of two tremorgenic agents: harmaline and oxotremorine. Neuroreport. 2000;11:2385–2390. doi: 10.1097/00001756-200008030-00010. [DOI] [PubMed] [Google Scholar]
- 109.Shih TM, Koviak TA, Capacio BR. Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev. 1991;15:349–362. doi: 10.1016/s0149-7634(05)80028-4. [DOI] [PubMed] [Google Scholar]
- 110.McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 111.Kadar T, Cohen G, Sahar R, Alkalai D, Shapira S. Long-term study of brain lesions following soman, in comparison to DFP and metrazol poisoning. Hum Exp Toxicol. 1992;11:517–523. doi: 10.1177/096032719201100613. [DOI] [PubMed] [Google Scholar]
- 112.Kadar T, Shapira S, Cohen G, Sahar R, Alkalay D, Raveh L. Sarin-induced neuropathology in rats. Hum Exp Toxicol. 1995;14:252–259. doi: 10.1177/096032719501400304. [DOI] [PubMed] [Google Scholar]
- 113.Dekundy A, Blaszczak P, Kaminski R, Turski WA. On the interactions between antimuscarinic atropine and NMDA receptor antagonists in anticholinesterase-treated mice. Arch Toxicol. 2001;74:702–708. doi: 10.1007/s002040000189. [DOI] [PubMed] [Google Scholar]
- 114.Dekundy A, Kaminski RM, Zielinska E, Turski WA. NMDA antagonists exert distinct effects in experimental organophosphate or carbamate poisoning in mice. Toxicol Appl Pharmacol. 2007;219:114–121. doi: 10.1016/j.taap.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 115.Savage EP, Keefe TJ, Mounce LM, Heaton RK, Lewis JA, Burcar PJ. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Environ Health. 1988;43:38–45. doi: 10.1080/00039896.1988.9934372. [DOI] [PubMed] [Google Scholar]
- 116.Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K. Chronic central nervous system effects of acute organophosphate pesticide intoxication, The Pesticide Health Effects Study Group. Lancet. 1991;338:223–227. doi: 10.1016/0140-6736(91)90356-t. [DOI] [PubMed] [Google Scholar]
- 117.McConnell R, Keifer M, Rosenstock L. Elevated quantitative vibrotactile threshold among workers previously poisoned with methamidophos and other organophosphate pesticides. Am J Ind Med. 1994;25:325–334. doi: 10.1002/ajim.4700250303. [DOI] [PubMed] [Google Scholar]
- 118.Wesseling C, Keifer M, Ahlbom A, McConnell R, Moon JD, Rosenstock L, Hogstedt C. Long-term neurobehavioral effects of mild poisonings with organophosphate and n-methyl carbamate pesticides among banana workers. Int J Occup Environ Health. 2002;8:27–34. doi: 10.1179/oeh.2002.8.1.27. [DOI] [PubMed] [Google Scholar]
- 119.Colosio C, Tiramani M, Maroni M. Neurobehavioral effects of pesticides: state of the art. Neurotoxicology. 2003;24:577–591. doi: 10.1016/S0161-813X(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 120.Müller-Vahl KR, Kolbe H, Dengler R. Transient severe parkinsonism after acute organophosphate poisoning. J Neurol Neurosurg Psychiatry. 1999;66:253–254. doi: 10.1136/jnnp.66.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shahar E, Andraws J. Extra-pyramidal parkinsonism complicating organophosphate insecticide poisoning. Eur J Paediatr Neurol. 2001;5:261–264. doi: 10.1053/ejpn.2001.0527. [DOI] [PubMed] [Google Scholar]
- 122.Arima H, Sobue K, So M, Morishima T, Ando H, Katsuya H. Transient and reversible parkinsonism after acute organophosphate poisoning. J Toxicol Clin Toxicol. 2003;41:67–70. doi: 10.1081/clt-120018273. [DOI] [PubMed] [Google Scholar]
- 123.Brahmi N, Gueye PN, Thabet H, Kouraichi N, Ben Salah N, Amamou M. Extrapyramidal syndrome as a delayed and reversible complication of acute dichlorvos organophosphate poisoning. Vet Hum Toxicol. 2004;46:187–189. [PubMed] [Google Scholar]
- 124.Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Sentenac-Roumanou H, Blanchet G. Involvement of glutamatergic system of amygdala in generalized seizures induced by soman: comparison with the hippocampus. C R Acad Sci III. 1991;313:421–426. [PubMed] [Google Scholar]
- 125.Lallement G, Carpentier P, Collet A, Baubichon D, Pernot-Marino I, Blanchet G. Extracellular acetylcholine changes in rat limbic structures during soman-induced seizures. NeuroToxicology. 1992;13:557–567. [PubMed] [Google Scholar]
- 126.Cassel GE, Fosbraey P. Measurement of the oxime HI-6 after peripheral administration in tandem with neurotransmitter levels in striatal dialysates: effects of soman intoxication. J Pharmacol Toxicol Methods. 1996;35:159–166. doi: 10.1016/1056-8719(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 127.Jacobsson SO, Sellström A, Persson SA, Cassel GE. Correlation between cortical EEG and striatal microdialysis in soman-intoxicated rats. Neurosci Lett. 1997;231:155–158. doi: 10.1016/s0304-3940(97)00552-1. [DOI] [PubMed] [Google Scholar]
- 128.Bourne JA, Fosbraey P, Halliday J. SCH 23390 affords protection against soman-evoked seizures in the freely moving guinea-pig: a concomitant neurochemical, electrophysiological and behavioural study. Neuropharmacology. 2001;40:279–288. doi: 10.1016/s0028-3908(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 129.Nallapaneni A, Liu J, Karanth S, Pope C. Modulation of paraoxon toxicity by the cannabinoid receptor agonist WIN 55,212-2. Toxicology. 2006;227:173–183. doi: 10.1016/j.tox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Nallapaneni A, Liu J, Karanth S, Pope C. Pharmacological enhancement of endocannabinoid signaling reduces the cholinergic toxicity of diisopropylfluorphosphate. NeuroToxicology. 2008;29:1037–1043. doi: 10.1016/j.neuro.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- 132.Pope CN, Chaudhuri J, Chakraborti TK. Organophosphate-sensitive cholinergic receptors: possible role in modulation of anticholinesterase-induced toxicity. In: Balasubramanian AS, Doctor BP, Taylor P, Quinn DM, editors. Enzymes of the Cholinesterase Family. Plenum; New York: 1995. pp. 305–312. [Google Scholar]
- 133.Liu J, Pope CN. Comparative presynaptic neurochemical changes in rat striatum following exposure to chlorpyrifos or parathion. J Toxicol Environ Health A. 1998;53:531–544. doi: 10.1080/009841098159123. [DOI] [PubMed] [Google Scholar]
- 134.Pope CN, Chakraborti TK. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology. 1992;73:35–43. doi: 10.1016/0300-483x(92)90168-e. [DOI] [PubMed] [Google Scholar]
- 135.Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol Appl Pharmacol. 1997;147:411–8. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- 136.Sultatos LG, Minor LD, Murphy SD. Metabolic activation of phosphorothioate pesticides: role of the liver. J Pharmacol Exp Ther. 1985;232:624–8. [PubMed] [Google Scholar]
- 137.Pond AL, Chambers HW, Coyne CP, Chambers JE. Purification of two rat hepatic proteins with A-esterase activity toward chlorpyrifos-oxon and paraoxon. J Pharmacol Exp Ther. 1998;286:1404–11. [PubMed] [Google Scholar]
- 138.Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity. J Toxicol Environ Health Part B. 1999;2:101–121. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 139.Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact. 2005;15:277–83. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 140.Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett. 2002;135:89–93. doi: 10.1016/s0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- 141.Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol Appl Pharmacol 2002. 2002;179:57–63. doi: 10.1006/taap.2001.9342. [DOI] [PubMed] [Google Scholar]
- 142.Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol Appl Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 143.Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sugiura T. Physiological roles of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Biofactors. 2009;35:88–97. doi: 10.1002/biof.18. [DOI] [PubMed] [Google Scholar]
- 145.Burkey TH, Quock RM, Consroe P, Ehlert FJ, Hosohata Y, Roeske WR, Yamamura HI. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur J Pharmacol. 1997;336:295–8. doi: 10.1016/s0014-2999(97)01255-7. [DOI] [PubMed] [Google Scholar]
- 146.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor, Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–12. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 147.Roberts LA, Christie MJ, Connor M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol. 2002;137:421–8. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agrò A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–9. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]