Abstract
Increased cardiac output in response to β-adrenergic receptor (β-AR) stimulation is achieved by rapid alteration of the activity of cardiac ion channels, pumps, and exchangers. Over the past decade, the discovery of macromolecular complexes that include the ion channels and pumps and the kinases that control their level of phosphorylation have led to an increased understanding of the molecular mechanisms behind the cardiac adrenergic response. This increased understanding has led to the discovery of a new long QT gene encoding an accessory protein in one of these macromolecular complexes. The following provides a brief review of the major components of the β-adrenergic pathway in the heart and discusses the direction of current and future research.
Introduction
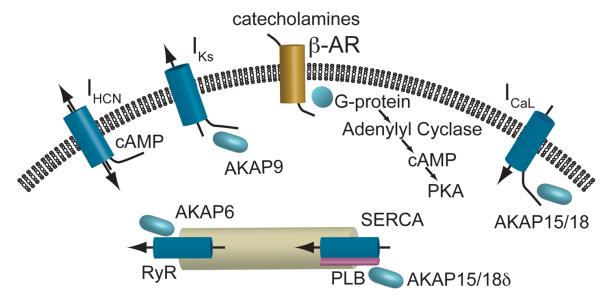
Increased cardiac output in response to β-adrenergic receptor (β-AR) stimulation is a fundamental component of the natural fight or flight response in human physiology. Stimulation of the sympathetic nervous system in response to exercise or emotional stress results in a rapid and dramatic increase in contractile force and heart rate which, in order to ensure adequate diastolic filling time between beats, is accompanied by a concomitant reduction of the ventricular action potential duration (APD) at the cellular level and the corresponding QT interval of the electrocardiogram (ECG). These adaptations are achieved by tightly coupling ion channel function to stimulation of the autonomic nervous system. In recent years, the physical link between autonomic stimulation and altered ion channel function has been elucidated by discovery of macromolecular signaling complexes that include both the ion channels themselves as well as the enzymes that control local kinase activity, shedding light on the mechanisms responsible for the rapid and robust response seen in cardiac cells. A number of ion channels and pumps are functionally altered by adrenergic stimulation; the major currents discussed here are shown schematically in Figure 1.
Figure 1.
A schematic representation of the major electrophysiological determinants of adrenergic response in cardiac myocytes. Catecholaminergic stimulation of β-receptors leads to increases in cAMP and PKA. Cyclic nucleotide binding to the HCN channels increases intrinsic pacing rate of nodal cells. PKA phosphorylation upregulates several targets in the Ca2+ signaling cascade to increase contractile force and the IKs channel to abbreviate the action potential duration to compensate for the increased heart rate.
Cardiac signaling targets
In cardiac myocytes, catecholamine binding to G-coupled β-adrenergic (β-AR) receptors initiates a signaling cascade increasing intercellular cyclic nucleotide and kinase concentrations that, in turn, alter the function of sarcolemmal and intracellular ion channels. The cyclic nucleotides themselves bind to some channels altering their function while PKA phosphorylation of other ion channels or their accessory proteins, that is modulated by a diverse set of A-kinase anchoring proteins (AKAP), imparts altered function to the majority of cardiac electrophysiological targets1.
First, the dramatic increase in heart rate is achieved, in part, by direct binding of cyclic nucleotides to hyperpolarization-activated cyclic nucleotide gated (HCN) channels that carry the ‘funny’ current that contributes to diastolic depolarization in nodal tissue2. Cyclic nucleotide binding increases IHCN during diastole as a result of a positive shift of the activation curve that more rapidly depolarizes the membrane leading to a decrease in the time necessary to reach threshold and initiate an action potential. This response is different than the rest of the major ion channels in the heart as it is mediated directly by binding of cyclic nucleotide, independent of serine and threonine phosphorylation.
Another major pathway affected by β-AR signaling is the control of intercellular Ca2+ and subsequently contractile force. This is achieved by upregulation of a number of components in the Ca2+ handling pathway of cardiac myocytes. First, L-type Ca2+ channels are phosphorylated by protein kinase A (PKA) leading to a shift in the voltage dependence of channel activation and an increase in peak current bringing more Ca2+ into the cell during each beat3. This phosphorylation is mediated by an A-kinase anchoring protein (AKAP), AKAP15/18, interacting with the intercellular domain of the channel bringing PKA to the site. Similarly, an increase in Ca2+ release from the sarcoplasmic reticulum (SR) is achieved via phosphorylation of the Ryanodine receptor complex further increasing intercellular Ca2+. Again an AKAP, AKAP6 (mAKAP), interacts with the Ryanodine receptor and recruits PKA to the site, which then leads to an increased Ca2+ release. Ca2+ release and its control by PKA is also implicated in sinoatrial node control of pacemaking2. With the vast increase in systolic Ca2+ influx comes a necessity to more rapidly remove Ca2+ during diastole so the muscle can relax before the next contraction. This is achieved by increased SR Ca2+ ATPase (SERCA) activity in the presence of β-adrenergic stimulation. At the molecular level, this is the result of alleviation of the normal inhibition of the ATPase by phospholamban (PLB). When PLB is phosphorylated its ability to decrease pump activity is removed.
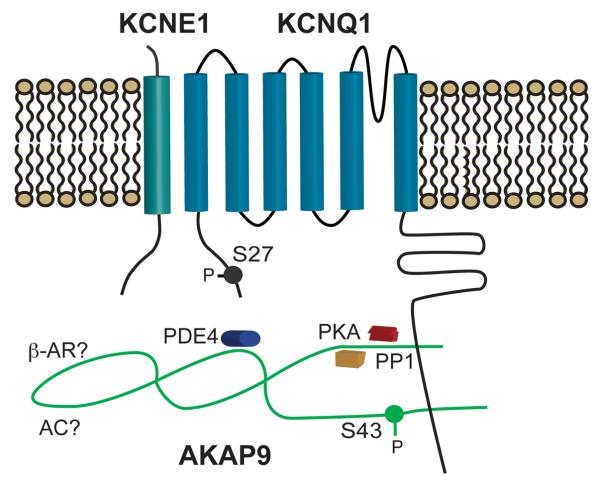
In order to allow for proper diastolic filling time at faster rates and to counter the increased inward current through Ca2+ channels, the slow inward rectifying potassium current IKs is also upregulated by β-AR signaling. The IKS channel has a strong adrenergic response and represents one of the best examples of a well-characterized macromolecular complex governing phosphorylation and ultimately the functional response to adrenergic stimulation. The response of the IKS channel requires the co-assembly of both α(KCNQ1) and β(KCNE1) subunits, as well as the binding of AKAP9 (Yotiao) to a leucine zipper motif in the pore forming subunit's carboxy terminal (C-T) domain (Figure 2)4. Mutations in any of these three proteins can lead to Long QT syndrome (variants 1 for KCNQ1, 5 for KCNE1, and 11 for AKAP9) and diminished adrenergic response, underlying these patients' susceptibility to arrhythmia during exercise. The participation of AKAP9 in the IKS complex is unique in that it has been shown to have both a passive and active role in the regulation of the channel. In expression system studies, the presence of AKAP9 is required to see the characteristic functional response observed in vivo independent of phosphorylation of the pore forming α-subunit. Not only does AKAP9 need to be present, but phosphorylation of a key residue (S43) in its amino terminus (N-T) is critical to the full functional response of the channel to cAMP. Direct binding of PKA, PP1, PP2a, and PDE4 allow this AKAP to tightly control both its, as well as its binding partners', phosphorylation state. Our understanding of the complexity of the IKS multi-protein complex continues to grow as does the understanding of its roles in the physiological response of the heart to adrenergic stimulation.
Figure 2.
A schematic diagram of the IKs macromolecular complex. IKs channels are comprised of α-(KCNQ1) and β-(KCNE1) subunits with a PKA phosphorylation on the N-terminus of KCNQ1 at position 27. The AKAP Yotiao (AKAP9) has a functionally important phosphorylation site at position 43 and interacts with the c-terminus of KCNQ1 to recruit several key enzymes, including PKA, PP1, and PDE4, to the channel complex.
Discussion and open questions
Many questions remain to be answered and are the focus of current and future investigations. For IKs, despite the progress in identifying the physiologically-critical components of the signaling pathway, fundamental questions remain to be answered regarding the basic physiological response: a rapid and reversible phosphorylation-dependent increase of cardiac repolarization reserve. For example, vital to the myocyte's increase in repolarization reserve is an increase in IKs current due to changes in channel voltage-dependent gating and in the maximal current when activated. What still needs to be addressed include the following two questions. (1) From a structure function standpoint, how does phosphorylation of a single residue in the KCNQ1 N-T domain alter the voltage dependence and kinetics of the IKs channel? (2) Is the increase in maximal IKs due, at least in part, to an increase in single channel conductance and/or to phosphorylation-dependent changes in membrane trafficking, and if so, through what mechanism(s)? In addition, there are still things to be learned about the components of the macromolecular complexes governing local cAMP signaling. With AKAP9, although functionally studied in brain and not yet heart, it has recently been shown that it also binds specific adenylyl cyclase subtypes, and with the recent discovery that it also binds a phosphodiesterase, it becomes clear that the local signaling environment of the IKs channel not only coordinates channel phosphorylation but also local cAMP concentrations. This points towards control of local cAMP as a novel pathway to systematically regulate the IKs channel. Additionally, at the molecular level, distinctions still clearly need to be made between molecular cAMP-dependent regulation of the IKs channel and the concomitant cAMP-dependent regulation of L-type calcium channels, one of the first critical targets to be identified in the response to adrenergic stimulation in the heart. For L-type Ca2+ channels, while long understood to robustly respond to cAMP, there remains a difficulty to reconstitute the cAMP-dependent regulation in heterologous expression systems. Lastly, the diversity of AKAPs used to influence the phosphorylation state of various cardiac targets leads to future examination about potential heterogeneity in local spatial and temporal signaling about each individual target.
From a clinical perspective, with the identification of multiple protein interactions coordinated by each individual AKAP, a major goal of research remains to identify the specific motifs on the AKAPs that coordinate these interactions and then to use these motifs in targeted genetic sequencing of patients with heritable arrhythmia syndromes who to date are phenotype positive but genotype negative. This technique led to the initial evidence of inherited Long QT syndrome as a result of mutation of AKAP9 only screening a small section of the large anchoring protein5. With the array of signaling molecules coordinated by AKAP9, for example, mutations in specific motifs might lead to channel upregulation (mutation of PDE4 site) or down-regulation (mutation of AC site) of IKs and thus contribute to exercise associated atrial arrhythmias as well as LQTS and possibly other syndromes. Mapping out these specific motifs may also allow for novel therapeutic targets that are specific to individual ion channel complexes and their phosphorylation state. A similar approach may be taken with the other AKAPs that interact with the critical components of adrenergic signaling, perhaps underlying other forms of heritable rhythm disorders associated with their respective ion channel or pump, including Long QT, familial atrial fibrillation, and catecholaminergic polymorphic ventricular tachycardia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61(4):394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47(2):157–170. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray PC, Scott JD, Catterall WA. Regulation of ion channels by cAMP-dependent protein kinase and A- kinase anchoring proteins. Curr.Opin.Neurobiol. 1998;8(3):330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 4.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295(5554):496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]