Abstract
Objective
The aim of the study was 1) to evaluate contrast enhancement patterns of subchondral cysts on magnetic resonance imaging and 2) to discuss possible radiological explanations of cyst enhancement based on existing theories of subchondral cyst formation in osteoarthritis.
Materials and Methods
The Multicenter Osteoarthritis (MOST) Study is a NIH-funded longitudinal observational study for individuals who have or are at high risk for knee osteoarthritis. All subjects with available non-enhanced and contrast enhanced MRI were included. The tibiofemoral and patellofemoral joints were divided in 14 subregions. The presence and size of subchondral cysts and bone marrow edema-like lesions (BMLs) were scored semiquantitatively in each subregion on non-contrast enhanced MRI from 0 to 3. Enhancement of subchondral cysts was evaluated on contrast enhanced MRI as grade 0 (absent), grade 1 (partial enhancement), or grade 2 (full enhancement). The adjacent articular cartilage was scored in each subregion on non-enhanced MRI as grade 0 (intact), grade 1 (partial thickness loss), or grade 2 (full thickness loss).
Results
Four hundred knees were included (1 knee per person, 5600 subregions). Subchondral cysts were detected in 260 subregions (4.6%). After intravenous contrast administration, 245 cysts (94.2%) showed full enhancement, 12 (4.6%) showed partial enhancement and 3 (1.2%) showed no enhancement. Enhancing BMLs were found in 237 (91.2%) subregions containing cysts, which were located adjacent or in the middle of BMLs. In 121 subregions (46.5%) having cysts, no adjacent full thickness cartilage loss was detected.
Conclusion
Most subchondral cysts demonstrated full or partial contrast enhancement, and were located adjacent or in the midst of enhancing BMLs. As pure cystic lesions are not expected to enhance on MRI, the term “subchondral cyst-like bone marrow lesion” might be appropriate to describe these lesions.
Keywords: MRI, knee, osteoarthritis, cyst
INTRODUCTION
Subchondral cysts are a common finding in osteoarthritic knees. There are two generally accepted theories on the etiology of subchondral cysts. The synovial fluid intrusion theory suggests that elevated intra-articular pressure forces joint fluid into the subchondral bone via fissured or ulcerated cartilage [1, 2], creating a cyst. According to this theory, cysts should be seen mostly deep within ulcerated or eroded cartilage where channels have been seen histologically [3]. The bony contusion theory postulates that subchondral cysts are a consequence of traumatic bone necrosis following impact of two opposing articular surfaces [4, 5]. Accordingly, cysts should be seen adjacent to or in the middle of bone marrow edema-like lesions which have histological features of bone trauma including areas of necrosis. Also, according to this theory, the communication with the synovial cavity in some cases would be a secondary phenomenon. A variety of terms such as “bone cysts”, “subarticular pseudocysts”, “geodes” and “synovial cysts” have been suggested for these lesions.
The majority of studies on the pathogenesis of subchondral cysts were performed before magnetic resonance imaging (MRI) was widely available for assessment of musculoskeletal disease [1, 2, 4–6]. MRI allows the detection of small lesions, which is crucial for understanding the pathogenesis of subchondral cysts, as large lesions are usually found in end-stage disease only. MRI is more sensitive to small subchondral cysts than x-ray, demonstrating well-defined rounded areas of fluid-like signal intensity on non-enhanced imaging [7, 8]. On contrast-enhanced MRI, it is widely accepted that the fluid-equivalent part of most cystic lesions demonstrates no enhancement after intravenous administration of paramagnetic contrast material.
The appearance of enhanced subchondral cysts has not been described well. For that matter, the percentage of subchondral cysts that exhibit contrast enhancement is unknown, as is the mechanism of enhancement. The purpose of this study was to determine the proportion of enhancing subchondral cysts and contrast-enhancement patterns, and to discuss possible radiological explanations for cyst enhancement, in light of the two established theories of subchondral cyst formation.
MATERIALS AND METHODS
Subjects
The MOST study includes 3,026 adults aged 50 to 79 years recruited at two sites, Birmingham, AL and Iowa City, IA through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. Subjects had knee osteoarthritis or were at high risk of knee osteoarthritis. Subjects considered at high risk for knee OA included those who were overweight or obese, those with knee pain, aching or stiffness on most of the last 30 days, a history of knee injury that made it difficult to walk for at least one week, or previous knee surgery. Subjects were excluded from MOST if they screened positive for rheumatoid arthritis, had ankylosing spondylitis, psoriatic arthritis, Reiter’s syndrome, had renal insufficiency that required hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without assistance, or were planning to move out of the area in the next three years. Detailed inclusion and exclusion criteria for the MOST study have been described previously [9]. We used a subset of MOST subjects who volunteered for a substudy to evaluate the relation of synovitis to knee pain and who received a contrast enhanced knee MRI. These subjects, as part of the parent study, also had non-enhanced MRI of the same knee generally acquired the same day as the enhanced MRI or within 30 days. Subjects with rheumatoid arthritis or any other form of inflammatory arthritis were excluded from the MOST study. Institutional Review Board approval was obtained at the University of California, University of Iowa and University of Alabama and Boston University Medical Center prior to initiating recruitment according to the research protocols. The protocol was in compliance with the Declaration of Helsinki and all subjects provided written informed consent prior to participation.
MR Imaging
The non-enhanced MRI was performed with a dedicated 1.0 T extremity system (OrthOne™, ONI Medical Systems, Wilmington, MA) with a 160 mm diameter circumferential send-receive extremity coil. The MRI protocol included axial and sagittal proton density-weighted fat-suppressed fast spin-echo sequences (PDFS) (TR=4800 ms, TE=35 ms, 3.0 mm slice thickness, 0.0 mm interslice gap, 32 slices, 140 mm × 140 mm field of view (FOV), matrix=288 × 192, number of excitations=2, echo train length=8). Subjects received also a 1.5 T MRI (General Electric Health Care, Milwaukee, WI), using a standard knee coil, after intravenous gadolinium injection (Magnevist - gadopentetate dimeglumine or Omniscan - gadodiamide at a dose of 0.2 ml (0.1 mmol)/kg body weight) within 0–30 days after the non-enhanced study. Axial and sagittal T1-weighted fat-suppressed sequences were acquired right away after gadolinium injection (TR=600ms, TE=13 ms, 3.0 slice thickness, 0.3 mm interslice gap, 28 slices, 160 mm × 160 mm FOV, matrix=256 × 256, number of excitations=3, echo train length=1).
MR Assessment
Images were interpreted in consensus by two musculoskeletal radiologists (MDC, MDM). Following the WORMS scoring system [10], the tibiofemoral joint was subdivided into 10 subregions and the patellofemoral joint was subdivided into 4 subregions. The subspinous subregion of the tibia, originally described in WORMS, was not included in this study as it is not covered by hyaline articular cartilage. Subchondral cysts were defined as well-delineated rounded areas of high-signal intensity on PDFS images. Their presence and size were scored semiquantitatively on PDFS images according to WORMS and based on the extent of subregional involvement (0=none; 1=<25% of the subregion; 2=25–50% of the subregion; 3=>50% of the subregion). Enhancement was scored on T1-weighted fat-suppressed contrast-enhanced images as grade 0=no enhancement of the cyst, grade 1=partial enhancement of the cyst, or grade 2=full enhancement of the cyst.
To investigate the pathogenesis of subchondral cyst formation, the adjacent cartilage morphology and the presence of bone marrow edema-like lesions (BMLs) were assessed in subregions where subchondral cysts were detected. BMLs were defined as ill-defined areas of increased signal intensity on PDFS and T2-weighted MRI sequences [8, 11, 12]. Adjacent BMLs were semiquantitatively scored on PDFS images according to WORMS, based on the extent of subregional involvement (0=none; 1=<25% of the subregion; 2=25–50% of the subregion; 3=>50% of the subregion). Adjacent cartilage was scored on the PDFS images as grade 0=intact cartilage, grade 1=partial thickness loss, or grade 2=full thickness loss.
RESULTS
Four hundred knees in 400 subjects (one knee per subject; 5600 subregions) were included (women: 46.1%, mean age 58.8 years ± 7.1, mean body mass index 29.5 kg/m2 ± 4.9). In 380 cases (95%), subjects received both non-enhanced and contrast enhanced MRI on the same day.
Subchondral cysts were detected in 260 subregions (4.6%) on PDFS images. Concerning subchondral cyst size, 220 subregions (84.6%) exhibited grade 1 cysts, 36 subregions (13.9%) grade 2 cysts and 4 (1.5%) subregions showed grade 3 cysts. After intravenous contrast administration (Figure 1–Figure 3), 245 cysts (94.2%) showed full contrast enhancement (grade 2), 12 (4.6%) showed partial contrast enhancement (grade 1) and 3 (1.2%) showed no contrast enhancement (grade 0).
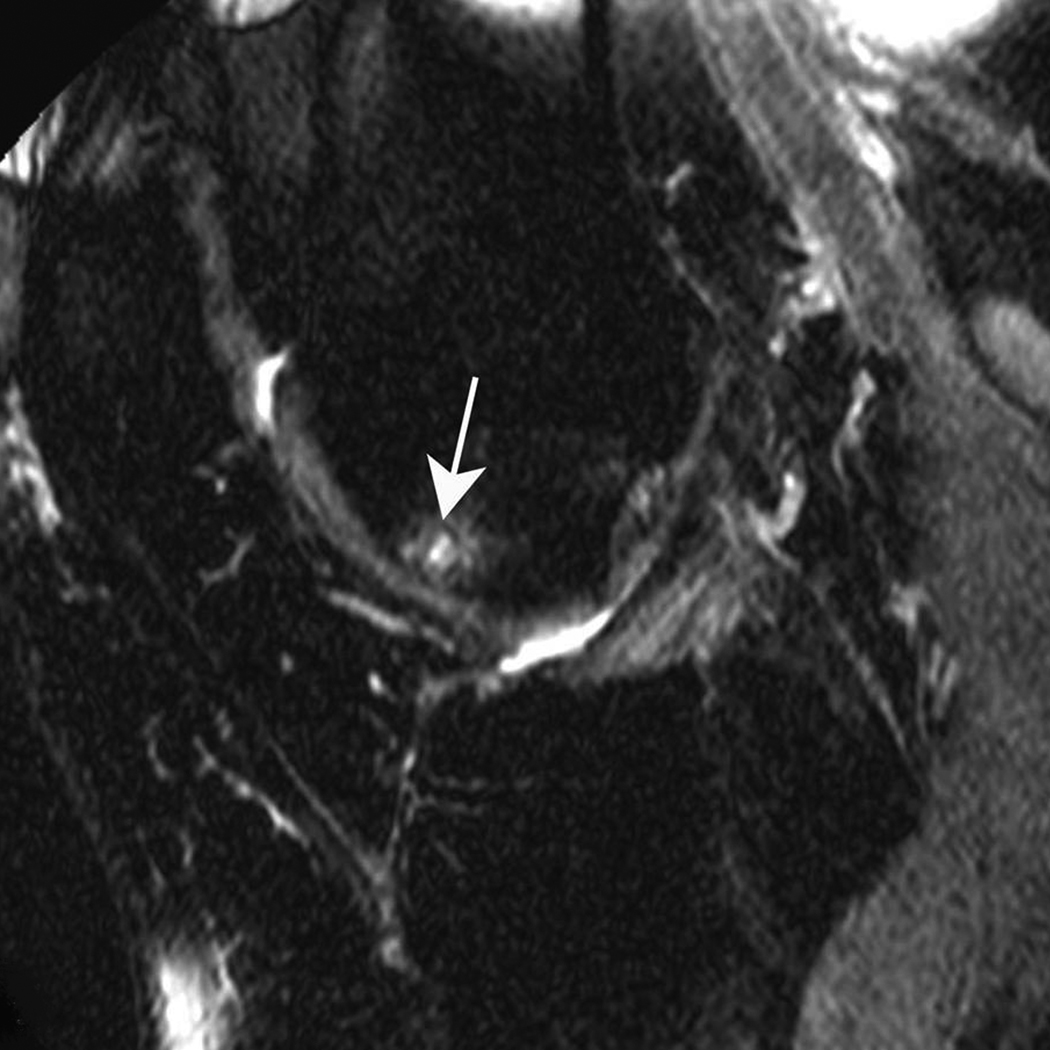
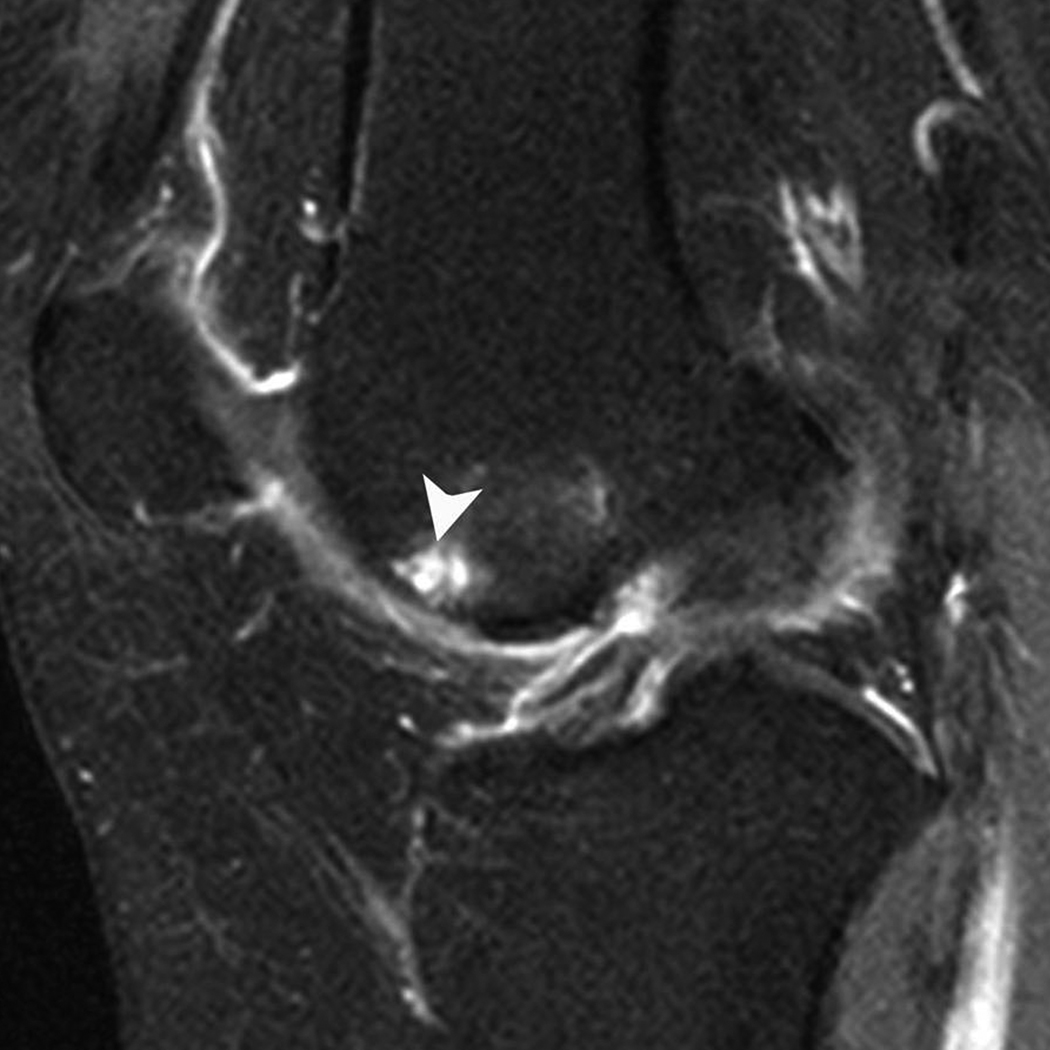
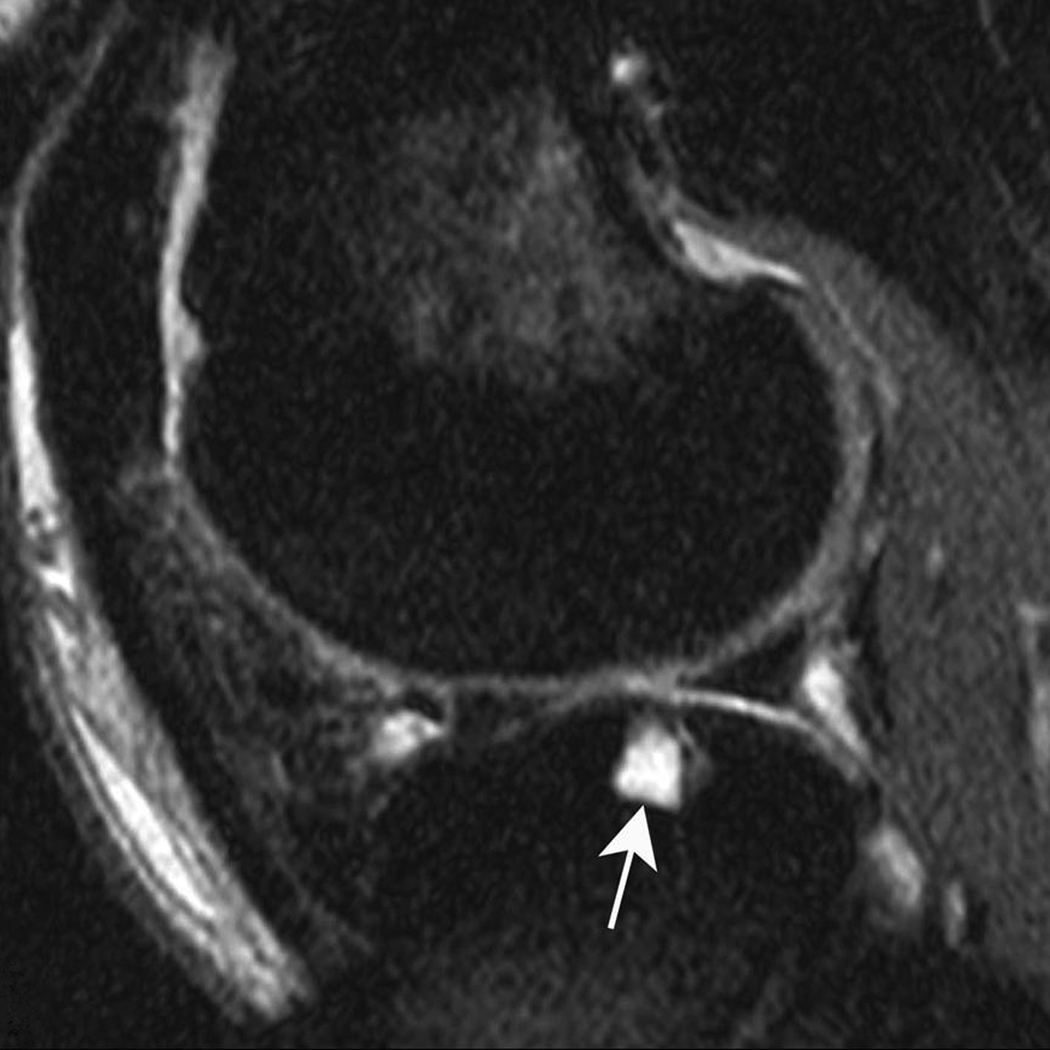
Figure 1. Subchondral cyst in the anterior subregion of the medial femur.
A) Sagittal PDFS image: small (grade 1) well-defined rounded area of high signal intensity representing a subchondral cyst (arrow) is shown. Note surrounding ill-defined area of high signal intensity, representing a BML. Only partial thickness loss of the adjacent cartilage is observed. B) Sagittal T1-weighted fat-suppressed contrast enhanced image acquired the same day shows full enhancement of cyst (arrowhead) and surrounding BML.
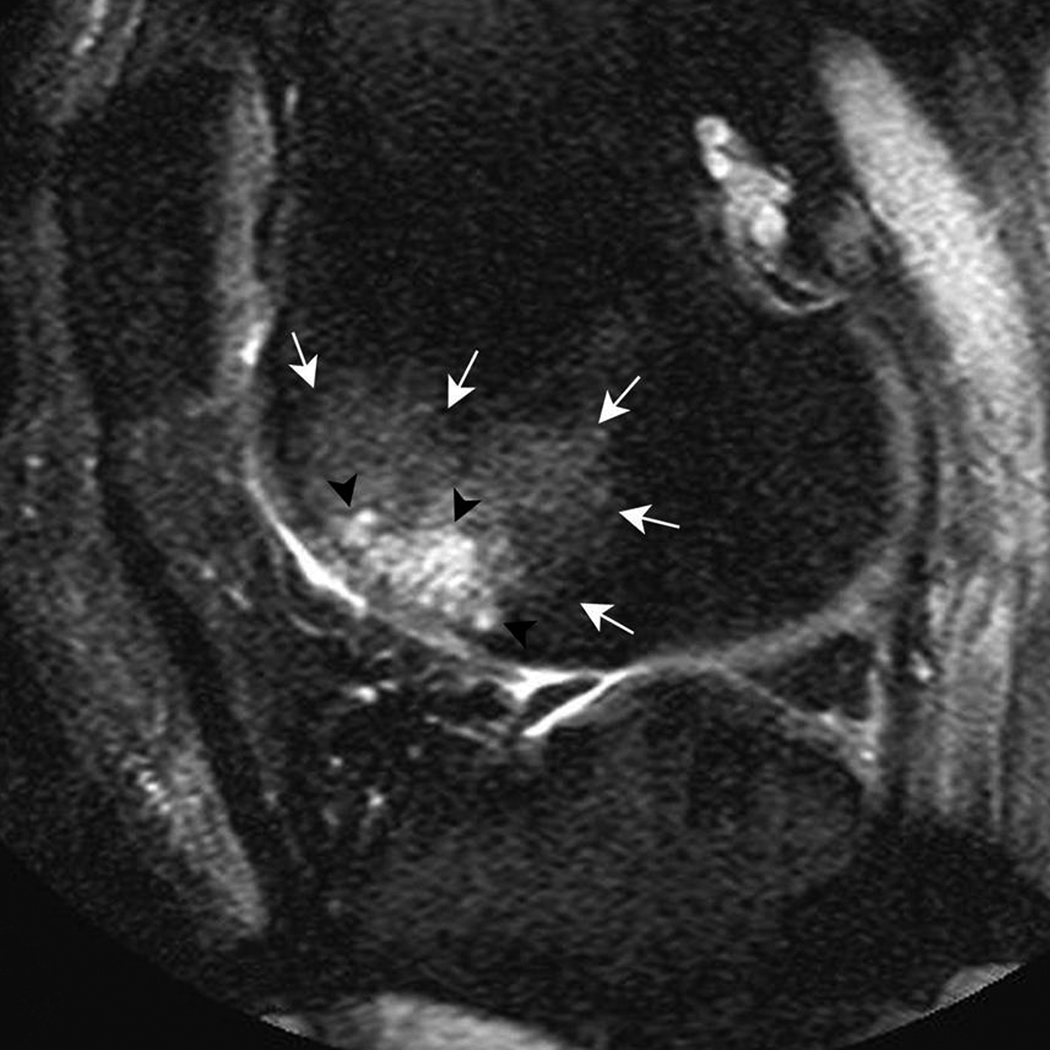
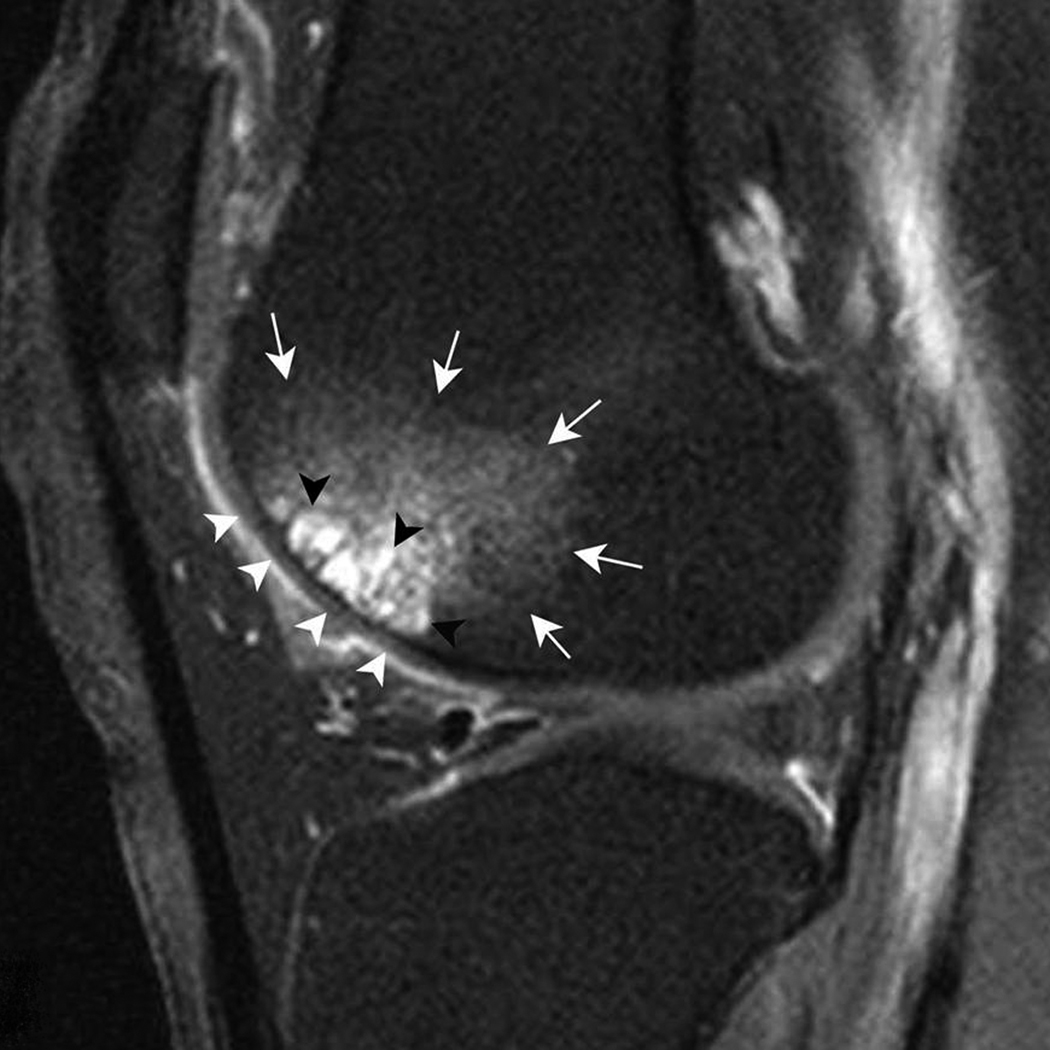
Figure 3. Multiple subchondral cysts in the anterior subregion of the lateral femur.
A) Sagittal PDFS image. A large area (grade 3) of multiple cystic lesions (black arrowheads) surrounded by a large BML (white arrows) is shown. B) Sagittal T1-weighted fat-suppressed contrast enhanced image acquired the same day depicts full enhancement of cysts (black arrowheads). Note enhancement of surrounding BML (white arrows) and intact adjacent cartilage (white arrowheads).
BMLs were detected in 91.2% of the subregions containing cysts (n=237). These cysts were adjacent to or in the middle of BMLs. Only 23 (8.8%) of the subregions with subchondral cysts did not exhibit concomitant BMLs. The distribution of subchondral cysts and BMLs is summarized in table 1. All BMLs detected on the PDFS images showed enhancement on T1-weighted fat-suppressed images after administration of contrast material.
Table 1.
Distribution of detected subchondral cysts in subregions with and without concomitant BMLs.
| Subchondral cysts (n=260) |
BMLs status in subregions presenting subchondral cysts. | |||
|---|---|---|---|---|
| Absent (Grade 0) | Grade 1 | Grade 2 | Grade 3 | |
| Grade 1 (n=220; 84.6%) | 20 (7.7%) | 65 (25%) | 74 (28.5%) | 61 (23.5%) |
| Grades 2 and 3 (n=40; 15.4%) | 3 (1.1%) | 8 (3.1%) | 5 (1.9%) | 24 (9.2%) |
In 11 subregions (4.2%) where subchondral cysts were detected, adjacent cartilage was intact (grade 0). In 110 subregions (42.3%), adjacent cartilage showed partial thickness defects (grade 1). In 139 subregions (53.5%), adjacent cartilage demonstrated full thickness defects (grade 2). The distribution of subchondral cysts in subregions with different grades of adjacent cartilage status is summarized in table 2.
Table 2.
Distribution of detected subchondral cysts in subregions with normal adjacent cartilage, partial thickness loss, and full thickness loss of adjacent cartilage.
| Subchondral cysts (n=260) |
Adjacent cartilage status in subregions presenting subchondral cysts. |
||
|---|---|---|---|
| Normal (Grade 0) |
Partial thickness loss (Grade 1) |
Full thickness loss (Grade 2) |
|
| Grade 1 (n=220; 84.6%) | 11 (4.2%) | 97 (37.3%) | 112 (43.1%) |
| Grades 2 and 3 (n=40; 15.4%) | 0 (0%) | 13 (5.0%) | 27 (10.4%) |
DISCUSSION
To our knowledge, this is the largest study evaluating contrast enhancement of subchondral cysts in patients with or at risk of knee osteoarthritis. Contrary to expectations, most subchondral cysts showed contrast enhancement after intravenous gadolinium administration. Previous histological studies of subchondral cysts have shown that these cavities may or may not communicate with the joint cavity, that they contain necrotic bone fragments, small fragments of articular cartilage, and foci of metaplastic cartilage, that they are surrounded by a layer of fibrous connective tissue, and that no epithelial components are found in the lining of these cavities [1, 4, 6, 7]. The presence of a surrounding fibrous connective tissue may explain the partial enhancement pattern of some lesions, which was always observed at the periphery of the cysts.
However, none of these components found at histology explain why the majority of subchondral cysts showed full enhancement after intravenous gadolinium administration. We hypothesize two theories to explain why these cavities are filling with gadolinium: 1) synovial fluid enters the cavities through chondral fissures or synovial tissue within the cysts and enhances (synovial fluid intrusion theory); 2) gadolinium diffuses into the cysts from surrounding subchondral areas of increased remodeling, i.e. adjacent BMLs (bony contusion theory). Some combination of these theories should also be considered.
According to the synovial fluid intrusion theory [1, 2], a subchondral cyst should develop only in subregions with full thickness cartilage loss where breaches of the articular surface allow synovial fluid and/or synovial tissue to intrude into the subchondral bone. Our results showed that in about half of the cases subchondral cysts appeared in subregions where adjacent cartilage did not show full thickness loss. Thus, the enhancing synovial intrusion pattern seems unlikely for those cysts. Even when subchondral cysts are detected in subregions near cartilage with areas of full thickness loss, the enhancing synovial intrusion pattern seems to be improbable for two reasons: 1) synovial tissue is rarely identified within the cavities in histological studies [1, 4, 6, 7] and 2) no synovial fluid enhancement is expected right away after intravenous gadolinium administration [13, 14].
According to the bony contusion theory [4, 5], a subchondral cyst forms independently of the adjacent cartilage status. Subchondral bone marrow edema-like lesions (BMLs) are a very common MRI finding in knee osteoarthritis, and represent non-characteristic histological abnormalities including bone marrow necrosis, bone marrow fibrosis, and trabecular abnormalities [8]. Rhaney and Lamb [4] have demonstrated the histologic similarity of subchondral cysts and the surrounding subchondral bone marrow, suggesting that subchondral cyst formation is secondary to subchondral bone marrow necrosis due to increased loading. We found that a large majority of subregions where subchondral cysts were detected exhibited concomitant BMLs, and all the BMLs enhanced in our study. Thus, we hypothesize that BMLs could represent the source of subchondral cyst enhancement observed in our study population.
Various terms for these lesions have been proposed in the literature. The term “subchondral cyst” is not appropriate, as these lesions are not lined by epithelial cells [6, 7]. Per MRI definition, the fluid-equivalent part of bone cysts should not demonstrate enhancement after intravenous administration of contrast material. Thus, our findings suggest that the term “subchondral cyst” is inappropriate to describe such lesions. A better term would be subchondral “cyst-like bone marrow lesion”.
Limitations of our study need mentioning. First and probably most important is that no histological correlation was performed. Even though MRI spin-echo sequences are widely accepted as an accurate technique to evaluate the articular cartilage [15, 16], we still do not have histological proof of cartilage status adjacent to subregions exhibiting subchondral cysts. Secondly, no T1-weighted images before intravenous administration of gadolinium were acquired. However, it is widely accepted that subchondral well-demarcated areas of high signal intensity on T2- and proton density-weighted images represent subchondral cysts [7, 8]. Although we did not acquire T1-weighted images prior to intravenous administration of contrast material, we were able to evaluate enhancement of these lesions. Thirdly, non-enhanced and contrast-enhanced MRI sequences were read simultaneously, and this may have caused bias in assessing detectability and enhancement of subchondral cysts, as well as the analysis of adjacent cartilage status.
In conclusion, the large majority of subchondral cysts unexpectedly showed enhancement after intravenous gadolinium administration. In about half of the cases, adjacent cartilage status did not show full thickness loss. Thus, enhancing synovial fluid intrusion seems unlikely for those cysts. Most cysts were observed in subregions with concomitant BMLs. Diffusion of gadolinium from the subchondral bone with BMLs into the cysts is likely to explain our findings. Also, we found that the enhancing subchondral cysts did not present with the typical MRI-defined patterns of cysts, and suggest that subchondral “cyst-like bone marrow lesion” might be a more appropriate term for these lesions.
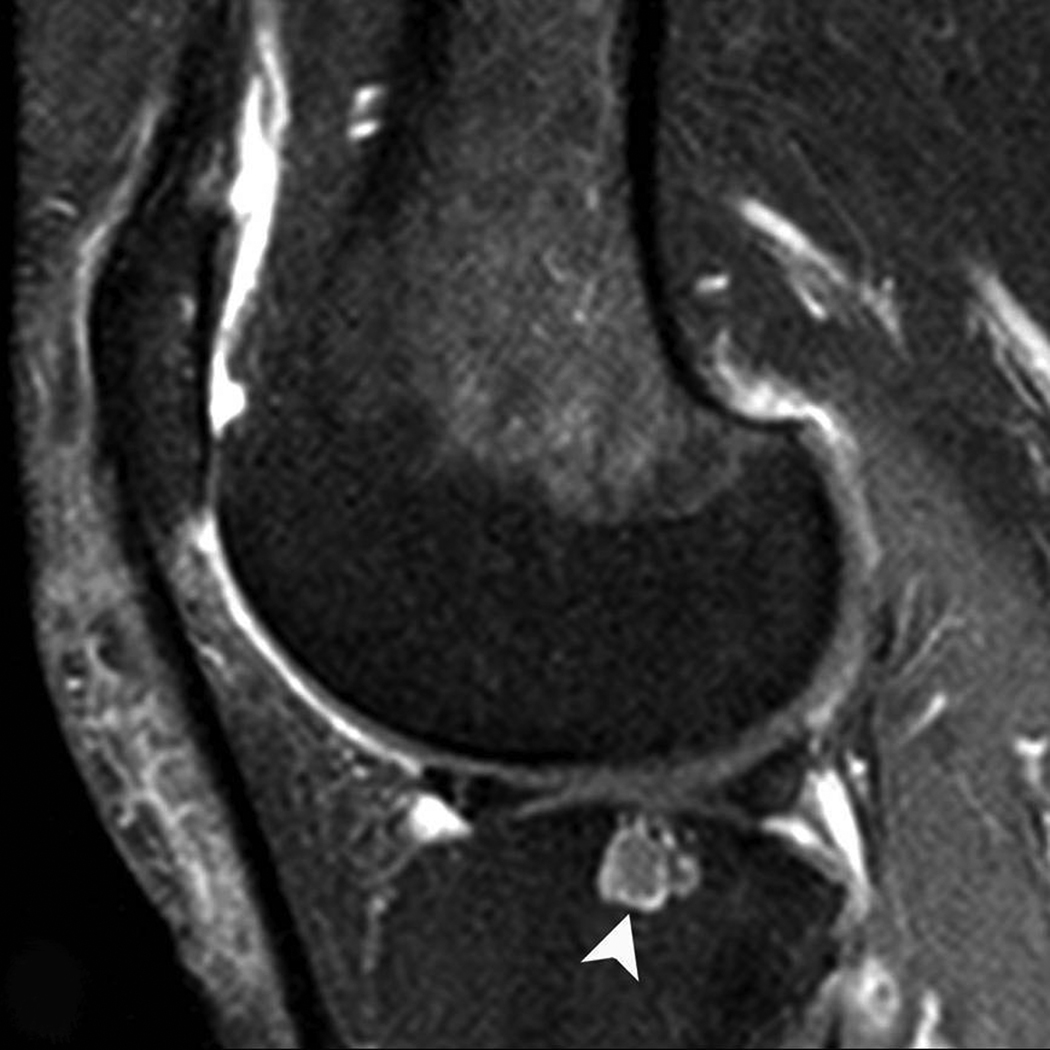
Figure 2. Subchondral cyst in the central subregion of the lateral tibia.
A) Sagittal PDFS image: medium-sized (grade 2) subchondral cyst (arrow) with no surrounding BML. Only partial thickness loss of the adjacent cartilage is observed. B) Sagittal T1-weighted fat-suppressed contrast enhanced image acquired the same day shows partial rim-enhancement of cyst (arrowhead).
ACKNOWLEDGMENTS
We wish to acknowledge the support of the staff of the MOST study. We would like to express our thanks to Drs. George El-Khoury, Cora E. Lewis, Yanyan Zhu, and M. Kassim Javaid for their help in acquiring the data and improving the manuscript. We further would like to thank the participants of the MOST study.
The MOST Study is supported by NIH grants from the National Institute on Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820).
The ancillary MOST study on contrast-enhanced MRI is supported by NIH grant R01 AR053161 (David Felson, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers
Conflict of interest statement: Ali Guermazi is president of Boston Imaging Core Lab LLC (BICL), a company providing radiological image assessment services. He is a shareholder of Synarc, Inc. Michel D. Crema, Frank W. Roemer, and Monica D. Marra are shareholders of BICL.
All other authors disclose no financial or personal relationship with other people or organizations that could inappropriately influence their work.
REFERENCES
- 1.Landells JW. The bone cysts of osteoarthritis. J Bone Joint Surg Br. 1953;35:B:643–B:649. doi: 10.1302/0301-620X.35B4.643. [DOI] [PubMed] [Google Scholar]
- 2.Freund E. The pathological significance of intra-articular pressure. Edinburgh Med J. 1940;47:192–203. [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang J, Bae WC, Shieu W, Lewis CW, Bugbee WD, Sah RL. Increased hydraulic conductance of human articular cartilage and subchondral bone plate with progression of osteoarthritis. Arthritis Rheum. 2008;58:3831–3842. doi: 10.1002/art.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhaney K, Lamb DW. The cysts of osteoarthritis of the hip; a radiological and pathological study. J Bone Joint Surg Br. 1955;37:B:663–B:675. doi: 10.1302/0301-620X.37B4.663. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson AB., Jr The Pathological Changes in Degenerative Arthritis of the Hip and Treatment by Rotational Osteotomy. J Bone Joint Surg Am. 1964;46:1337–1352. [PubMed] [Google Scholar]
- 6.Resnick D, Niwayama G, Coutts RD. Subchondral cysts (geodes) in arthritic disorders: pathologic and radiographic appearance of the hip joint. AJR Am J Roentgenol. 1977;128:799–806. doi: 10.2214/ajr.128.5.799. [DOI] [PubMed] [Google Scholar]
- 7.Pouders C, De Maeseneer M, Van Roy P, Gielen J, Goossens A, Shahabpour M. Prevalence and MRI-anatomic correlation of bone cysts in osteoarthritic knees. AJR Am J Roentgenol. 2008;190:17–21. doi: 10.2214/ajr.07.2098. [DOI] [PubMed] [Google Scholar]
- 8.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 9.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–797. doi: 10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–1535. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 12.Kornaat PR, Kloppenburg M, Sharma R, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol. 2007;17:3073–3078. doi: 10.1007/s00330-007-0711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahlensieck M, Peterfy CG, Wischer T, et al. Indirect MR arthrography: optimization and clinical applications. Radiology. 1996;200:249–254. doi: 10.1148/radiology.200.1.8657921. [DOI] [PubMed] [Google Scholar]
- 14.Winalski CS, Aliabadi P, Wright RJ, Shortkroff S, Sledge CB, Weissman BN. Enhancement of joint fluid with intravenously administered gadopentetate dimeglumine: technique, rationale, and implications. Radiology. 1993;187:179–185. doi: 10.1148/radiology.187.1.8451409. [DOI] [PubMed] [Google Scholar]
- 15.Bredella MA, Tirman PF, Peterfy CG, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172:1073–1080. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 16.Sonin AH, Pensy RA, Mulligan ME, Hatem S. Grading articular cartilage of the knee using fast spin-echo proton density-weighted MR imaging without fat suppression. AJR Am J Roentgenol. 2002;179:1159–1166. doi: 10.2214/ajr.179.5.1791159. [DOI] [PubMed] [Google Scholar]