Abstract
The purpose of this study was to assess the pharmacokinetics of gabapentin in healthy Greyhound dogs after single oral doses targeted at 10 and 20 mg/kg PO. Six healthy Greyhounds were enrolled (3 males, 3 females). Blood was obtained at predetermined times for the measurement of gabapentin plasma concentrations by liquid chromatography/mass spectrometry. Pharmacokinetic parameters were determined with computer software.
The actual mean (and range) doses administered were 10.2 (9.1–12.0) mg/kg and 20.5 (18.2 – 24) mg/kg for the 10 mg/kg and 20 mg/kg targeted dose groups. The mean CMAX for the 10 and 20 mg/kg groups were 8.54 and 13.22 μg/mL at 1.3 and 1.5 h, and the terminal half-lives were 3.3 and 3.4 h, respectively. The relative bioavailability of the 10 mg/kg group was 1.13 compared to the 20 mg/kg group. Gabapentin was rapidly absorbed and eliminated in dogs indicating frequent dosing is needed to maintain minimum targeted plasma concentrations.
Keywords: Analgesic, Anticonvulsant, Veterinary, Dog, Pharmacology
Gabapentin is an anticonvulsant and analgesic drug producing its pharmacological effects through incompletely understood mechanisms. Gabapentin has minimal direct effects on gamma-aminobutyric acid (GABA) receptors (Cheng and Chiou, 2006). Gabapentin binds to the α2δ subunit of voltage gated calcium channels acting pre-synaptically to decrease the release of excitatory neurotransmitters and it may increase brain concentrations of GABA or antagonize AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors.
Gabapentin has been recommended or used in dogs, but few data are available on its pharmacokinetics, safety or efficacy in the species (Govendire et al., 2005; Platt et al., 2006; Lamont, 2008; Cashmore et al., 2009). Previous studies have examined the pharmacokinetics of gabapentin in Beagles at supra-therapeutic doses (Vollmer et al., 1986; Radulovic et al., 1995; Rhee et al., 2008). The purpose of this study was to assess the pharmacokinetics of gabapentin in dogs at clinically relevant doses. The study included healthy Greyhounds (3 males, 3 females) aged 1.5–3 years and weighing 25–42 kg. The Institutional Animal Care and Use Committee at Kansas State University approved the study.
Gabapentin was administered PO at a targeted dose of 10 mg/kg, to the nearest whole capsule (300 and 400 mg capsules, Apotex Inc.) on day 1 and at a targeted dose of 20 mg/kg, to the nearest whole capsule on day 2. Animals were fasted for 12 h prior to dosing. Immediately after dosing all animals were offered a treat (Milk-Bone, Del Monte Foods); animals not eating the treat were administered 5 mL water PO to ensure swallowing of the capsule(s). Dogs were offered food 4 h after dosing and food was removed 12 h prior to the second dose.
Blood samples (9 mL) were collected into heparin tubes prior to drug administration and 15, 30 and 45 min and 1, 1.5, 2, 4, 6, 8, 12 and 24 h after administration from a jugular catheter (Venocath-16, Abbott Ireland). Plasma was separated by centrifugation (2 000 g for 15 min) and stored at −70 °C.
Plasma concentrations of gabapentin, m/z 172.1→154.1 (Spectrum Chemical), and the internal standard (IS) pregabalin, m/z 160.0.1→142.0, (Lyrica, Pfizer) were determined by liquid chomatography (Shimadzu Prominence, Shimadzu Scientific Instruments) with mass spectrometry (API 2000, Applied Biosystems) (Table 1). The standard curve was linear from 0.1–25 μg/mL. The accuracy and coefficient of variation of the analytical method for gabapentin were 102 ± 9% and 8%, respectively, at 0.1, 1, and 25 μg/mL in replicates of five each. Plasma, 0.1 mL, 0.1 mL IS (5 μg/mL), and 0.4 mL methanol with 0.1% formic acid were combined then vortexed for 5 s. The samples were centrifuged for 10 min (10 000 g) and 20 μL of the supernatant injected.
Table 1.
Mass spectrometer settings and the mobile phase gradient for the determination of gabapentin and the internal standard (IS) pregabalin
| Mass spectrometer settings | |
| Source temperature (C) | 300 |
| Dwell time (ms) | 200 |
| Ion source gas 1 (psi) | 20 |
| Ion source gas 2 (psi) | 20 |
| Curtain gas (psi) | 10 |
| Collision gas 1 (psi) | 6 |
| Ion Spray voltage (V) | 4500 |
| Entrance potential (V) | 10 |
| Declustering potential (V) | 50 |
| Collision energy (V) | 20 (gabapentin), 17 (IS) |
| Collision cell exit potential (V) | 10 |
| Mode of analysis | Positive ionization |
| Mobile phase gradient | |
| 0–0.5 min | 100% B |
| 0.5 – 2.5 min | linear gradient to 70% B: 30% A |
| 2.5 – 3 min | hold at 70% B: 30% A |
| 3 – 3.5 min | linear gradient to 100% B |
| 3.5 – 5 min | hold at 100% B |
| A = Methanol with 0.1% formic acid | |
| B = 0.1% formic acid in water | |
Separation was achieved at 40 °C using a C18 column (Supelco Discovery, 50 mm × 2.1 mm × 5 μm, Sigma-Aldrich). The mobile phase consisted of A: methanol with 0.1% formic acid and B: 0.1 % formic acid, with a flow rate of 0.3 mL/min (Table 1). Pharmacokinetic analysis was performed with computer software (WinNonlin 5.2, Pharsight Corporation) and the calculated pharmacokinetic parameters are included in Table 2.
Table 2.
Abbreviations for the pharmacokinetic parameters
| AUC extrapolated | percent of the AUC extrapolated to infinity |
| AUCinf/Dose | the area under the curve from 0 to infinity per dose administered |
| AUCinf | area under the curve from time 0 to infinity |
| AUMCinf | area under the first moment curve from time 0 to infinity |
| Cl/F | plasma clearance per fraction of the dose absorbed |
| CMAX | maximum plasma concentration |
| CMAX/Dose | maximum plasma concentration per dose administered |
| T ½λz | terminal half-life |
| λz | first-order rate constant |
| MRTinf | mean residence time extrapolated to infinity |
| TMAX | time to maximum plasma concentration |
| Vz/F | apparent volume of distribution of the area fraction of the dose absorbed |
| Relative F | relative fraction of the dose absorbed for each oral dose rate |
The actual doses of gabapentin administered on day 1 were 9.1–12.0 mg/kg and 18.2–24.0 mg/kg on day 2 (Table 3). Gabapentin was rapidly absorbed and eliminated (Table 3, Figs. 1–3). The terminal half-life for the 10 and 20 mg/kg doses were 3.3 and 3.4 h, respectively.
Table 3.
Pharmacokinetic parameters of oral gabapentin in Healthy Greyhound dogs
| 10 mg/kg targeted dose | 20 mg/kg targeted dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | ||||||||
| Mean | Min | Median | Max | Mean | Min | Median | Max | ||
| Dose | mg/kg | 10.2 | 9.1 | 9.9 | 12.0 | 20.5 | 18.2 | 19.7 | 24.0 |
| Non-compartmental pharmacokinetic parameters | |||||||||
| AUC extrapolated | % | 4.00 | 0.92 | 5.98 | 11.17 | 3.08 | 0.56 | 4.52 | 10.32 |
| AUCinf/Dose | h*μg/mL | 4.77 | 3.92 | 4.81 | 5.61 | 4.21 | 3.00 | 4.22 | 6.29 |
| AUCinf | h*μg/mL | 48.77 | 39.77 | 49.50 | 63.02 | 86.02 | 60.00 | 83.01 | 151.24 |
| AUMCinf | h*h*μg/mL | 264.76 | 182.92 | 274.78 | 374.51 | 489.41 | 315.59 | 447.60 | 964.42 |
| Cl/F | mL/min/kg | 3.49 | 2.97 | 3.49 | 4.26 | 3.96 | 2.65 | 3.97 | 5.56 |
| CMAX | μg/mL | 8.54 | 5.32 | 9.68 | 10.90 | 13.22 | 10.70 | 12.95 | 18.20 |
| CMAX/Dose | μg/mL | 0.84 | 0.56 | 0.84 | 1.10 | 0.65 | 0.56 | 0.63 | 0.77 |
| T½λz | h | 3.25 | 2.63 | 3.35 | 3.68 | 3.41 | 3.07 | 3.39 | 3.91 |
| λz | 1/h | 0.213 | 0.189 | 0.207 | 0.263 | 0.203 | 0.177 | 0.205 | 0.226 |
| MRTinf | h | 5.43 | 4.60 | 5.40 | 6.78 | 5.69 | 5.01 | 5.40 | 6.94 |
| TMAX | h | 1.31 | 0.75 | 1.50 | 2.00 | 1.51 | 1.00 | 1.75 | 2.00 |
| Vz/F | L/kg | 0.983 | 0.850 | 0.942 | 1.256 | 1.170 | 0.835 | 1.207 | 1.476 |
| Relative F | 1.13 | 0.83 | 1.28 | 1.41 | N/A | N/A | N/A | N/A | |
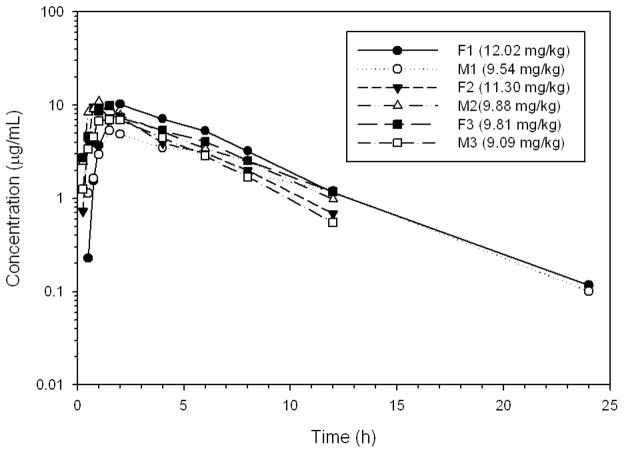
Figure 1.
Plasma concentrations of gabapentin in six healthy Greyhounds after oral administration of a target dose of 10 mg/kg to the nearest whole capsule (actual dose administered in parenthesis).
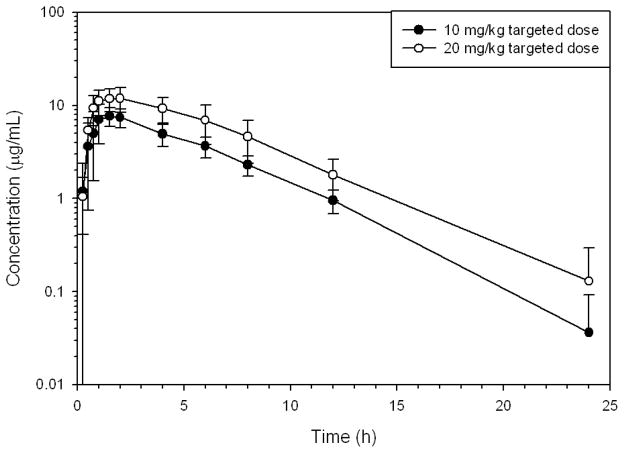
Figure 3.
Mean ± SD plasma concentrations of gabapentin in six healthy Greyhounds after oral administration of targeted doses of 10 and 20 mg/kg to the nearest whole capsule.
The relative fraction of the dose absorbed for each oral dose rate (F) of the 10 mg/kg dose, compared to the 20 mg/kg dose was 1.13. The mean peak plasma concentrations in the 10 and 20 mg/kg dose groups were 8.54 and 13.22 μg/mL, respectively. The CMAX in humans is not proportional to the dose as decreases in bioavailability occur with increased doses resulting in less than proportional increases in plasma concentrations1. Sample size analysis indicates a sample size of eight dogs is needed to statistically compare the CMAX dose proportionality with an alpha of 0.05 and a power of 0.8.
The efficacy of gabapentin in Greyhounds was not evaluated. Efficacy in humans is associated with 2 μg/mL plasma concentrations, but the effective concentrations are unknown in the dog. Gabapentin exceeded 2 μg/mL in the 10 mg/kg dose group in 6/6 dogs at 6 h, 4/6 dogs at 8 h and 0/6 dogs at 12 h after dosing. Gabapentin exceeded 2 μg/mL in the 20 mg/kg dose group in 6/6 dogs at 8 h, and 2/6 dogs at 12 h after dosing. These data suggest 10–20 mg/kg every 8 h would maintain 2 μg/mL plasma concentrations in dogs.
The study was a non-randomized block design with doses administered on consecutive days, therefore day to day variability, carryover, or treatment order could have affected the pharmacokinetics. Comparisons of the two doses must be made cautiously, but the study does provide preliminary data. Another limitation of the study was the lack of IV drug administration. A commercially available injectable solution was not available at the time of the study. Additionally, budgetary constraints limited the number of crossovers that could be conducted. The bioavailability, mean absorption time, clearance and volume of distribution can only be determined with IV studies. Further studies including IV administration are needed to completely understand the pharmacokinetics of gabapentin in dogs.
The current study was not designed to assess the safety of gabapentin. Loose stools occurred in three dogs on the second day of the study but it is not clear whether this was related to the drug administration or another cause. The dogs were offered a treat which is not a routine component of their diet. The dogs were also transported from their runs to the study location 24 h prior to the beginning of the study. Loose stools have been observed in these dogs in other studies, after transport from their runs to the study location, but before the study started (unpublished observations). Further studies are needed assessing the effects of multiple doses of gabapentin in dogs. In conclusion, gabapentin was rapidly absorbed and eliminated in dogs indicating multiple doses are needed per day in order to maintain targeted plasma concentrations.
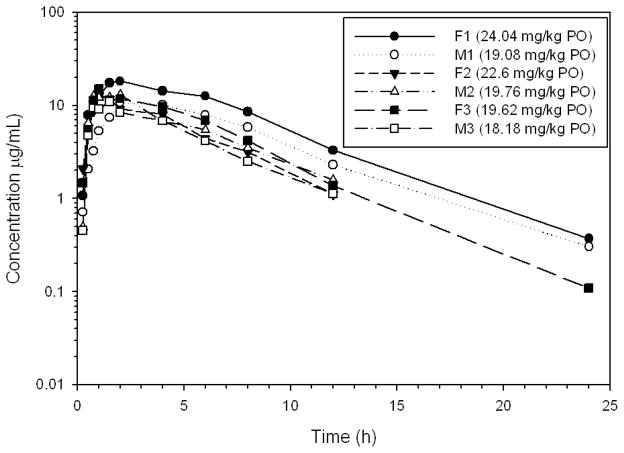
Figure 2.
Plasma concentrations of gabapentin in six healthy Greyhounds after oral administration of a target dose of 20 mg/kg to the nearest whole capsule (actual dose administered in parenthesis).
Acknowledgments
The authors would like to thank Megan Montgomery for her technical help throughout the study. The authors would also like to thank the Animal Resources Facilities at Kansas State University for the excellent work in maintaining the health of the animals. The authors thank the Department of Anatomy and Physiology, the Analytical Pharmacology Laboratory, the Veterinary Research Scholars Program at Kansas State University (funded by NIH NCRR 5T35RR007064-10), and the Merck-Merial Research Grants Program for providing financial support of the study.
Footnotes
See Neurontin, package insert: www.pfizer.com/files/products/uspi_neurontin.pdf.
Conflict of interest statement
Dr KuKanich has been a consultant for Bayer Animal Health, Pfizer Animal Health, and Farnam Animal Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cashmore RG, Harcourt-Brown TR, Freeman PM, Jeffery ND, Granger N. Clinical diagnosis and treatment of suspected neuropathic pain in the dogs. Australian Veterinary Journal. 2009;87:45–50. doi: 10.1111/j.1751-0813.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Chiou LC. Mechanisms of the antinociceptive action of gabapentin. Journal of Pharmacological Sciences. 2006;100:471–86. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- Govendir M, Perkins M, Malik R. Improving seizure control in dogs with refractory epilepsy using gabapentin as an adjunctive agent. Australian Veterinary Journal. 2005;83:602–8. doi: 10.1111/j.1751-0813.2005.tb13269.x. [DOI] [PubMed] [Google Scholar]
- Lamont LA. Adjunctive analgesic therapy in veterinary medicine. Veterinary Clinics of North America Small Animal Practice. 2008;38:1187–203. doi: 10.1016/j.cvsm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Platt SR, Adams V, Garosi LS, Abramson CJ, Penderis J, De Stefani A, Matiasek L. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Veterinary Record. 2006;159:881–4. [PubMed] [Google Scholar]
- Radulovic LL, Türck D, von Hodenberg A, Vollmer KO, McNally WP, DeHart PD, Hanson BJ, Bockbrader HN, Chang T. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metabolism and Disposition. 1995;23:441–8. [PubMed] [Google Scholar]
- Rhee YS, Park S, Lee TW, Park CW, Nam TY, Oh TO, Jeon JW, Han SB, Lee DS, Park ES. In Vitro/in vivo relationship of gabapentin from a sustained-release tablet formulation: a pharmacokinetic study in the beagle dog. Archives of Pharmacological Research. 2008;31:911–7. doi: 10.1007/s12272-001-1246-x. [DOI] [PubMed] [Google Scholar]
- Vollmer KO, von Hodenberg A, Kölle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36:830–9. [PubMed] [Google Scholar]