Abstract
Objectives
The metabolic and genetic correlates of circulating insulin-like growth factor-1 (IGF-1) and its main circulating carrier, IGF-1-binding-protein-3 (IGFBP-3), are unclear.
Methods and Results
We measured serum IGF-1 and IGFBP-3 concentrations in a sample of the Framingham Heart Study (N=3977, aged 40±9 years, 46% male), and evaluated their relations to cardiovascular risk factors using multivariable regression. Serum IGF-1 was inversely correlated with age, body mass index, total cholesterol, the presence of diabetes, alcohol consumption and glomerular filtration rate (all p<0.01); whereas the ratio of IGF-1:IGFBP-3 was lower in women and inversely related to age, triglycerides, high density lipoprotein cholesterol, systolic blood pressure and alcohol consumption (all p<0.0001). Circulating IGF-1 correlated negatively with insulin resistance (homeostatic model assessment) (r=−0.1; p<0.0001) and was lower in participants with more components of the metabolic syndrome (Adult Treatment Panel III criteria) (p<0.0001). Additive genetic factors (heritability) accounted for 43% and 39% of the variation of IGF-1 and IGFBP-3 respectively (both p<10−27).
Conclusions
Our cross-sectional observations in a large community-based sample link lower circulating IGF-1 to greater metabolic risk burden, and underscore substantial genetic influences on IGF-1 concentrations. Prospective studies are warranted to elucidate if lower IGF-1 concentrations predict greater metabolic risk longitudinally.
Keywords: insulin-like growth factor, insulin resistance, metabolic syndrome, heritability
Insulin-like growth factor-1 (IGF-1) is a growth hormone that regulates cell metabolism, growth, proliferation and apoptosis in multiple organ systems.1 It is abundant in the circulation, where it binds to insulin-like growth factor binding proteins (IGFBPs). IGFBP-3 is the major circulating carrier of IGF-1 and regulates the biological effects of IGF-1 by sequestering IGF-1 into a circulating reservoir, thereby reducing the free fraction of bioactive IGF-1 in the blood.2
In the cardiovascular system, the IGF axis is postulated to be an important mediator of cardiovascular risk and disease. Indeed, low IGF-1 concentrations have been shown to predict cardiovascular mortality among elderly participants of the Rancho Bernardo Study.3 However, the associations of circulating IGF-1 concentrations with known cardiovascular risk factors and genetic influences remain unclear. Prior studies examining the clinical correlates of IGF-1 and IGFBP-3 have yielded mixed results. Studies relating IGF-1 concentrations to blood pressure have noted positive,4 negative5 and no6 associations. Similarly, clinical reports have been inconsistent regarding the associations between IGF-1 or IGFBP-3 and diabetes mellitus (DM),7, 8 dyslipidemia,9, 10 or obesity.5, 11–16 Most previous studies were limited to relatively small or highly selected samples, and data from large-scale community-based samples are still needed. Further, despite evidence of a significant genetic contribution to circulating IGF-1 concentrations from twin studies,17–19 data are limited regarding the heritability of IGF-1 and IGFBP-3 in the general community.
Accordingly, we systematically assessed the clinical correlates, estimated the heritability and performed genome-wide linkage analyses of circulating IGF-1, IGFBP-3 and their ratio in a large community-based sample. Given the previously reported associations of IGF-1 and IGFBP-3 with individual components of the metabolic syndrome,20 we hypothesized that these markers would reflect the burden of metabolic risk in the community. Specifically, we hypothesized that lower IGF-1 and higher IGFBP-3 circulating concentrations, possibly reflecting lower unbound (free) IGF, would be related to greater burden of metabolic risk. We further hypothesized that a significant proportion of the variability of circulating IGF-1 and IGFBP-3 in the general community would be attributable to additive genetic effects.
METHODS
Participants
The Framingham Heart Study21 is an ongoing community-based cohort investigation of cardiovascular risk, first established in 1948, that is currently studying three generations of participants. The most contemporary generation of participants (Generation 3, recruited in 2002–2004) was included in the current study sample. We also included participants of the Omni generation 2 cohort, a minority cohort from Framingham similarly recruited in 2003–2005. All participants underwent detailed medical interviews, physical examinations and laboratory investigations according to standardized protocols. Of 4273 eligible participants, 296 were excluded due one of more of the following: prevalent cardiovascular disease (N=66) or renal impairment (serum creatinine > 2mg/dl; N=2), missing IGF-1/IGFBP-3 measurements (N=99) or missing covariates (N=162). A total of 3977 participants made up the final sample. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board at the Boston University Medical Center.
Measurement of IGF-1 and IGFBP-3
Blood samples were collected according to rigorous protocol in the morning following overnight fast. Samples were centrifuged and aliquotted immediately for storage at −70 °C. Serum IGF-1 was measured by standard immunoassay (R&D Systems Quantikine Human IGF-I Cat#DG100, SG100 and PDG100; ELISA method) with a lower detection limit of 9.4 ng/ml. This assay involves pretreatment with an acid dissociation solution to eliminate IGFBP-3 interference. Serum IGFBP-3 was measured by standard immunoassay (R&D Systems Quantikine Human IGFBP-3 Cat#DGB300; ELISA method) with a lower detection limit of 75.99 ng/mL and an upper limit of 5000 ng/ml. Quality control measures included running duplicate samples and checking reproducibility according to strict protocol. The intra-assay coefficients of variation were 5.3% for the IGF-1 assay and 9.1% for the IGFBP-3 assay.
Statistical Analyses
Distribution of IGF-1 and IGFBP-3
Serum IGF-1 and IGFBP-3 concentrations were normally distributed, whereas the distribution of their ratio was skewed. Normal quantile transformation was used to normalize the distribution of the IGF-1:IGFBP-3 ratio.
Clinical correlates
The following clinical covariates were identified on the basis of published studies and biological plausibility:6, 9–14, 22−24 age, sex, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension (SBP ≥140 mmHg and/or DBP ≥90 mmHg or the use of anti-hypertensive medications), DM (fasting blood sugar ≥126 mg/dl or the use of anti-diabetic medications), total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, smoking status, alcohol consumption and estimated glomerular filtration rate (GFR, calculated using the MDRD equation).
To investigate the association of IGF-1, IGFBP-3 or the ratio of IGF-1:IGFBP-3 with clinical covariates, significant predictors were first identified for each biomarker using multivariable linear regression with stepwise forward selection (P≤0.10 for model entry). To account for relatedness among participants, generalized estimating equations (GEE; using Compound Symmetry Correlation Matrix) were then used to assess the association between each biomarker and the clinical covariates that were statistically significant in the initial regression analyses.
To assess the associations of IGF-1, IGFBP-3 and their ratio with insulin resistance and the metabolic syndrome, the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the equation HOMA-IR =(FPG × FPI)/22.5 where FPG =fasting plasma glucose in mmol/l and FPI =fasting plasma insulin in mU/l measured by ELISA (Linco Research, Millipore Bioscience Division, intra-assay coefficient of variation of 2.7%, with no cross-reactivity with human proinsulin at concentrations up to 100nM).25 The presence of insulin resistance was defined as HOMA-IR ≥75th percentile in the sample. The updated Adult Treatment Panel III criteria were used to define the metabolic syndrome.20 The diagnosis was met in the presence of ≥3 of: waist circumference ≥102 cm (≥40 inches) in men or ≥88 cm (≥35 inches) in women; triglycerides ≥150 mg/dL (1.7 mmol/L) or on drug treatment for elevated triglycerides; HDL <40 mg/dL (1.03 mmol/L) in men, <50 mg/dL (1.3 mmol/L) in women; SBP ≥130 mm Hg SBP or DBP ≥85 mm Hg or on antihypertensive drug treatment in a patient with a history of hypertension; and fasting blood glucose ≥100 mg/dL or on drug treatment for elevated glucose. We further related each biomarker with the number of components of the metabolic syndrome present using generalized linear models adjusting for age and sex.
Heritability estimates
Heritability was estimated from variance component models using Sequential Oligogenic Linkage Analysis Routines (SOLAR). Two heritability estimates were provided for IGF-1 and IGFBP-3: (i) adjusting for age and sex; and (ii) adjusting for all significantly associated clinical covariates.
Genetic Linkage
Multipoint quantitative trait linkage analyses were conducted with SOLAR on 687 autosomal microsatellite markers using the variance-components models adjusting for (i) age and sex; and (ii) all significantly associated clinical covariates. Linkage was assessed by comparing models that incorporated genetic marker information to models that did not incorporate genetic information (i.e., identity by descent data). Results were expressed as the logarithm-of-the-odds (LOD) score from each model.
RESULTS
Sample characteristics
Our community-based sample consisted of predominantly young to middle-aged adults with low to moderate prevalence of known cardiovascular risk factors and a slight preponderance of women (Table 1). Age- and sex- stratified concentrations of IGF-1, IGFBP-3 and IGF-1:IGFBP-3 ratio are provided in the online Supplementary Table I.
Table 1.
Sample characteristics
| Clinical variables | Men (N=1837) | Women (N=2140) |
|---|---|---|
| Age, years | 40±9 | 40±9 |
| Height, m | 1.78±0.07 | 1.64±0.06 |
| Weight, kg | 88.1±15.8 | 69.5±16.4 |
| Body mass index, kg/m2 | 27.8±4.6 | 25.8±6.0 |
| Systolic blood pressure, mmHg | 121±13 | 113±14 |
| Diastolic blood pressure, mmHg | 78±9 | 73±9 |
| Hypertension, % | 20 | 12 |
| Diabetes mellitus, % | 3 | 2 |
| Smoking, % | 16 | 15 |
| Alcohol consumption, ounces per month | 13.6±18 | 5.9±8.0 |
| Glomerular filtration rate, ml/min/1.73m2 | 99.7±17.2 | 99.5±18.8 |
| Total cholesterol, mg/dl | 193±37 | 185±34 |
| HDL cholesterol, mg/dl | 47±12 | 61±16 |
| LDL cholesterol, mg/dl | 120±31 | 104±30 |
| Triglycerides (median, 25th–75th percentile), mg/dl | 107 (72–160) | 80 (59–114) |
| HOMA-IR | 1.20±1.02 | 0.97±0.75 |
| Insulin resistance, % | 31 | 20 |
| IGF-1, ng/ml | 128±39 | 129±45 |
| IGFBP-3, ng/ml | 2905±1079 | 3097±1096 |
| IGF-1:IGFBP-3 ratio (median, 25th–75th percentile) | 0.044 (0.034–0.057) | 0.042 (0.032–0.054) |
Values are mean±SD unless otherwise indicated.
HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance, calculated using the equation HOMA-IR = (FPI × FPG)/22.5 where FPI = fasting plasma insulin in mU/l and FPG = fasting plasma glucose in mmol/l25; The presence of insulin resistance was defined as HOMA-IR at or above the 75th percentile of 1.25; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3; IQR, interquartile range.
To calculate the molar ratio of IGF-1 to IGFBP-3 using molar weights of 7.65 kDa for IGF-1 and 30.5 kDa45 for IGFBP-3, multiply ratio values by 3.987.
Clinical correlates
In multivariable analysis (Table 2), IGF-1 concentrations were negatively associated with age, diabetes, total cholesterol, body mass index, alcohol consumption and renal function. IGFBP-3 concentrations were similarly negatively associated with age and body mass index, but were also lower in men and positively associated with SBP, HDL cholesterol and triglycerides in multivariable-adjusted models. The ratio of IGF-1:IGFBP-3 was higher in men and negatively associated with age, SBP, triglycerides, HDL cholesterol and alcohol consumption.
Table 2.
Clinical correlates of insulin-like growth factor-1 (IGF-1), IGF binding protein-3 (IGFBP-3) and their ratio
| Dependent variable | Independent variable | Beta coefficient* | 95% CI | P value |
|---|---|---|---|---|
| IGF-1, ng/ml | Age, per 1SD | −17.24 | (−18.90, −15.58) | <0.0001 |
| Sex, men vs women | 2.29 | −0.57, 5.16 | 0.1160 | |
| Diabetes, yes vs no | −10.95 | (−18.27, −3.63) | 0.0034 | |
| Total cholesterol, per 1 SD | −2.10 | (−3.60, −0.60) | 0.0061 | |
| HDL cholesterol, per 1SD | −1.56 | (−3.08, −0.03) | 0.0453 | |
| Body mass index, per 1SD | −4.41 | (−5.79, −3.03) | <0.0001 | |
| Smoking, yes vs no | −3.19 | (−6.54, 0.16) | 0.0617 | |
| Alcohol consumption, per 1SD | −3.79 | (−4.84, −2.74) | <0.0001 | |
| GFR, per 1SD | −2.69 | (−4.10, −1.29) | 0.0002 | |
|
| ||||
| IGFBP-3, ng/ml | Age, per 1SD | −177.21 | (−217.51, −136.92) | <0.0001 |
| Sex, men vs women | −190.61 | (−270.35, −110.86) | <0.0001 | |
| Systolic BP, per 1SD | 54.30 | (16.00, 92.59) | 0.0055 | |
| HDL cholesterol, per 1SD | 75.59 | (31.13, 120.06) | 0.0009 | |
| Triglycerides, per 1 SD | 124.75 | (72.68, 176.82) | <0.0001 | |
| Body mass index, per 1SD | −86.44 | (−127.49, −45.39) | <0.0001 | |
| Alcohol consumption, per 1SD | 32.12 | (−7.65, 71.90) | 0.1134 | |
| GFR, per 1SD | −36.30 | (−72.98, 0.37) | 0.0524 | |
|
| ||||
| IGF-1:IGFBP-3 ratio | Age, per 1SD | −0.1366 | (−0.1720,−0.1012) | <0.0001 |
| Sex, men vs women | 0.2571 | (0.1828, 0.3315) | <0.0001 | |
| Systolic BP, per 1SD | −0.0741 | (−0.1094, −0.0388) | <0.0001 | |
| Triglycerides, per 1SD | −0.1415 | (−0/1971, −0.0860) | <0.0001 | |
| HDL cholesterol, per 1SD | −0.1002 | (−0.1414, −0.0591) | <0.0001 | |
| Alcohol consumption, per 1SD | −0.1106 | (−0/1468, 0.0744) | <0.0001 | |
IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3; GFR, glomerular filtration rate; BP, blood pressure; HDL, high density lipoprotein;
Beta coefficients represent the expected change in mean IGF1, IGFBP3 or transformed ratio for each standard deviation increment of the indicated continuous variable (or each category of indicated discrete variable), adjusting for the other variables in the model.
To calculate the molar ratio of IGF-1 to IGFBP-3 using molar weights of 7.65 kDa for IGF-1 and 30.5 kDa45 for IGFBP-3, multiply ratio values by 3.987.
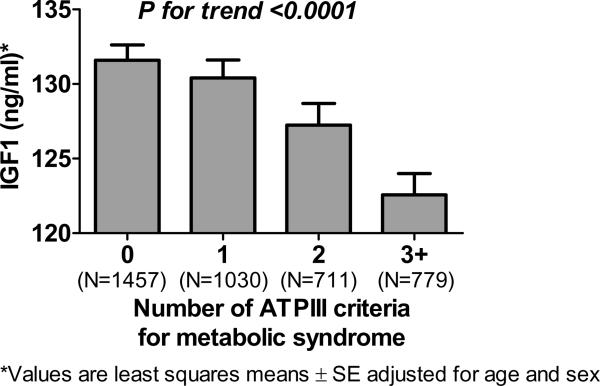
Adjusting for age and sex, HOMA-IR was inversely correlated with IGF-1 (r=−0.096; p<0.0001) and the IGF-1:IGFBP-3 ratio (r=−0.056; p=0.0008). The presence of insulin resistance, defined as HOMA-IR at or above the 75th percentile of 1.25, was accordingly associated with reduced IGF-1 concentrations compared to those without insulin resistance, adjusting for age and sex (122±45 ng/ml vs 132±42 ng/ml; p=0.0006). There was also a strong association between reduced IGF-1 concentrations and the presence of the metabolic syndrome (p<0.0001, adjusted for age and sex). Similar findings were obtained with the IGF-1:IGFBP-3 ratio (p=0.0004) but not with IGFBP-3 concentrations alone (p=0.95). As shown in Figure 1, IGF-1 concentrations were lower in participants with more components of the metabolic syndrome (p for trend < 0.0001 adjusting for age and sex).
Figure 1. Association between circulating concentrations of insulin-like growth factor-1 (IGF-1) and the number of components of the metabolic syndrome.
Bar graphs represent the least squares means ± SE of IGF-1 in ng/ml, adjusted for age and sex.
Genetic correlates
Both IGF-1 and IGFBP-3 were heritable, with estimates of 43% and 39%, respectively in adjusted analyses (Table 3). In linkage analyses adjusted for clinical covariates, none of the peak LOD scores reached the standard genome-wide significance threshold LOD score of 3. For IGF-1, the maximum LOD score was 2.41 (at chromosome 12, 8 cM) adjusting for age and sex; and 2.48 (at chromosome 1, 36 cM) adjusting for significantly associated clinical correlates. For IGFBP-3, the maximum LOD score was 1.94 (at chromosome 7, 70 cM) adjusting for age and sex; and 1.76 (at chromosome 8, 165 cM) adjusting for significantly associated clinical correlates.
Table 3.
Heritability estimates for insulin-like growth factor-1 (IGF-1) and IGF binding protein-3 (IGFBP-3)
| Biomarker | Model | Heritability | 95% CI | P value |
|---|---|---|---|---|
| IGF-1 | Age- & sex- adjusted | 0.43 | (0.346,0.515) | 1.6E-28 |
| Multivariable- adjusted | 0.43 | (0.346,0.515) | 1.2E-27 | |
|
| ||||
| IGFBP-3 | Age- & sex- adjusted | 0.39 | (0.310,0.462) | 6.6E-29 |
| Multivariable- adjusted | 0.39 | (0.315,0.471) | 2.7E-30 | |
IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3
DISCUSSION
Principal findings
Our study provides epidemiologic evidence for an association between the IGF-1 axis and metabolic risk burden, as well as the estimated contribution of genetic factors to circulating IGF-1 and its main binding protein IGFBP-3 in the general population. Among young to middle-aged adults in the community, reduced concentrations of circulating IGF-1 and its unbound fraction (IGF-1:IGFBP-3 ratio) are related to the metabolic syndrome and its components, adjusting for age and sex. After accounting for these clinical correlates, there was a significant contribution of heritable factors to the variability of IGF-1 and IGFBP-3 concentrations in the general community. These cross-sectional observations are consistent with the notion that the IGF-1 axis may be an important mediator of metabolic risk in the community. Prospective studies are warranted to elucidate if lower IGF-1 concentrations predict increased risk of cardiovascular events longitudinally.
Clinical correlates of circulating IGF-1 & IGFBP-3
Age and sex
Numerous previous studies have reported a negative association between age and concentrations of IGF-1 or IGFBP-3.13, 14, 22–24 This observation is consistent with the established role of the IGF axis in organ growth and the known parallel decline in growth hormone concentrations with age.26 Many previous studies were limited to women22–24 or men16 alone. Nonetheless the current findings of similar IGF-1 concentrations in men and women, but higher IGFBP-3 in women are consistent with some10, 27 but not all12–14 previous studies. Of note, the latter studies12–14 included predominantly post-menopausal women in whom exogenous hormone use may have led to suppression of circulating IGF-1 – a phenomenon consistently observed in previous studies28 but occurring by unclear mechanisms. In contrast, our sample was comprised of predominantly premenopausal women.
Obesity
The relationship between obesity and concentrations of IGF-1 is complex. Increased body fat and decreased muscle mass has been associated with hyposecretion of growth hormone, a known regulator of hepatic IGF-1 production.1 Thus, a negative association between IGF-1 and body mass index, as found in the current study and others,11–13 may be expected. However, adipocytes can also produce IGF-1,29 while obesity-related increases in insulin secretion may stimulate hepatic synthesis of IGF-1.30
Insulin resistance and diabetes mellitus
Given the known insulin-like effects of IGF-1 on glucose uptake31 an association between lower IGF-1 concentrations and increasing insulin resistance may be expected. Our large sample size provided statistical power to detect this association, albeit a modest correlation and of uncertain clinical significance. However, similar modest associations (r<0.2) of known clinical importance include the correlation between severity or duration of systemic hypertension and degree of left ventricular hypertrophy.32 The current results are also consistent with previous studies33, 34 and are highly biologically plausible. Prospective studies have shown that reduced IGF-1 concentrations predict the development of impaired glucose tolerance and DM,35 while exogenous IGF-1 administration reduces serum glucose concentrations in insulin resistance36 and DM.37, 38
Lipids
The inverse association of IGF-1 with total cholesterol concentrations and, conversely, the positive association of IGFBP-3 with plasma lipids are consistent with the known role of IGF-1 in lipid metabolism,38 the largely inhibitory effect of IGFBP-3 on IGF-1 bioactivity2 and observations from previous cross-sectional studies.9, 10 These observations are also corroborated by previous reports of increased risk of atherosclerosis among individuals with low IGF-1 and high IGFBP-3.39
Blood pressure
IGF-1 has been convincingly shown to favorably influence blood pressure via nitric oxide-dependent effects40 and to play a critical role in vascular remodeling41 in experimental studies. A direct association between IGFBP-3 and blood pressure found in the current study and others6 supports the notion that alterations in the relative binding of IGF-1 to IGFBP-3 may be related to risk of hypertension and associated target organ damage.
Metabolic syndrome
The association between low IGF-1:IGFBP-3 ratio and the metabolic syndrome has recently been elegantly demonstrated in the multi-ethnic NHANES III population.33 Our findings lend support to the authors' call for prospective studies to elucidate if lower IGF-1 or IGF-1:IGFBP-3 concentrations predict increased risk of cardiovascular events longitudinally. We further adjust for select lifestyle factors in the current study, and emphasize the genetic underpinnings to this association.
Genetic correlates of circulating IGF-1 & IGFBP-3
Heritability
Twin studies have estimated that genetic effects account for 38%19 to >80%17, 18 of the variation in serum IGF-1 and IGFBP-3 concentrations. Our current heritability estimates are consistent with these data and importantly extend the estimates to adults in the general population. The estimated heritability of IGF-1 and IGFBP-3 in the general community is comparable to that of common cardiovascular risk factors such as blood pressure, lipids and BMI.
Linkage
Genome-wide linkage analyses interestingly mapped to chromosomal regions other than that containing the known genes encoding IGF-1 (12q22–q23) and IGFBP-3 (7p13–p12) themselves. Thus, while genetic factors contribute to variation in circulating concentrations of these biomarkers, this genetic contribution does not appear to be related to variation in the IGF-1 or IGFBP-3 gene itself, but may instead be related to genes affecting the pathways through which these biomarkers are regulated. Supporting this concept, the most frequently investigated polymorphism relating to the IGF-1 gene -- a simple tandem repeat polymorphism lying 1kb 5' to the IGF-1 gene transcriptional start site -- has not been convincingly shown to affect circulating IGF-1 concentrations.42 Intriguingly, the regions of suggestive linkage for IGF-1 identified in the current study (12p13 and 1p36) lie in quantitative trait loci known to be involved in blood pressure regulation and associated with hypertension (BP53_H and BP9_H respectively).43, 44 These findings are hypothesis-generating but require confirmation in further studies.
Strengths and limitations
The strengths of the current investigation include the large, unselected community-based sample; systematic and complete ascertainment of clinical covariates, availability of extensive pedigrees and use of multivariable-adjusted analyses.
The cross-sectional design of our study precludes any causal inferences; prospective studies would be needed for that purpose. Since direct measurement of free (biologically active) IGF-1 was not available in our sample, we used the IGF-1:IGFBP-3 ratio as an estimate of free IGF-1, as previously suggested45 and applied in several studies.12, 13, 33, 46–48 However, this ratio does not account for IGF-II or proteolysis of IGFBP-3, and thus may not always reflect free IGF-1 concentrations.49, 50 Our predominantly white, young to middle-aged sample limits the generalizability of findings to non-white and older populations. However, there was less potential for confounding by non-cardiovascular co-morbidities and concurrent medications in our sample.
Conclusions
Our cross-sectional observations in a large community-based sample link lower concentrations of circulating IGF-1 or its unbound fraction (IGF-1:IGFBP-3 ratio) to greater metabolic risk burden, and demonstrate substantial heritability for serum IGF-1 concentrations. These findings suggest that the IGF-1 axis may play an important role in predisposition to cardiometabolic disease in the community. Longitudinal studies are warranted to elucidate if lower IGF-1 concentrations predict risk of incident cardiovascular events.
Supplementary Material
Acknowledgments
Sources of funding This work was supported by the National Heart, Lung and Blood Institute (contract No. N01-HC-25195), NIH grants R01-HL-077477 (RSV).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 4.Andronico G, Mangano MT, Nardi E, Mule G, Piazza G, Cerasola G. Insulin-like growth factor 1 and sodium-lithium countertransport in essential hypertension and in hypertensive left ventricular hypertrophy. J Hypertens. 1993;11:1097–1101. doi: 10.1097/00004872-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosen T, Lindstedt G, Lundberg PA, Bengtsson BA. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 6.Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total IGF-I, free IGF-I, and IGFB-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol. 1998;18:277–282. doi: 10.1161/01.atv.18.2.277. [DOI] [PubMed] [Google Scholar]
- 7.Sesti G, Sciacqua A, Cardellini M, Marini MA, Maio R, Vatrano M, Succurro E, Lauro R, Federici M, Perticone F. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. 2005;28:120–125. doi: 10.2337/diacare.28.1.120. [DOI] [PubMed] [Google Scholar]
- 8.Rajpathak SN, Gunter MJ, Wylie-Rosett J, Ho GY, Kaplan RC, Muzumdar R, Rohan TE, Strickler HD. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev. 2009;25:3–12. doi: 10.1002/dmrr.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceda GP, Dall'Aglio E, Magnacavallo A, Vargas N, Fontana V, Maggio M, Valenti G, Lee PD, Hintz RL, Hoffman AR. The insulin-like growth factor axis and plasma lipid levels in the elderly. J Clin Endocrinol Metab. 1998;83:499–502. doi: 10.1210/jcem.83.2.4548. [DOI] [PubMed] [Google Scholar]
- 10.Nystrom FH, Ohman PK, Ekman BA, Osterlund MK, Karlberg BE, Arnqvist HJ. Population-based reference values for IGF-I and IGF-binding protein-1: relations with metabolic and anthropometric variables. Eur J Endocrinol. 1997;136:165–172. doi: 10.1530/eje.0.1360165. [DOI] [PubMed] [Google Scholar]
- 11.Colletti RB, Copeland KC, Devlin JT, Roberts JD, McAuliffe TL. Effect of obesity on plasma insulin-like growth factor-I in cancer patients. Int J Obes. 1991;15:523–527. [PubMed] [Google Scholar]
- 12.Chang S, Wu X, Yu H, Spitz MR. Plasma concentrations of insulin-like growth factors among healthy adult men and postmenopausal women: associations with body composition, lifestyle, and reproductive factors. Cancer Epidemiol Biomarkers Prev. 2002;11:758–766. [PubMed] [Google Scholar]
- 13.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors (United States) Cancer Causes Control. 2005;16:917–927. doi: 10.1007/s10552-005-2702-3. [DOI] [PubMed] [Google Scholar]
- 14.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]
- 15.Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum free and total insulin-like growth factor-I, insulin-like growth factor binding protein-1 and insulin-like growth factor binding protein-3 Levels in healthy elderly individuals. Relation to self-reported quality of health and disability. Gerontology. 1998;44:277–280. doi: 10.1159/000022026. [DOI] [PubMed] [Google Scholar]
- 16.Teramukai S, Rohan T, Eguchi H, Oda T, Shinchi K, Kono S. Anthropometric and behavioral correlates of insulin-like growth factor I and insulin-like growth factor binding protein 3 in middle-aged Japanese men. Am J Epidemiol. 2002;156:344–348. doi: 10.1093/aje/kwf069. [DOI] [PubMed] [Google Scholar]
- 17.Kao PC, Matheny AP, Jr., Lang CA. Insulin-like growth factor-I comparisons in healthy twin children. J Clin Endocrinol Metab. 1994;78:310–312. doi: 10.1210/jcem.78.2.8106617. [DOI] [PubMed] [Google Scholar]
- 18.Verhaeghe J, Loos R, Vlietinck R, Herck EV, van Bree R, Schutter AM. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in cord serum of twins: genetic versus environmental regulation. Am J Obstet Gynecol. 1996;175:1180–1188. doi: 10.1016/s0002-9378(96)70025-x. [DOI] [PubMed] [Google Scholar]
- 19.Harrela M, Koistinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, Toivanen L, Koskenvuo M, Leinonen P, Koistinen R, Seppala M. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Dawber TR, Meadors GF, Moore FE., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue LR, Hunter SJ, Sherblom AP, Rosen C. Age-related changes in serum insulin-like growth factor-binding proteins in women. J Clin Endocrinol Metab. 1990;71:575–579. doi: 10.1210/jcem-71-3-575. [DOI] [PubMed] [Google Scholar]
- 23.Holmes MD, Pollak MN, Hankinson SE. Lifestyle correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:862–867. [PubMed] [Google Scholar]
- 24.Lukanova A, Toniolo P, Akhmedkhanov A, Hunt K, Rinaldi S, Zeleniuch-Jacquotte A, Haley NJ, Riboli E, Stattin P, Lundin E, Kaaks R. A cross-sectional study of IGF-I determinants in women. Eur J Cancer Prev. 2001;10:443–452. doi: 10.1097/00008469-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 27.Kaklamani VG, Linos A, Kaklamani E, Markaki I, Mantzoros C. Age, sex, and smoking are predictors of circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3. J Clin Oncol. 1999;17:813–817. doi: 10.1200/JCO.1999.17.3.813. [DOI] [PubMed] [Google Scholar]
- 28.Cardim HJ, Lopes CM, Giannella-Neto D, da Fonseca AM, Pinotti JA. The insulin-like growth factor-I system and hormone replacement therapy. Fertil Steril. 2001;75:282–287. doi: 10.1016/s0015-0282(00)01691-5. [DOI] [PubMed] [Google Scholar]
- 29.Wabitsch M, Heinze E, Debatin KM, Blum WF. IGF-I- and IGFBP-3-expression in cultured human preadipocytes and adipocytes. Horm Metab Res. 2000;32:555–559. doi: 10.1055/s-2007-978685. [DOI] [PubMed] [Google Scholar]
- 30.Boni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260:E846–851. doi: 10.1152/ajpendo.1991.260.6.E846. [DOI] [PubMed] [Google Scholar]
- 31.Russell-Jones DL, Bates AT, Umpleby AM, Hennessy TR, Bowes SB, Hopkins KD, Jackson N, Kelly J, Shojaee-Moradie F, Jones RH, Sonksen PH. A comparison of the effects of IGF-I and insulin on glucose metabolism, fat metabolism and the cardiovascular system in normal human volunteers. Eur J Clin Invest. 1995;25:403–411. doi: 10.1111/j.1365-2362.1995.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 32.Khattar RS, Acharya DU, Kinsey C, Senior R, Lahiri A. Longitudinal association of ambulatory pulse pressure with left ventricular mass and vascular hypertrophy in essential hypertension. J Hypertens. 1997;15:737–743. doi: 10.1097/00004872-199715070-00005. [DOI] [PubMed] [Google Scholar]
- 33.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Malarstig A, Brismar K, Hamsten A, Fisher RM, Hellenius ML. IGF-I/IGFBP-3 ratio: a mechanistic insight into the metabolic syndrome. Clin Sci (Lond) 2009;116:507–512. doi: 10.1042/CS20080382. [DOI] [PubMed] [Google Scholar]
- 34.Perticone F, Maio R, Sciacqua A, Perticone M, Laino I, Miceli S, Mazzaferro D, Pascale A, Andreozzi F, Giorgio S. Insulin-like growth factor-1 and glomerular filtration rate in hypertensive patients. J Hypertens. 2009;27:613–617. doi: 10.1097/hjh.0b013e32831fda24. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359:1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 36.Morrow LA, O'Brien MB, Moller DE, Flier JS, Moses AC. Recombinant human insulin-like growth factor-I therapy improves glycemic control and insulin action in the type A syndrome of severe insulin resistance. J Clin Endocrinol Metab. 1994;79:205–210. doi: 10.1210/jcem.79.1.8027228. [DOI] [PubMed] [Google Scholar]
- 37.Moses AC, Young SC, Morrow LA, O'Brien M, Clemmons DR. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45:91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 38.Pratipanawatr T, Pratipanawatr W, Rosen C, Berria R, Bajaj M, Cusi K, Mandarino L, Kashyap S, Belfort R, DeFronzo RA. Effect of IGF-I on FFA and glucose metabolism in control and type 2 diabetic subjects. Am J Physiol Endocrinol Metab. 2002;282:E1360–1368. doi: 10.1152/ajpendo.00335.2001. [DOI] [PubMed] [Google Scholar]
- 39.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 40.Pete G, Hu Y, Walsh M, Sowers J, Dunbar JC. Insulin-like growth factor-I decreases mean blood pressure and selectively increases regional blood flow in normal rats. Proc Soc Exp Biol Med. 1996;213:187–192. doi: 10.3181/00379727-213-44049. [DOI] [PubMed] [Google Scholar]
- 41.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260–2265. doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher O, Gibson L, Johnson N, Altmann DR, Holly JM, Ashworth A, Peto J, Silva Idos S. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:2–19. [PubMed] [Google Scholar]
- 43.Rice T, Rankinen T, Province MA, Chagnon YC, Perusse L, Borecki IB, Bouchard C, Rao DC. Genome-wide linkage analysis of systolic and diastolic blood pressure: the Quebec Family Study. Circulation. 2000;102:1956–1963. doi: 10.1161/01.cir.102.16.1956. [DOI] [PubMed] [Google Scholar]
- 44.Glenn CL, Wang WY, Benjafield AV, Morris BJ. Linkage and association of tumor necrosis factor receptor 2 locus with hypertension, hypercholesterolemia and plasma shed receptor. Hum Mol Genet. 2000;9:1943–1949. doi: 10.1093/hmg/9.13.1943. [DOI] [PubMed] [Google Scholar]
- 45.Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf) 1994;41:85–93. doi: 10.1111/j.1365-2265.1994.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 46.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 47.DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1444–1451. [PubMed] [Google Scholar]
- 48.Colao A, Spiezia S, Di Somma C, Pivonello R, Marzullo P, Rota F, Musella T, Auriemma RS, De Martino MC, Lombardi G. Circulating insulin-like growth factor-I levels are correlated with the atherosclerotic profile in healthy subjects independently of age. J Endocrinol Invest. 2005;28:440–448. doi: 10.1007/BF03347225. [DOI] [PubMed] [Google Scholar]
- 49.Frystyk J. Free insulin-like growth factors -- measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14:337–375. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Frystyk J. Aging somatotropic axis: mechanisms and implications of insulin-like growth factor-related binding protein adaptation. Endocrinol Metab Clin North Am. 2005;34:865–876. viii. doi: 10.1016/j.ecl.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.