Abstract
Adipose tissue expresses all components of the renin-angiotensin system including angiotensinogen (AGT). Recent studies have highlighted a potential role of AGT in adipose tissue function and homeostasis. However, some controversies surround the regulatory mechanisms of AGT in obese adipose tissue. In this context, we here demonstrated that the AGT messenger RNA (mRNA) level in human subcutaneous adipose tissue was significantly reduced in obese subjects as compared with nonobese subjects. Adipose tissue AGT mRNA level in obese mice was also lower as compared with their lean littermates; however, the hepatic AGT mRNA level remained unchanged. When 3T3-L1 adipocytes were cultured for a long period, the adipocytes became hypertrophic with a marked increase in the production of reactive oxygen species. Expression and secretion of AGT continued to decrease during the course of adipocyte hypertrophy. Treatment of the 3T3-L1 and primary adipocytes with reactive oxygen species (hydrogen peroxide) or tumor necrosis factor α caused a significant decrease in the expression and secretion of AGT. On the other hand, treatment with the antioxidant N-acetyl cysteine suppressed the decrease in the expression and secretion of AGT in the hypertrophied 3T3-L1 adipocytes. Finally, treatment of obese db/db mice with N-acetyl cysteine augmented the expression of AGT in the adipose tissue, but not in the liver. The present study demonstrates for the first time that oxidative stress dysregulates AGT in obese adipose tissue, providing a novel insight into the adipose tissue–specific interaction between the regulation of AGT and oxidative stress in the pathophysiology of obesity.
1. Introduction
Overactivity of the systemic renin-angiotensin system (RAS) is one of the central mechanisms for obesity-related metabolic disorders [1,2]. Notably, the major components of the RAS are expressed in various tissues including the heart, blood vessels, adipose tissue, and brain [3]; these comprise tissue RAS. A series of products are produced locally from angiotensinogen (AGT), the unique precursor of angiotensin peptides, and play a critical role in cardiovascular homeostasis [3,4].
Although AGT is produced mainly by the liver, adipose tissue is also considered as a source of AGT production [5]. In agreement with this notion, the adipose tissue expresses all components of the RAS, including AGT, renin, angiotensin I–converting enzyme, and angiotensin II type 1 receptor, in humans and rodents [6,7]. A previous study has demonstrated that AGT-deficient mice are low in blood pressure and body fat mass [8]. Moreover, adipocyte-specific transgenic overexpression of AGT on an AGT-deficient background was shown to augment plasma AGT level and rescue hypotension and leanness [9]. These results indicate that adipose tissue–derived AGT does contribute to the circulating AGT level and adipogenesis.
In rodent experiments, the AGT messenger RNA (mRNA) level in white adipose tissue has been shown to be regulated by the nutritional status; however, that in the liver was independent of the nutritional status [10,11]. In human cross-sectional studies, the AGT mRNA level in adipose tissue was shown to be higher in obese subjects [6,12]. On the other hand, another study reported that the AGT mRNA level in adipose tissue was significantly lower in obese individuals [13]. Elevation of AGT expression in adipose tissue in obese individuals thus remains controversial [14].
Several studies have shown that increased oxidative stress is a manifestation of obesity-related metabolic derangement [15–17]. In fact, in humans, oxidative stress is critically associated with atherosclerosis, hypertension, and diabetes mellitus [18,19]. Oxidative stress is also related with the RAS. Angiotensin II is a potent inducer of reactive oxygen species (ROS) in a variety of tissues [20–22]. In the liver and kidney, increased ROS has been reported to increase AGT gene expression [23–26]. Also in obese adipose tissue, generation of ROS is exaggerated and is involved in adipose tissue dysfunction [17,27]. However, whether increased ROS may affect adipose AGT production remains to be elucidated.
In the present study, we demonstrated that the AGT mRNA level was reduced in obese adipose tissue in humans and mice and in hypertrophied 3T3-L1 adipocytes. In this context, we tested the hypothesis that increased oxidative stress would modulate AGT in obese adipose tissue.
2. Materials and methods
2.1. Subcutaneous abdominal adipose tissue biopsies in human subjects
The present study was performed according to the Declaration of Helsinki and approved by the Ethical Committee on Human Research of Kyoto University Graduate School of Medicine (2004, no. 553). Written informed consent was obtained from all subjects before the study.
Subcutaneous abdominal adipose tissue biopsies were obtained from 46 Japanese subjects (24 men and 22 women; age [mean ± SD], 46 ± 2.1 years). The body mass index (BMI) of the subjects ranged from 19 to 52 (mean ± SD, 30 ± 1.6) kg/m2. All subjects had been on stable therapy with lipid-lowering, antihypertensive, or hypoglycemic agents for at least 1 month before admission and continued with the same doses throughout the study period. Patients who received angiotensin I–converting enzyme inhibitors, angiotensin II receptor blockers, and steroid-related drugs were carefully excluded. For the study, subcutaneous abdominal adipose depots of the study subjects were excised from the periumbilical region under local anesthesia. The samples were immediately frozen in liquid nitrogen and stored at −80°C until use.
2.2. Mouse experiments
Male ob/ob mice (age, 12 weeks) were purchased from Oriental BioService (Kyoto, Japan) and housed in the animal facility of Kyoto University. Male db/db mice (age, 10 weeks) were purchased from Japan SLC (Hamamatsu, Japan) and housed in Seoul National University. The mice were allowed free access to food and water. For in vivo antioxidant treatment, the db/db mice were injected with N-acetyl cysteine (NAC; 150 mg/kg body weight; Sigma-Aldrich Japan, Tokyo, Japan) or the vehicle (phosphate-buffered saline) into the peritoneal cavity once daily for 1 week. All experimental procedures were approved by the Kyoto University Graduate School of Medicine Animal Research Committee and the Seoul National University Animal Experiment Ethics Committee.
2.3. Cell culture and isolation of primary adipocytes
3T3-L1 fibroblasts were cultured and differentiated into adipocytes as described previously [28]. Briefly, the 2-day postconfluent cells (designated as day 0) were incubated for 2 days with 10% fetal bovine serum (FBS)/Dulbecco modified Eagle medium (DMEM), 0.5 mmol/L 3-isobutyl-1-methylxanthine, 0.25 µmol/L dexamethasone, and 1 µg/mL insulin. The cells were then incubated for 2 days in 10% FBS/DMEM with insulin and, thereafter, incubated in 10% FBS/DMEM that was changed on every alternate day. Oil red O staining was performed as described [29].
Primary adipocytes were isolated from epididymal fat pads of 9-week-old male C57BL/6J mice (purchased from Oriental BioService, Kyoto, Japan). Epididymal fat pads were harvested, minced into 2- to 3-mm pieces, and digested using 0.8 mg/mL collagenase (Sigma-Aldrich Japan) in DMEM for 30 minutes at 37°C in a shaking water bath. After the digestion with collagenase, cells were filtered through a 250-µm nylon filter and centrifuged at 1000 rpm for 30 seconds. The suspended mature adipocytes were separated from the pelleted stromovascular fraction and washed 3 times in DMEM for experiments.
2.4. Determination of adipocyte size
The cells were fixed with 2% osmium tetroxide and passed through a 250-µm nylon filter to remove the fibrous elements, and the cells were washed extensively with isotonic saline. A total of 10 000 cells was analyzed using the Coulter Multisizer III (Beckman Coulter, High Wycombe, England) [30].
2.5. Quantitative real-time polymerase chain reaction
Total RNA was extracted from human and mouse adipose tissue by using a QIAGEN RNeasy Mini Kit (QIAGEN Japan, Tokyo, Japan) and from cultured adipocytes by using Trizol Reagent (Invitrogen, Carlsbad, CA). Complementary DNA was then synthesized by using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Taqman polymerase chain reactions (PCRs) for human AGT, mouse AGT, mouse monocyte chemoattractant protein 1 (MCP-1), mouse interleukin 6 (IL-6), and mouse tumor necrosis factor α (TNFα) were performed using the ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA). The sequences of probes and primers are summarized in Table 1.
Table 1.
Sequences of Taqman PCR primers and probes
| Gene name Genbank accession no. |
Forward primer Reverse primer |
Probe (5′-FAM, 3′-TAMRA) |
|---|---|---|
| Human AGT | GGTGGAGGGTCTCACTTTCCA | CCCTCAACTGGATGAAGAAACTGTCTCC |
| NM_000029 | ATGGTCAGGTGGATGGTCCG | |
| Mouse Agt | ACACCTACGTTCACTTCCAAG | ATGAGAGGTTTCTCTCAGCTGCCTGGA |
| NM_007428 | CCGAGATGCTGTTGTCCAC | |
| Mouse Ccl2 (MCP-1) | TTGGCTCAGCCAGATGC | CCCCACTCACCTGCTGCTACTCATTCA |
| NM_011333 | CCAGCCTACTCATTGGGATCA | |
| Mouse Il6 (IL-6) | ATGAAGTTCCTCTCTGCAAGAG | CACCAGCATCAGTCCCAAGAAGGCA |
| NM_031168 | GTAGGGAAGGCCGTGGTTG | |
| Mouse Tnf (TNFα) | TCTCTTCAAGGGACAAGGCTG | CCCGACTACGTGCTCCTCACCCA |
| NM_013693 | ATAGCAAATCGGCTGACGGT |
The sequences of primers and probes for each gene used in the present study are summarized.
2.6. Enzyme-linked immunosorbent assay
The AGT protein level in the culture media was measured by sandwich-type enzyme-linked immunosorbent assay (ELISA) as described [31]. Similarly, the MCP-1 and IL-6 protein levels were detected by using an ELISA kit (R&D Systems, Minneapolis, MN).
2.7. Determination of ROS
The ROS activity was determined by the nitroblue tetrazolium (NBT) assay [32]. Reduced NBT (formazan) was dissolved in 50% acetic acid, and the absorbance of the supernatant was determined at 560 nm.
2.8. Statistical analysis
The data are presented as the mean ± SE. Unpaired Student t test was used for comparisons with the control group. The differences were accepted as significant at a level of P < .05.
3. Results
3.1. AGT mRNA expression level in adipose tissue from obese humans and mice
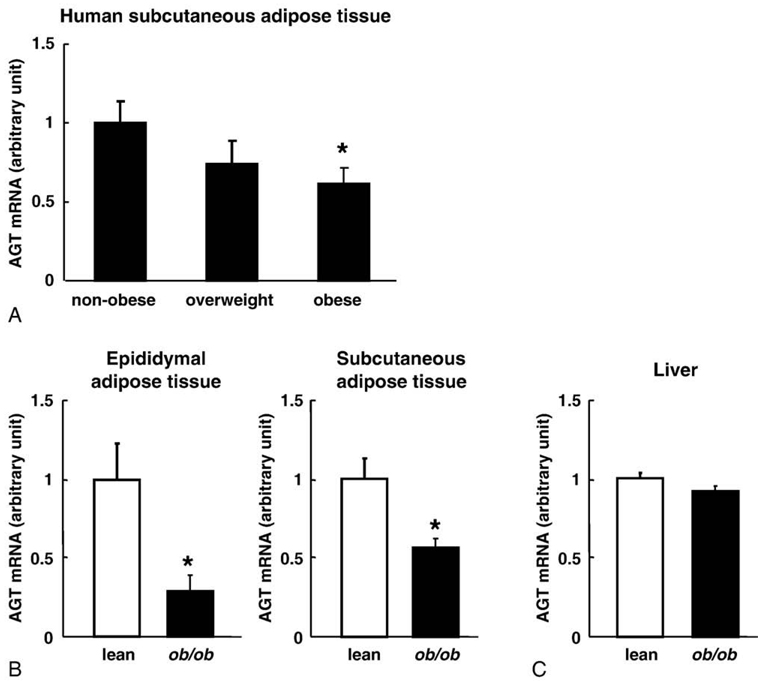
To explore the impact of obesity on AGT gene expression in human adipose tissue, we performed subcutaneous abdominal adipose tissue biopsies from 46 subjects with a wide range of BMI. The AGT mRNA level was significantly reduced by 61% in the obese subjects as compared with the nonobese subjects (Fig. 1A).
Fig. 1.
The AGT mRNA levels in obese adipose tissue from humans and mice. A, The relation between the AGT mRNA level in subcutaneous abdominal adipose tissue and the degree of obesity in humans: nonobese (BMI <25), n = 20; overweight (25 ≤ BMI < 30), n = 13; obese (BMI ≥30), n = 13. B, Comparison of the adipose tissue AGT mRNA levels in 12-week-old male ob/ob mice (n = 4; mean body weight, 60 ± 0.7 g) and their lean littermates (n = 4; mean body weight, 29 ± 0.3 g). Left: epididymal adipose tissue depots. Right: subcutaneous abdominal adipose tissue depots. C, Comparison of the hepatic AGT mRNA level between the ob/ob mice (n = 4) and their lean littermates (n = 4). The mRNA level was examined by real-time PCR and normalized to that of 18S ribosomal RNA (rRNA). The data are expressed as the mean ± SE. *P <.05 as compared with the nonobese subjects or the lean littermates.
To verify the obesity-related decrease in adipose AGT expression, we analyzed adipose tissue from genetically obese mice. In 12-week-old male ob/ob mice (mean body weight, 60 ± 0.7 g), the AGT mRNA level was significantly decreased in both epididymal (29%) and subcutaneous (57%) adipose depots as compared with their lean littermates (mean body weight, 29 ± 0.3 g) (Fig. 1B). On the other hand, the AGT mRNA levels in the liver remained unaltered in both groups (Fig. 1C).
Similar results were observed in case of the diet-induced obese (DIO) mice (12-week-old male C57BL/6J mice fed with a high-fat/high-sucrose diet for 4 weeks). The AGT mRNA level in the adipose tissue of the DIO mice (mean body weight, 40 ± 0.8 g) was significantly lower than that in the adipose tissue of their lean littermates (mean body weight, 30 ± 0.4 g) (P <.05); however, the hepatic AGT mRNA level remained unchanged in both groups (Yasue et al, unpublished observations). These results indicate that the AGT mRNA level was decreased exclusively in the obese adipose tissue in both humans and mice.
3.2. AGT expression during the course of hypertrophy in the 3T3-L1 adipocytes
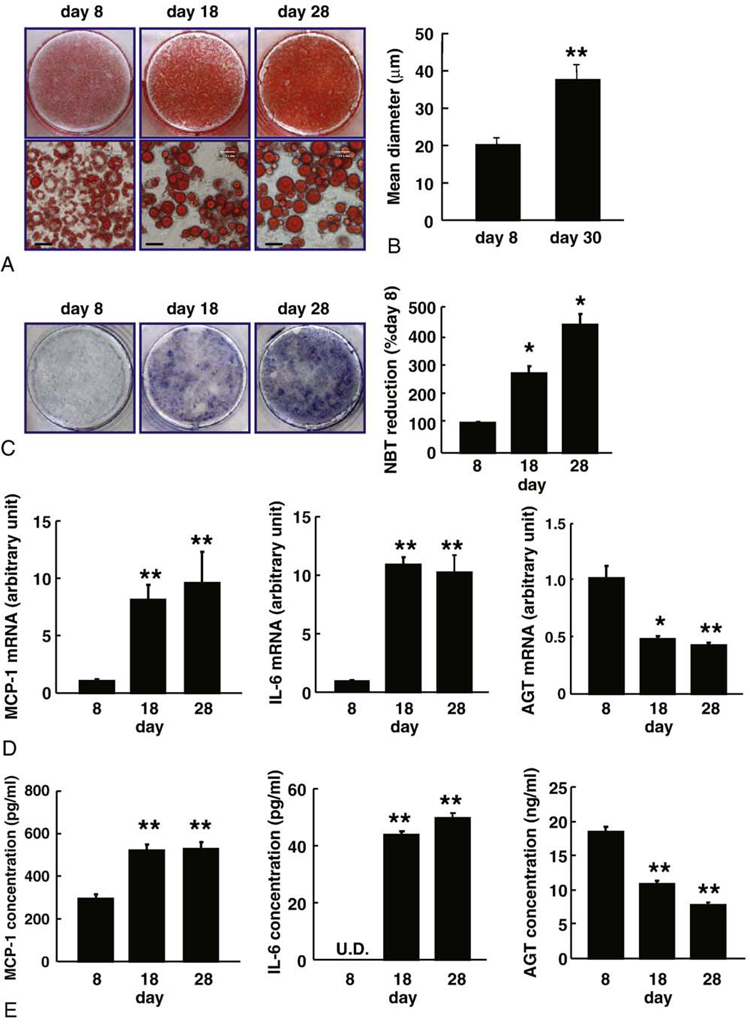
To explore the mechanism by which AGT is decreased in obese adipose tissue, we analyzed hypertrophied adipocytes. 3T3-L1 fibroblasts were completely differentiated into adipocytes for 8-day incubation with induction media [28]. Consistent with a previous report [33], the AGT mRNA level in differentiated 3T3-L1 adipocytes (day 8) was significantly elevated by 15-folds in comparison with 3T3-L1 fibroblasts (day 0). For generating hypertrophied adipocytes, 3T3-L1 adipocytes were cultured up to 30 days after the induction of differentiation. Oil red O staining exhibited a gradual increase in lipid accumulation from day 8 to day 28. The adipocytes displayed unilocular lipid droplets on days 18 and 28 (Fig. 2A).
Fig. 2.
The AGT expression during the course of hypertrophy in the 3T3-L1 adipocytes. A, Oil red O staining of the 3T3-L1 adipocytes on days 8, 18, and 28 after induction of differentiation. Bar = 30 µm. B, Size of the 3T3-L1 adipocytes on days 8 and 30. Adipocyte size was measured using a Coulter Multisizer III. C, The ROS production during adipocyte hypertrophy. The ROS production was assessed by the NBT assay. Dark blue formazan was dissolved, and the absorbance was determined at 560 nm (n = 3). D, The MCP-1, IL-6, and AGT mRNA levels in the 3T3-L1 adipocytes on days 8, 18, and 28 (n = 4). The mRNA level was examined by real-time PCR and normalized to that of 18S rRNA. E, The AGT protein concentration in the culture media. The MCP-1, IL-6, and AGT concentrations in the 3T3-L1 adipocytes on days 8, 18, and 28 were analyzed by ELISA (n = 4). Results are representatives of at least 3 independent experiments. The data are expressed as the mean ± SE. *P < .05 and **P < .01 as compared with the value of day 8. U.D. indicates undetectable.
The mean diameter of the adipocytes as assessed by the Coulter Multisizer III was 20.2 µm on day 8 and 37.5 µm on day 30 (Fig. 2B). During the course of adipocyte hypertrophy, ROS production increased 2.7-folds (day 18) and 4.3-folds (day 28) in comparison with the levels on day 8 (Fig. 2C). The mRNA and protein levels of MCP-1 and IL-6 were elevated substantially on days 18 and 28 (Fig. 2D and E, respectively). The AGT mRNA level was significantly lower on days 18 (48%) and 28 (42%) than that on day 8 (Fig. 2D). The AGT concentration in the culture media was decreased on days 18 (59% of the initial value) and 28 (42% of the initial value) (Fig. 2E). These results suggest that AGT expression and secretion were decreased in the hypertrophied adipocytes.
3.3. Impact of TNFα on the expression and secretion of AGT in adipocytes
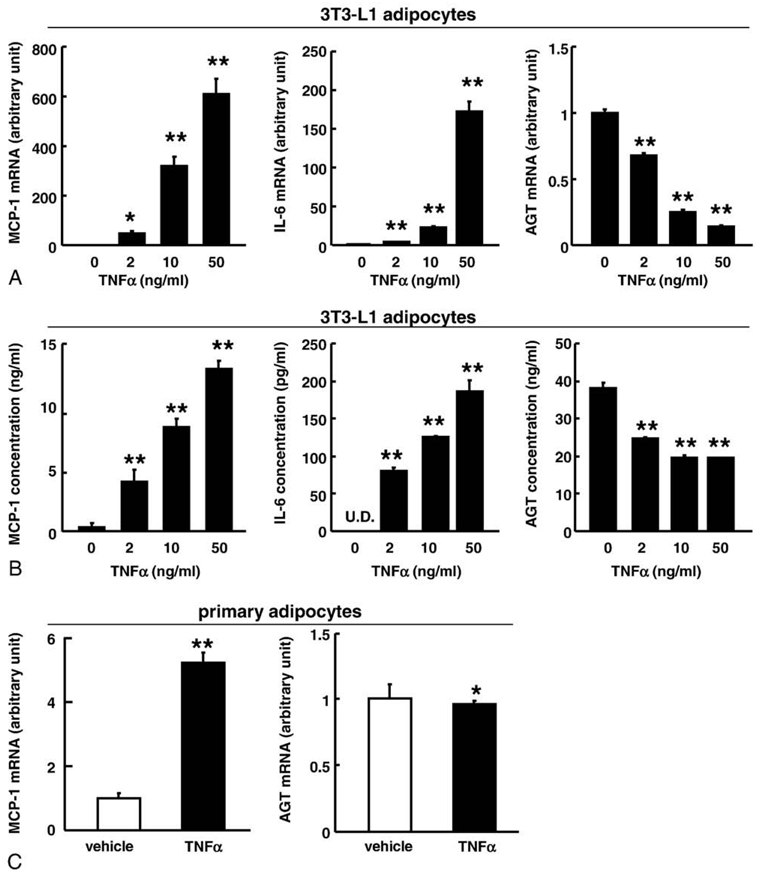
Tumor necrosis factor α plays a critical role in the pathophysiology of inflammation and oxidative stress [27,34,35]. To explore the impact of TNFα on the expression and secretion of AGT in adipocytes, the differentiated 3T3-L1 adipocytes (day 8) were treated with TNFα (Sigma-Aldrich Japan) for 24 hours. Treatment with TNFα decreased the AGT mRNA level in a dose-dependent manner along with a concomitant increase in the MCP-1 and IL-6 mRNA levels (Fig. 3A). The AGT protein level in the culture media decreased in parallel to the AGT mRNA level (Fig. 3B).
Fig. 3.
Impact of TNFα on the expression and secretion of AGT in the 3T3-L1 adipocytes. A, The AGT, MCP-1, and IL-6 mRNA level in the 3T3-L1 adipocytes (day 8) treated with TNFα for 24 hours (n = 4). The mRNA level was examined by real-time PCR and normalized to that of 18S rRNA. B, The AGT protein concentration in the culture media in the 3T3-L1 adipocytes (day 8) treated with TNFα for 24 hours (n = 4). The protein level was assessed by ELISA. C, The AGT and MCP-1 mRNA level in the primary adipocytes treated with TNFα (10 ng/mL) for 24 hours (n = 4). The mRNA level was examined by real-time PCR and normalized to that of 18S rRNA. Results are representatives of at least 3 independent experiments. The data are expressed as the mean ± SE. *P < .05 and **P < .01 as compared with the control value.
We also investigated the effects of TNFα on primary adipocytes. Similarly to 3T3-L1 adipocytes, treatment with TNFα (10 ng/mL) for 24 hours slightly but significantly decreased AGT mRNA level and substantially increased MCP-1 mRNA level (Fig. 3C).
3.4. Impact of oxidative stress on the expression and secretion of AGT in adipocytes
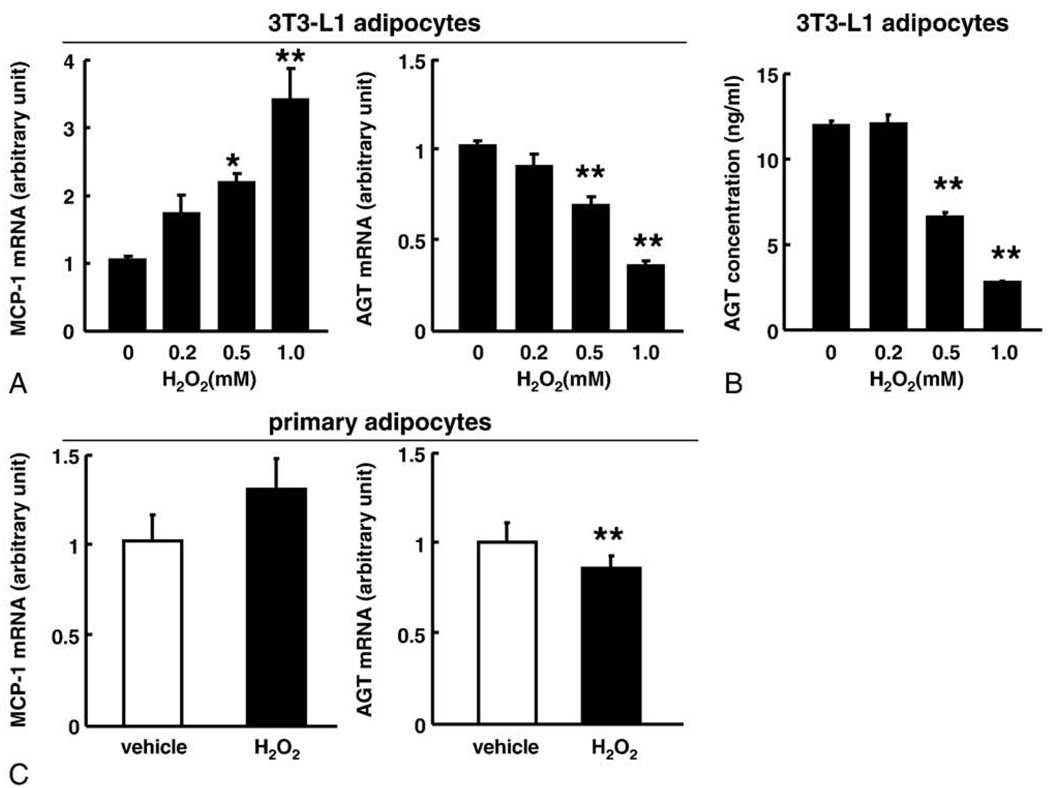
To explore the impact of oxidative stress on the expression and secretion of AGT in adipocytes, differentiated 3T3-L1 adipocytes (day 8) were exposed to a specific ROS molecule, hydrogen peroxide (H2O2), for 24 hours [17,36]. Incubation of adipocytes with H2O2 significantly increased the MCP-1 mRNA level, consistent with a previous report [17]. In contrast, H2O2 diminished the AGT mRNA level up to 35% of the initial value in a dose-dependent manner (Fig. 4A). The AGT protein level in the culture media also decreased up to 23% of the initial value (Fig. 4B).
Fig. 4.
Impact of oxidative stress on the expression and secretion of AGT in the 3T3-L1 adipocytes. A, The AGT and MCP-1 mRNA level in the 3T3-L1 adipocytes (day 8) treated with H2O2 for 24 hours (n = 4). The mRNA level was examined by real-time PCR and normalized to that of 18S rRNA. B, The AGT protein level in the culture media of the 3T3-L1 adipocytes (day 8) treated with H2O2 for 24 hours (n = 4). The protein concentration was assessed by ELISA. C, The AGT and MCP-1 mRNA level in the primary adipocytes treated with H2O2 (1 mmol/L) for 24 hours (n = 4). The mRNA level was examined by real-time PCR and normalized to that of 18S rRNA. Results are representatives of at least 3 independent experiments. The data are expressed as the mean ± SE. *P < .05 and **P < .01 as compared with the control value.
Similar to 3T3-L1 adipocytes, H2O2 treatment (1 mmol/L, 24 hours) significantly decreased AGT mRNA level in primary adipocytes (Fig. 4C). The H2O2 treatment tended to increase the MCP-1 mRNA level.
3.5. Effect of antioxidant treatment on the expression and secretion of AGT in adipocytes
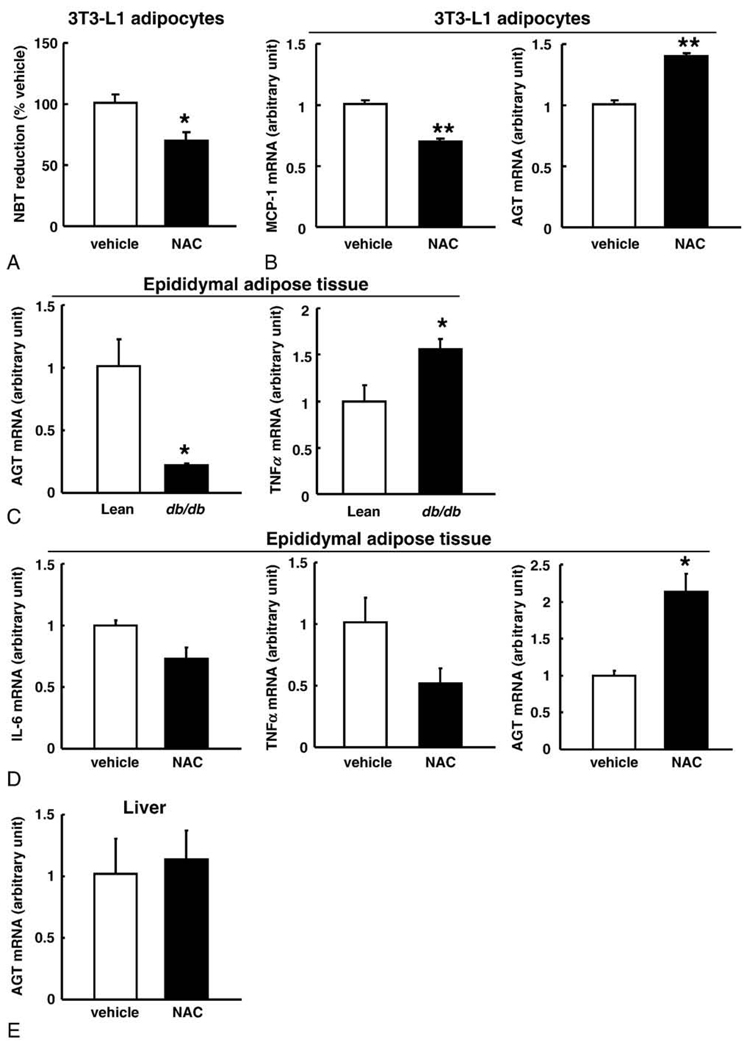
We examined whether inhibition of ROS generation could nullify the decrease in AGT gene expression and AGT secretion in obese adipose tissue. First, we treated 3T3-L1 adipocytes with the antioxidant NAC (10 mmol/L) for 10 days (days 8–18). Without the NAC treatment, the adipocytes had become hypertrophic and increased ROS production in this period (Fig. 2B and C). The NBT assay revealed that NAC treatment significantly suppressed ROS production (Fig. 5A). Although ROS production was reported to potentiate adipocyte differentiation in early phase [37], the ROS suppression with the NAC treatment in our experiments did not cause morphologic changes in hypertrophied adipocytes compared with the vehicle treatment. The NAC treatment inhibited the increase in MCP-1 expression (Fig. 5B). The AGT mRNA level was significantly elevated with NAC treatment (Fig. 5B).
Fig. 5.
Effect of antioxidant treatment on the expression and secretion of AGT in the adipocytes. A, Suppression of ROS generation in the 3T3-L1 adipocytes treated with NAC (10 mmol/L) for 10 days (n = 3). The ROS was estimated by the NBT assay. B, The MCP-1 and AGT mRNA levels in the 3T3-L1 adipocytes incubated with NAC (10 mmol/L) (n = 8). The mRNA level was examined by real-time PCR and normalized to that of 18S rRNA. Results are representatives of at least 3 independent experiments. C, Comparison of the AGT and TNFα mRNA levels between 10-week-old male db/db mice (n = 4; mean body weight, 48 ± 1.5 g) and their lean littermates (n = 4; mean body weight, 28 ± 1.0 g) in epididymal adipose tissue. D, The level of IL-6, TNFα, and AGT mRNA in the epididymal adipose tissue depots of obese db/db mice treated with NAC (150 mg/kg body weight) or vehicle (phosphate-buffered saline) once daily for 1 week (n = 3). E, The AGT mRNA level in the liver of obese db/db mice treated with NAC or vehicle for 1 week (n = 3). The mRNA level was examined by real-time PCR and normalized to that of cyclophilin mRNA. The data are expressed as the mean ± SE. *P < .05 and **P < .01 as compared with the control value.
To test whether such phenomenon is reproducible in obese adipose tissue where ROS production is exaggerated [17], we administered NAC to obese db/db mice once daily for 1 week. Similar to the obese ob/ob mice and DIO mice, in obese db/db mice (mean body weight, 48 ± 1.5 g), the AGT mRNA level in epididymal adipose tissue was markedly decreased to 22% as compared with their lean littermates (mean body weight, 28 ± 1.0 g) (Fig. 5C). In contrast, the TNFα mRNA level in epididymal adipose tissue was significantly higher in db/db mice than in their lean littermates (Fig. 5C). Both systemic and local (adipose tissue) oxidative stress was elevated substantially in obese db/db mice [38]. Notably, in db/db mice, NAC treatment significantly reduced the oxidative stress also in adipose tissue [38].
In the NAC treatment group, the AGT mRNA level in the epididymal adipose depots increased significantly by 2.1-folds compared with that in the vehicle group, whereas the IL-6 (P = .052) and TNFα (P = .10) mRNA levels tended to decrease in the NAC treatment group (Fig. 5D). On the other hand, the hepatic AGT mRNA level remained unchanged in both groups (Fig. 5E).
4. Discussion
The major finding of the present study is that oxidative stress dysregulates AGT in adipose tissue in obese humans and rodents. The AGT mRNA level was decreased in both obese adipose tissue and hypertrophied adipocytes, in which oxidative stress was exaggerated. Exposure of oxidative stress decreased AGT expression not only in the adipocyte cell line but also in primary adipocytes. The decrease in AGT expression was rescued by treatment with the antioxidant both in vivo and in vitro. Such obesity-associated changes in AGT in the adipose tissue were not observed in the liver.
The AGT regulation in obese adipose tissue has long been analyzed, but results were inconsistent [6,12,13]. We here demonstrated that the AGT mRNA level in adipose tissue was reduced in both obese humans and mice (Fig. 1). In obese mice, there seem to be no apparent depot-specific (subcutaneous and epididymal adipose depots) or strain-specific (ob/ob, db/db, and DIO mice) differences in the fall of AGT in adipose tissue. The AGT mRNA level was decreased also in hypertrophied 3T3-L1 adipocytes (Fig. 2). These results are consistent with a previous report using differentiation system of human adipocytes in primary culture, where the AGT mRNA level increased in differentiation process, but decreased in further culture process [39].
In previous experiments, several hormonal and metabolic changes associated with obesity influence AGT expression in adipocytes; however, due to species differences and experimental conditions, there are controversies around the results [39–42]. On the other hand, our results indicate that AGT expression is decreased in obese adipose tissue. Reactive oxygen species (H2O2) decreased AGT expression in both 3T3-L1 adipocytes and primary adipocytes (Fig. 4). On the other hand, elimination of ROS with antioxidant increased AGT expression not only in hypertrophied 3T3-L1 adipocytes but also in adipose tissue from obese mice (Fig. 5). The oxidative stress–mediated decrease in adipose AGT is reproduced in our various experiments.
Several studies have suggested the augmentation of AGT by oxidative stress in the liver and kidney. In the liver, angiotensin II is known to enhance AGT expression via ROS generation [23], resulting in a positive feedback loop of AGT production [43]. In addition, oxidative stress mediated by hyperglycemia and hypertension has been shown to augment the expression of AGT in the rodent kidney [25,26]. In turn, elevated expression of AGT has been shown to activate renal RAS and considerably contribute to renal injury [26]. On the other hand, our data support a notion that oxidative stress “decreases” expression and secretion of AGT in obese adipose tissue, implying that regulation of AGT in adipose tissue may be distinct from other tissues in response to oxidative stress.
The clinical or pathophysiologic implications of decreased AGT in obese adipose tissue still remain unclear. Although further studies are warranted, the notion that adipose tissue RAS is involved in the control of adipogenesis and adipose tissue mass [44] tempts us to speculate that tissue-specific decrease of AGT in obese adipose tissue may serve as a defense against further exacerbation of adiposity. In obese adipose tissue, exaggerated oxidative stress affects the expression of a variety of genes [17]. Representatively, ROS induces the proinflammatory TNFα but suppresses the anti-inflammatory adiponectin in murine adipose tissue [17]. Glutathione peroxidase 3 (GPx3), an antioxidant enzyme secreted from the adipose tissue and kidney, is known to be decreased by oxidative stress exclusively in adipose tissue in obese db/db mice [38]. Notably, in vivo administration of an antioxidant was shown to rescue the decrease in GPx3 expression only in adipose tissue, but not in the kidney [38]. In this context, AGT shares close similarity with adiponectin and GPx3 in terms of the response to oxidative stress in adipose tissue.
Tissue-specific dysregulation of AGT has also been observed in inflammatory response [45,46]. Hepatic AGT is shown to increase by inflammatory stimuli via the acute-phase responsive element (APRE) on the promoter region of the AGT gene [43,47]. In rats treated with lipopolysaccharide, AGT mRNA level was shown to increase in the liver, aorta, and adrenal gland, but remained unchanged in the kidney [45]. Furthermore, in transgenic mice with cardioselective overexpression of TNFα, expression of AGT was decreased exclusively in the heart [46]. In the present study, we demonstrated that TNFα decreased the expression and secretion of AGT in 3T3-L1 adipocytes (Fig. 3). Considering that chronic, low-grade inflammation is a manifestation of obese adipose tissue [48,49], our results suggest that AGT is inversely regulated by inflammation in obese adipose tissue.
Previous works have raised a possibility that the inflammatory responses to AGT in adipose tissue and liver are controlled by distinct mechanisms [50]. In cultured adipocytes, inflammatory signals transcriptionally decrease AGT by the inhibition of APRE [50]; however, in cultured hepatocytes, nuclear factor–κB signaling augments AGT by the activation of APRE [47]. Importantly, the intracellular signaling involved in oxidative stress and inflammation interact and share, at least in part, common pathways in a tissue-specific manner [18,19]. In this context, tissue-specific dysregulation of AGT by oxidative stress is reminiscent of the case in inflammatory signals; and a possible link between the dysregulation of AGT and oxidative stress in obese adipose tissue may provide a fresh clue to dissect the pathophysiology of obesity. For example, we would suggest the one possibility that oxidative stress–induced suppression of adipose tissue RAS via the decrease in AGT may control adipose tissue function including adipocyte differentiation, lipolysis, and local blood flow [44].
In summary, the present study demonstrates for the first time that oxidative stress dysregulates AGT in obese adipose tissue in humans and rodents as well as in cultured adipocytes with hypertrophy. Our results support a concept that oxidative stress–dependent decrease in AGT may be a unique facet of dysfunction in obese adipose tissue.
Acknowledgment
We are grateful to A Katsurada (Departments of Medicine and Physiology, and Hypertension and Renal Center of Excellence, Tulane University Health Sciences Center, New Orleans, LA), M Tabuchi (Department of Pharmacology, Kinki University School of Medicine, Osaka-sayama, Japan), M Okada (Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan), and M Kasuga (Research Institute, International Medical Center of Japan, Tokyo, Japan) for help and discussion. We also thank A Ryu, S Maki, and M Nagamoto for assistance and Y Kobayashi and T Fukui for discussion.
This work was supported in part by Grants-in-Aid (MEXT, Japan) B2 and S2, Takeda Medical Research Foundation, Smoking Research Foundation, Lilly Research Foundation, Research on Measures for Intractable Diseases (Health and Labor Science Research Grant), Special Coordination Funds for Promoting Science and Technology (JST), Research Grant of National Cardiovascular Center, Sankyo Research Foundation, the Korea Research Foundation Grant (KRF-2008-005-J00203), and the National Research Laboratory Program (ROA-2004-000-10359-0) funded by the Korean Government.
Footnotes
The authors of this manuscript have nothing to declare.
Institutional approval: The human study was approved by the ethics committee for human research of the Kyoto University Graduate School of Medicine (2004, no. 553). Written informed consent was obtained from all subjects prior to the study. All animal experimental procedures were approved by the Kyoto University Graduate School of Medicine Animal Research Committee and the Seoul National University Animal Experiment Ethics Committee.
References
- 1.Engeli S. Role of the renin-angiotensin-aldosterone system in the metabolic syndrome. Contrib Nephrol. 2006;151:122–134. doi: 10.1159/000095324. [DOI] [PubMed] [Google Scholar]
- 2.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77(6 Pt 2):I4–I13. [PubMed] [Google Scholar]
- 4.Raizada V, Skipper B, Luo W, Griffith J. Intracardiac and intrarenal renin-angiotensin systems: mechanisms of cardiovascular and renal effects. J Investig Med. 2007;55:341–359. doi: 10.2310/6650.2007.00020. [DOI] [PubMed] [Google Scholar]
- 5.Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988;11(6 Pt 2):591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- 6.Giacchetti G, Faloia E, Mariniello B, Sardu C, Gatti C, Camilloni MA, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15:381–388. doi: 10.1016/s0895-7061(02)02257-4. [DOI] [PubMed] [Google Scholar]
- 7.Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 8.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 9.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 10.Frederich RC, Jr, Kahn BB, Peach MJ, Flier JS. Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- 11.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 12.Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes Res. 2000;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- 13.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 14.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, et al. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 15.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 16.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 19.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress–induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 21.Das DK, Maulik N, Engelman RM. Redox regulation of angiotensin II signaling in the heart. J Cell Mol Med. 2004;8:144–152. doi: 10.1111/j.1582-4934.2004.tb00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachse A, Wolf G. Angiotensin II–induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 23.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type–dependent effects on the nuclear factor–kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000;212:155–169. [PubMed] [Google Scholar]
- 24.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- 25.Brezniceanu ML, Liu F, Wei CC, Tran S, Sachetelli S, Zhang SL, et al. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007;71:912–923. doi: 10.1038/sj.ki.5002188. [DOI] [PubMed] [Google Scholar]
- 26.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–927. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 28.Frost SC, Lane MD. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- 29.Fujimoto M, Masuzaki H, Tanaka T, Yasue S, Tomita T, Okazawa K, et al. An angiotensin II AT1 receptor antagonist, telmisartan augments glucose uptake and GLUT4 protein expression in 3T3-L1 adipocytes. FEBS Lett. 2004;576:492–497. doi: 10.1016/j.febslet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Sakai T, Sakaue H, Nakamura T, Okada M, Matsuki Y, Watanabe E, et al. Skp2 controls adipocyte proliferation during the development of obesity. J Biol Chem. 2007;282:2038–2046. doi: 10.1074/jbc.M608144200. [DOI] [PubMed] [Google Scholar]
- 31.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52:1457–1463. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- 33.Saye JA, Cassis LA, Sturgill TW, Lynch KR, Peach MJ. Angiotensinogen gene expression in 3T3-L1 cells. Am J Physiol. 1989;256(2 Pt 1):C448–C451. doi: 10.1152/ajpcell.1989.256.2.C448. [DOI] [PubMed] [Google Scholar]
- 34.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 35.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamigaki M, Sakaue S, Tsujino I, Ohira H, Ikeda D, Itoh N, et al. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339:624–632. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, et al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008;22:2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E731–F740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- 40.Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am J Physiol. 1997;273(1 Pt 2):R236–R242. doi: 10.1152/ajpregu.1997.273.1.R236. [DOI] [PubMed] [Google Scholar]
- 41.Aubert J, Safonova I, Negrel R, Ailhaud G. Insulin down-regulates angiotensinogen gene expression and angiotensinogen secretion in cultured adipose cells. Biochem Biophys Res Commun. 1998;250:77–82. doi: 10.1006/bbrc.1998.9185. [DOI] [PubMed] [Google Scholar]
- 42.Harte A, McTernan P, Chetty R, Coppack S, Katz J, Smith S, et al. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation. 2005;111:1954–1961. doi: 10.1161/01.CIR.0000161954.17870.5D. [DOI] [PubMed] [Google Scholar]
- 43.Morgan L, Broughton Pipkin F, Kalsheker N. Angiotensinogen: molecular biology, biochemistry and physiology. Int J Biochem Cell Biol. 1996;28:1211–1222. doi: 10.1016/s1357-2725(96)00086-6. [DOI] [PubMed] [Google Scholar]
- 44.Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose reninangiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyui N, Tamura K, Yamaguchi S, Nakamaru M, Ishigami T, Yabana M, et al. Tissue angiotensinogen gene expression induced by lipopolysaccharide in hypertensive rats. Hypertension. 1997;30:859–867. doi: 10.1161/01.hyp.30.4.859. [DOI] [PubMed] [Google Scholar]
- 46.Flesch M, Hoper A, Dell’Italia L, Evans K, Bond R, Peshock R, et al. Activation and functional significance of the renin-angiotensin system in mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2003;108:598–604. doi: 10.1161/01.CIR.0000081768.13378.BF. [DOI] [PubMed] [Google Scholar]
- 47.Ron D, Brasier AR, Habener JF. Transcriptional regulation of hepatic angiotensinogen gene expression by the acute-phase response. Mol Cell Endocrinol. 1990;74:C97–C104. doi: 10.1016/0303-7207(90)90221-s. [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor–alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ron D, Brasier AR, McGehee RE, Jr, Habener JF. Tumor necrosis factor–induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP) J Clin Invest. 1992;89:223–233. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]