Abstract
Developmental plasticity in helminth life cycles serves, in most cases, to increase the probability of transmission between hosts, suggesting that the necessity to achieve transmission is a prominent selective pressure in the evolution of this phenomenon. Some evidence suggests that digenean trematodes from the genus Schistosoma are also capable of limited developmental responses to host factors. Here we review the currently available data on this phenomenon and attempt to draw comparisons with similar processes in the life cycles of other helminths. At present the biological significance of developmental responses by schistosomes under laboratory conditions remains unclear. Further work is needed to determine whether developmental plasticity plays any role in increasing the probability of schistosome transmission and life cycle propagation under adverse conditions, as it does in other helminth life cycles.
Keywords: Schistosoma, Blood fluke, Helminth, Development, Transmission
1. Introduction
In comparison to other infectious agents such as protists and viruses, helminths are metazoans with significantly larger genomes that encode for complex programmes of development. One aspect of the increased developmental complexity of helminths is that, for a given species, different developmental outcomes may be possible at certain stages in the life cycle. In the context of a helminth infection, developmental plasticity on the part of the pathogen may have important consequences for the host, determining the type and degree of pathology that develops, or whether pathology occurs at all. Variability of infection outcome is a hallmark of helminth infections (Maizels et al., 1993) and variability in helminth development between individual hosts may contribute to the variability of infection outcomes observed in an affected host population.
In most cases, developmental responses in helminth life cycles serve to increase the probability of transmission between hosts, suggesting that the necessity to achieve transmission is a dominant selective force in the evolution of plastic developmental programmes in helminths. The most widely studied and best understood examples of developmental plasticity are the various facultative forms of arrested larval development and hypobiosis observed in the life cycles of many nematodes. Comparisons have been drawn between developmental arrest in parasitic species and the well known dauer pathway of the free-living nematode Caenorhabditis elegans (Riddle and Albert, 1997). Arrested development of parasitic nematodes has received extensive and scholarly review by Michel (1974) and Gibbs (1986). Here we provide only a brief overview of this important subject and the reader is referred to these sources for more detailed information. Other examples of developmental plasticity can be found in nematodes. Paratenesis, the use of transport hosts to facilitate transmission, involves plasticity in host usage and a form of facultative arrested development. Members of the Strongyloididae display unique developmental plasticity in the form of developmental switching between free-living and parasitic forms.
While developmental plasticity is most thoroughly documented in nematodes, notable examples are also known from various platyhelminths. We briefly review examples from the Platyhelminthes and draw parallels, where possible, with developmental plasticity in nematodes. Finally, we review the evidence for the occurrence of developmental plasticity in schistosomes. The prevailing view is that schistosome development does not vary between individual hosts but proceeds in a similar fashion in all infected individuals. Clearly, schistosome development is not subject to the dramatic developmental plasticity observed in the life cycles of Toxocara or Alaria (see below), but limited developmental responses to host factors can be observed under laboratory conditions. It remains to be seen whether these developmental responses have implications for the epidemiology of these medically significant pathogens.
2. The dauer larva of C. elegans and homologous stages of parasitic nematodes
The dauer larvae of C. elegans and other free-living nematodes are facultative arrested third stage (L3) larvae that are adapted for dispersal and survival when environmental conditions (food availability, pheromone concentration, temperature) are unsuitable for life cycle completion (Riddle and Albert, 1997). Dauer formation is initiated in response to environmental factors and many of the genes controlling the dauer response in C. elegans have been identified (Riddle and Albert, 1997). Compared with reproductive L3 larvae, the C. elegans dauer displays anatomical, behavioural and metabolic adaptations that facilitate survival and dispersal (Riddle and Albert, 1997): for example, radial shrinkage of the body and closure of the buccal capsule and anus occurs, feeding is suspended, nictation behaviour is initiated and tricarboxylic acid cycle activity is reduced. It is now clear that the infectious larval stages (which are also frequently L3 stages) of many parasitic nematodes are morphologically, behaviourally and metabolically analogous to the C. elegans dauer larva (Hawdon and Schad, 1991; Hotez et al., 1993; Blaxter and Bird, 1997). Homologues of some of the genes involved in the C. elegans dauer programme have been identified in the genomes of parasitic nematodes (Gomez-Escobar et al., 1997, 1998, 2000). Given the conserved nature of many of the genes involved in dauer formation, their representation in the genomes of parasitic nematodes is not surprising, but it will be of considerable interest to determine whether these genes function in the development of infectious larvae. Most parasitic species, however, are obligate parasites, the infectious dauer larva being a constitutive, rather than facultative, component of the life cycle, resembling dauer-constitutive mutants of C. elegans (Hotez et al., 1993), and dauer larva formation in many nematode parasites does not therefore constitute a form of developmental plasticity. As discussed below, many parasitic nematodes, including some important pathogens, exhibit an additional facultative state of diapause after initiation of the parasitic phase of the life cycle. Given that the dauer programme has already been completed in these organisms, it is unclear whether this second arrested state represents a reiteration of the dauer pathway, involving the same or similar genes, or an additional parasite-specific developmental programme, involving different genes, for which there is no analogue in C. elegans.
3. Facultative arrested development in parasitic nematodes
Gibbs (1986) defined two broad categories of facultative developmental arrest in nematodes, distinguished by the factors believed to provide the impetus for arrest: (i) seasonally induced arrest and (ii) immune-mediated arrest. Seasonally induced arrest occurs in response to environmental factors, such as temperature, and resembles the diapause of many insects (Horak, 1981). Immunemediated arrest appears to occur in response to poorly defined or undefined host factors, which may in some cases be immunological and in other cases not. We therefore prefer the term host-induced arrest and while we discuss it here as a single entity, we acknowledge that this category may represent a heterogeneous group of different phenomena, as the host factors involved and the developmental responses to those factors are varied. Facultative developmental arrest occurs in a wide variety of nematodes from several clades (Blaxter et al., 1998), some examples of which are provided in Table 1.
Table 1.
Facultative developmental arrest in nematodes
| Genus | Cladea | Type of arrest | Reference |
|---|---|---|---|
| Dictyocaulus | V | Seasonal | Ayalew et al., 1974; Michel, 1974 |
| Ostertagia | V | Seasonal | Armour and Bruce, 1974 |
| Cooperia | V | Seasonal | Sommerville, 1960; Michel et al., 1970 |
| Hyostrongylus | V | Seasonal | Burden et al., 1970; Connan, 1971 |
| Obeliscoides | V | Seasonal | Fernando et al., 1971 |
| Haemonchus | V | Seasonal | Eysker, 1981 |
| Nematospiroides | V | Host-induced | Behnke and Parish, 1979 |
| Nematodirus | V | Seasonal | Waller and Thomas, 1983 |
| Oesophagostomum | V | Seasonal? | Gordon, 1949; Rossiter, 1964 |
| Cyathostome spp. | V | Seasonal | Gibson, 1953; Chiejina and Mason, 1977 |
| Ancylostoma | V | Seasonal and host-induced? | Schad et al., 1973; Schad, 1982 |
| Uncinaria | V | Seasonal? | Olsen and Lyons, 1962, 1965 |
| Strongyloides | IV | Host-induced | Moncol and Batte, 1966 |
| Ascaridia | III | Seasonal | Ikeme, 1970 |
| Toxocara | III | Host-induced | Sprent, 1958, 1961; Michel, 1974 |
| Neoascaris | III | Host-induced | Warren and Needham, 1969 |
| Habronema | III | Seasonal | Schwartz et al., 1931 |
Clades defined by Blaxter et al. (1998).
Facultative, seasonally induced arrest, frequently referred to as hypobiosis, occurs in parasitic nematodes where a significant portion of the life cycle occurs outside the host and is subject to potentially adverse environmental conditions. Hypobiosis is therefore particularly prevalent in the Strongylida, where it has been most extensively studied (Gibbs, 1986). Seasonally induced arrest has evolved to synchronise release of free-living larvae with environmental conditions suitable for their survival and subsequent development to infectious stages (Schad et al., 1973). To achieve synchronisation, development within the host is suspended at a pre-adult stage when environmental conditions unsuitable for free-living larval development prevail, and resumed as suitable environmental conditions return. This developmental response is particularly prevalent at high and low latitudes, where excessive cold and heat/dryness at specific times of year mitigates against survival of free-living larval stages. Environmental factors experienced by infectious larvae determine whether development is arrested after infection, and environmental temperatures are particularly relevant in this regard (Armour and Bruce, 1974; Schad, 1982; Fernandez et al., 1999). There is also a significant genetic component to the propensity of a nematode population to undergo hypobiosis (Smeal and Donald, 1981). Resumption of development in seasonally arrested larvae appears to occur spontaneously after a fixed period of arrest (Blitz and Gibbs, 1971), although roles for host factors in stimulating reactivation of arrested larvae have not been ruled out (Gibbs, 1986).
The selective advantage of this form of developmental plasticity where seasonally adverse environmental conditions occur is obvious, as parasites unable to continue transmission from one favourable season to the next would soon be eliminated from the population.
Host-induced arrest has also evolved to synchronise generation of infectious stages with conditions suitable for their survival, transmission and development. In contrast to seasonally induced arrest, however, the signals for host-induced arrest do not emanate from the environment but are host factors that exert their effect after infection. In some cases, immunity against the invading nematode has been implicated as a host factor that induces arrest (Behnke and Parish, 1979), and suppression of immunity can allow for resumption of nematode development (Behnke and Parish, 1979). In other species, obvious patterns of developmental arrest are clear, but the exact identity of the host factors signalling arrest is unknown. For example, in Toxocara canis, patent infections develop only in young dogs, while in older animals somatic migration ensues and larvae arrest in various tissues throughout the body (Sprent, 1958, 1961; Michel, 1974). In female hosts, arrested T. canis larvae reactivate when the host becomes pregnant and opportunity for transmission to a susceptible generation of new hosts arises, by transplacental and transmammary routes. Reactivated larvae are also capable of establishing a patent infection in the dam around the time of parturition, providing yet another opportunity for transmission. In this example, host factors to which parasite developmental responses occur are associated with host age, pregnancy and lactation. Developmental responses to these host factors increase the probability of transmission to new hosts and would be expected to confer a significant selective advantage to the parasite.
Transmammary infection has been demonstrated in other nematode species, for example in Neoascaris vitulorum (Warren and Needham, 1969), Ancylostoma caninum (Enigk and Stoye, 1968), Uncinaria lucasi (Olsen and Lyons, 1962, 1965) Strongyloides ransomi (Moncol and Batte, 1966) and Strongyloides stercoralis (Shoop et al., 2002). In these cases, host-induced arrest is presumably involved in establishment of arrested larvae in the tissues of the dam so that these larvae can subsequently provide a source of infectious larvae in the milk. The term amphiparatenesis has been used to describe this phenomenon, where adult hosts act essentially as paratenic hosts and juveniles act as definitive hosts (Shoop and Corkum, 1987). The specific host factors that induce parasite arrest in the adult female host are unknown, but presumably are associated with host age and/or sex. Resumption of development and passage into the milk is logically believed to be stimulated by host factors associated with lactation, such as systemic endocrine and local growth factors, and in some cases there is experimental evidence to support this (Stoye and Krause, 1976; Arasu, 2001).
As with seasonally induced arrest, the role of host-induced arrest in increasing the probability of transmission to young susceptible hosts is obvious and would presumably confer a significant selective advantage to the parasite.
4. Paratenesis in nematodes
Paratenic hosts are not permissive to parasite development but act as hosts for arrested infectious larvae, participating in their dispersal and transmission to permissive hosts. In most examples, paratenesis involves a developmental response by the parasite to host factors related to the species of the host. A form of host-induced arrest occurs in the paratenic host, host factors eliciting a developmental response in the parasite that results in cessation of development and induction of diapause. T. canis again provides an example of this form of developmental plasticity, where rodents act as paratenic hosts (Sprent, 1958; Michel, 1974). The utilisation of prey species as paratenic hosts to facilitate transmission to definitive predator hosts provides another example of how developmental plasticity is utilised to increase the probability of transmission.
5. Developmental switching in Strongyloididae
Members of the Strongyloididae display remarkable developmental plasticity that results in life cycles with both parasitic and free-living phases. For example, in Strongyloides ratti, parasitic female worms in the small intestine produce offspring by mitotic parthenogenesis (Viney, 1994) that are capable of either developing into free-living males and females or into infectious female L3 larvae that are analogous to C. elegans dauer larvae. Further emphasising the developmental and physiological homology between the infectious L3 larva of Strongyloides spp. and the C. elegans dauer larva, laser ablation studies have demonstrated that development of the infectious L3 larva in S. stercoralis is under control of the ASF and ASI amphidial neurons (Ashton et al., 1998), homologues of the ADF and ASI neurons that control dauer formation in C. elegans.
Experiments with naïve, immune and immunosuppressed hosts demonstrate that immunity to Strongyloides infection is a host factor that favours development of free-living forms of both sexes and favours an increase in the ratio of male to female progeny (Gemmill et al., 1997; Harvey et al., 2000). Further, environmental temperature influences development of the female progeny to either free-living or infectious forms and the sensitivity of females to this environmental factor is increased by previously experienced immune-related host factors (Harvey et al., 2000). The apparent net effect of these developmental responses is that free-living development is favoured when susceptible hosts are not available, increasing the probability of dispersal and transmission to new, susceptible hosts.
6. Developmental plasticity in platyhelminths
The levels of developmental plasticity outlined above for the Nematoda are not generally recognised to occur in the Platyhelminthes. One explanation for this is that developmental responses such as hypobiosis do not occur in the life cycles of platyhelminths of veterinary and medical importance. However, there is ample evidence that developmental plasticity does occur in the life cycles of many parasitic flatworms, so the conclusion that platyhelminths are incapable of developmental responses similar to those exhibited by nematodes is not justified.
Obvious analogues of the dauer pathway have yet to be recognised in flatworms and it is possible that, strictly defined, dauer development is a nematode-specific phenomenon. However, that platyhelminths are capable of entering diapause, be it similar to dauer formation or not, is demonstrated by, for example, the metacercaria stage of many trematodes, an arrested infectious larval stage that is functionally analogous to infectious larvae of nematodes. As with the majority of infectious nematode larvae however, the metacercaria is an obligatory stage in the life cycles of most trematodes and does not constitute a form of facultative diapause.
Some trematodes exhibit developmental responses with obvious parallels to those outlined above for T. canis. For example Alaria marcianae, and some other members of the Diplostomatidae, are now known to be capable of transmammary transmission (Harris et al., 1967; Shoop and Corkum, 1984, 1987), achieved by mesocercariae migrating to the mammary gland of the lactating female host. This represents another example of amphiparatenesis (Shoop and Corkum, 1987) and, as discussed above for nematodes, host factors related to lactation and developmental responses by parasites to those factors are presumably involved. Furthermore, A. marcianae can also utilize paratenic hosts in a manner similar to that of Toxocara. When a mesocercaria-infected frog intermediate host is preyed upon by a cat or raccoon definitive host, migration is initiated and development to metacercariae and adult parasites occurs. However, when a mesocercaria-infected frog is preyed upon by a reptile, bird or mammal other than the definitive cat or raccoon host, the mesocercariae penetrate the tissues of the paratenic host and await its ingestion by the appropriate definitive host. As with T. canis, host factors related to species and plastic developmental responses by the parasite to those factors are presumably involved in this life cycle.
Other examples of developmental plasticity can be found in archaic trematodes, the Aspidogastrea (Rohde, 1994). In some species, e.g. Aspidogaster conchicola, vertebrates serve only as facultative hosts and the life cycle can be completed by parasitism of the molluscan host alone (Rohde, 1994). For other aspidogastreans, such as Lobatostoma manteri, the vertebrate host is obligatory but the larval parasites are able to survive outside the host for prolonged periods if necessary (Rohde, 1994). These life cycles superficially resemble that of Strongyloides spp., but there is no evidence yet that precise developmental switching in response to environmental or host factors regulate the developmental responses of aspidogastreans.
7. Developmental responses in schistosomes
Evidence that host immune factors may influence schistosome development was first presented by Coker (1957) who demonstrated that, contrary to expectations, administration of immunosuppressive doses of corticosteroid to Schistosoma mansoni-infected mice inhibited the establishment of the parasite. Similar results were produced by Weinmann and Hunter (1960), although in both of these cases it was not possible to discriminate between a direct effect of steroids on the parasite and an indirect effect mediated via the immune system. Evidence in support of the latter explanation was provided by Doenhoff et al. (1978) and by Harrison and Doenhoff (1983). In these studies, corticosteroids, other immunosuppressants and depletion of T lymphocytes were all found to also delay schistosome maturation and the onset of oviposition in S. mansoni-infected mice, suggesting that T lymphocytes are a host factor that elicits developmental responses in S. mansoni.
The importance of the adaptive immune system as a host factor in normal schistosome development in mice was further delineated by Amiri et al. (1992), who found that schistosome fecundity was reduced in animals that are homozygous for the prkdcscid allele, a mutation that reduces levels of both B and T lymphocytes. In these animals, normal schistosome fecundity could be restored by administration of exogenous tumour necrosis factor (TNF), a proinflammatory cytokine (Amiri et al., 1992).
These results prompted us to next examine S. mansoni development in animals that completely lack an adaptive immune system (Davies et al., 2001). For this purpose, mice that are homozygous for targeted deletions of recombination activating gene-1 (RAG-1) were used (Mombaerts et al., 1992). Deletion of the RAG-1 genes blocks V (D) J recombination, prevents expression of all rearranged antigen receptors and renders the animal deficient in all B and T lymphocytes. Surprisingly, S. mansoni parasites isolated from these animals display a severe phenotype, characterised by overall reduction in body size, delayed development and a reduction in fecundity (Davies et al., 2001). Further experiments confirmed that a T lymphocyte population that expresses the CD4 cell surface molecule is the dominant host factor in determining the outcome of schistosome development (Davies et al., 2001). Administration of cytokines alone, including TNF, was not sufficient to rescue the profoundly attenuated phenotype of S. mansoni in RAG-1−/− mice (Davies, Lim and McKerrow, unpublished).
Despite the grossly abnormal phenotype of S. mansoni in RAG-1−/− animals (Davies et al., 2001), the available data suggest that parasite development is merely altered and that the parasites are not crippled or dying. For example, there is no difference in parasite recovery rates between wild type mice and RAG-1−/− mice (Davies, Lim and McKerrow, unpublished). Further, the small numbers of eggs produced by S. mansoni in RAG-1−/− mice are viable and the miracidia that hatch from them are infectious for Biomphalaria glabrata snails. In one experiment, exposure of 79 B. glabrata to 160 miracidia derived from RAG-1−/− mice produced infections in 17 snails, an infection rate of 22% and similar to rates obtained with miracidia from wild type animals in our laboratory. Cercariae produced by these snails are also infectious for mice (Davies, Lim and McKerrow, unpublished), indicating that the entire S. mansoni life cycle can be maintained in immunodeficient hosts.
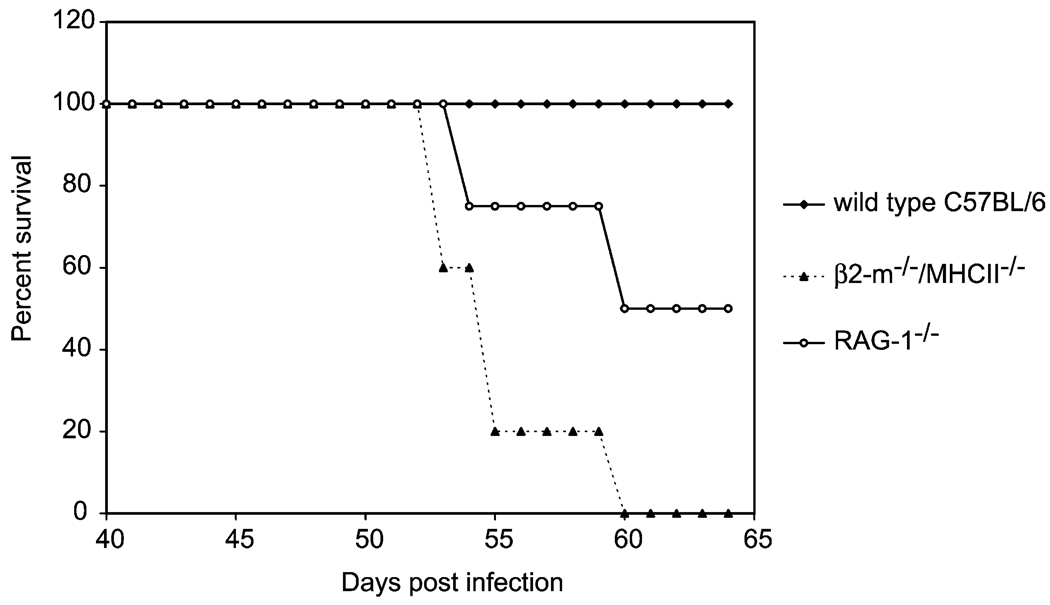
The selective advantage of a developmental response to immunodeficient hosts that results in the development of smaller, less fecund parasites is not immediately obvious. However, some insights may be gained from the examination of survival rates of infected RAG-1−/− mice and those of other immunodeficient animals that support normal S. mansoni development. One such example is the doubly deficient β2-microglobulin (β2-m)−/− /major histocompatability complex (MHC) class mouse (Grusby et al., 1993), which lacks all conventional T cells but supports normal schistosome development, possibly because of an unusual CD4+ T cell population that is retained at the site of parasite development in the liver (Davies et al., 2001). The absence of CD4+ T helper (TH) cells in both RAG-1−/− and β2-m−/− /MHC II−/− mice renders them unable to form granulomas around schistosome eggs that lodge in the liver (Pearce and MacDonald, 2002) and both are therefore equally susceptible to hepatocellular damage during schistosome infection (Byram et al., 1979). However, the greatly reduced rates of egg production in RAG-1−/− animals significantly prolongs their survival and the survival of their parasite burdens (Fig. 1). Whether prolonging survival of an immunodeficient host confers some advantage to the parasite, such as increasing the probability of transmission to a snail intermediate host, remains to be seen but this is a hypothesis we are currently testing.
Fig. 1.
Survival of wild type, β2-m−/− /MHCII−/− and RAG-1−/− mice infected with S. mansoni. Groups (four to five mice per group) of age- and sex-matched wild type, β2-m−/− /MHCII−/− and RAG-1−/− mice with C57BL/6 background were exposed to 100 S. mansoni cercariae and survival was monitored for 9 weeks post infection. Results are representative of two independent experiments.
Evidence for developmental responses by schistosomes to other host factors has come from studies that manipulate levels of the host cytokine interleukin (IL)-7 (Wolowczuk et al., 1999; Roye et al., 2001; Saule et al., 2002). Infection of IL-7−/− mice with S. mansoni results in a dramatically abnormal parasite phenotype (Wolowczuk et al., 1999) similar to that observed in RAG-1−/− mice (Davies et al., 2001). These results extended previous findings that IL-7 is expressed in the skin at the time of schistosome infection and that administration of exogenous IL-7 to infected mice enhanced schistosome survival when compared to control animals (Wolowczuk et al., 1997). Similarly, overexpression of IL-7 in the skin of transgenic mice also enhanced schistosome survival (Roye et al., 2001). One interpretation of these results is that IL-7 is a host factor that directly influences the growth and survival of S. mansoni (Wolowczuk et al., 1999). Alternatively, the effects of IL-7 deficiency on parasite development may be mediated indirectly, as IL-7 is critical for lymphocyte development and IL-7−/− mice are lymphopenic (von Freeden-Jeffry et al., 1995). Parasite development is also impaired in IL-7 receptor-deficient mice (Davies, Lim and McKerrow, unpublished), which produce IL-7 but are unable to respond to it, suggesting that the parasite phenotype in IL-7−/− mice is an indirect result of lymphocyte deficiency.
Observations by Wahab et al. (1971) regarding the interaction between host thyroid hormones and S. mansoni development in mice are of interest. Pharmacologically decreasing thyroid hormone levels produced worms that were smaller than normal, while increasing thyroid hormone levels produced worms that were bigger than those from control animals and which initiated oviposition earlier in infection (Wahab et al., 1971). The results suggest that S. mansoni has additional developmental capacity that is not realised in normal mice. These observations were recently repeated, manipulation of thyroid hormone signalling being achieved by administration of exogenous thyroid hormones, by restricting access to dietary iodine, and by targeted deletion of thyroid hormone receptor genes (Saule et al., 2002). Again, augmentation of thyroid hormone levels resulted in the development of worms that were larger than normal and this effect was enhanced further by simultaneous treatment with IL-7 (Saule et al., 2002). While intriguing, it is not possible to determine whether these effects are mediated directly on the parasite or whether the effects are indirect. Thyroid hormone in particular has profound, global effects on the host, affecting metabolic rate (Wahab et al., 1971), growth and development, reproduction and metabolism of carbohydrates, fats and proteins. Further, thyroid hormones exert specific effects within the immune system on processes such as lymphocyte development (Arpin et al., 2000).
While developmental responses by schistosomes to immune factors are clearly observable under laboratory conditions, the question of whether this phenomenon bears any relevance to schistosome infections under field conditions has not yet been addressed. Theoretically, a mechanism that allows for enhanced host survival in immunocompromised hosts (Fig. 1) could convey a selective advantage, particularly to parasites infecting host populations that display a high frequency of compromised immune function. In areas where schistosome infection is endemic, two possible causes of immune dysfunction are prevalent—(i) malnutrition (Desai et al., 1980), and (ii) co-infection with other pathogens (Keusch and Migasena, 1982; Ashford et al., 1992; Chunge et al., 1995; Thiong’o et al., 2001; Keiser et al., 2002)—suggesting that schistosome infection and compromised immune function might coincide in the same patients at high frequency. In a study that examined the relationship between human immunodeficiency virus (HIV) infection and S. mansoni egg excretion rates in a population of co-infected patients in Western Kenya (Karanja et al., 1997), it was shown that HIV infection and subsequent depletion of circulating CD4+ T cell numbers correlated with a reduction in faecal egg output. This observation suggests that developmental responses to immune factors might also occur in humans. However, it is possible that impairment of egg excretion in these patients was the cause of reduced faecal egg output, not reduced egg production, as egg transit across the wall of the intestine is facilitated by immune responses (Doenhoff et al., 1978).
8. Conclusions
Evidence from several areas suggests that schistosomes are capable of developmental responses to host immune factors. Given the prevalence of developmental plasticity in helminths to both environmental and host factors, perhaps these findings are to be expected. In other helminths, developmental responses have apparently evolved to increase the probability of parasite survival and transmission. Further work is now needed to (i) fully characterise schistosome responses to host factors, (ii) fully characterise the host factors that elicit developmental responses in schistosomes, and (iii) determine the biological relevance of these responses to the clinical manifestations and epidemiology of schistosomiasis in the field.
Acknowledgements
The authors thank Murray Selkirk, Rick Maizels, Malcolm Kennedy and Kleoniki Gounaris for organising a highly successful meeting. The authors are supported by the National Institutes of Health (F32 AI10424 and P30 DK26743 (UCSF Liver Center) to S.J.D.) and by the Sandler Family Foundation (to J.H.M).
References
- Amiri P, Locksley RM, Parslow TG, Sadick M, Rector E, Ritter D, McKerrow JH. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice (see comments) Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Arasu P. In vitro reactivation of Ancylostoma caninum tissue-arrested third-stage larvae by transforming growth factor-beta. J. Parasitol. 2001;87:733–738. doi: 10.1645/0022-3395(2001)087[0733:IVROAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Armour J, Bruce RG. Inhibited development in Ostertagia ostertagi infections—a diapause phenomenon in a nematode. Parasitology. 1974;69:161–174. doi: 10.1017/s0031182000048009. [DOI] [PubMed] [Google Scholar]
- Arpin C, Pihlgren M, Fraichard A, Aubert D, Samarut J, Chassande O, Marvel J. Effects of T3R alpha 1 and T3R alpha 2 gene deletion on T and B lymphocyte development. J. Immunol. 2000;164:152–160. doi: 10.4049/jimmunol.164.1.152. [DOI] [PubMed] [Google Scholar]
- Ashford RW, Craig PS, Oppenheimer SJ. Polyparasitism on the Kenya coast. 1. Prevalence, and association between parasitic infections. Ann. Trop. Med. Parasitol. 1992;86:671–679. doi: 10.1080/00034983.1992.11812724. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J. Parasitol. 1998;84:691–695. [PubMed] [Google Scholar]
- Ayalew L, Frechette JL, Malo R, Beauregard C. Seasonal fluctuation and inhibited development of populations of Dictyocaulus filaria in ewes and lambs. Can. J. Comp. Med. 1974;38:448–456. [PMC free article] [PubMed] [Google Scholar]
- Behnke JM, Parish HA. Nematospiroides dubius: arrested development of larvae in immune mice. Exp. Parasitol. 1979;47:116–127. doi: 10.1016/0014-4894(79)90013-4. [DOI] [PubMed] [Google Scholar]
- Blaxter M, Bird D. Parasitic nematodes. In: Riddle DL, Blumenthal T, Meyer B, Priess J, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 851–878. [PubMed] [Google Scholar]
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Blitz NM, Gibbs HC. An observation on the maturation of arrested Haemonchus contortus larvae in sheep. Can. J. Comp. Med. 1971;35:178–179. [PMC free article] [PubMed] [Google Scholar]
- Burden DJ, Harding JD, Kendall SB. The biology of Hyostrongylus rubidus. 3. Effect of host age on a primary infection. J. Comp. Pathol. 1970;80:601–605. doi: 10.1016/0021-9975(70)90058-7. [DOI] [PubMed] [Google Scholar]
- Byram JE, Doenhoff MJ, Musallam R, Brink LH, von Lichtenberg F. Schistosoma mansoni infections in T-cell deprived mice, and the ameliorating effect of administering homologous chronic infection serum. II. Pathology. Am. J. Trop. Med. Hyg. 1979;28:274–285. doi: 10.4269/ajtmh.1979.28.274. [DOI] [PubMed] [Google Scholar]
- Chiejina SN, Mason JA. Immature stages of Trichonema spp as a cause of diarrhoea in adult horses in spring. Vet. Rec. 1977;100:360–361. doi: 10.1136/vr.100.17.360. [DOI] [PubMed] [Google Scholar]
- Chunge RN, Karumba N, Ouma JH, Thiongo FW, Sturrock RF, Butterworth AE. Polyparasitism in two rural communities with endemic Schistosoma mansoni infection in Machakos District, Kenya. J. Trop. Med. Hyg. 1995;98:440–444. [PubMed] [Google Scholar]
- Coker CM. Effect of cortisone on natural immunity to Schistosoma mansoni in mice. Proc. Soc. Exp. Biol. Med. 1957;96:1–3. doi: 10.3181/00379727-96-23377. [DOI] [PubMed] [Google Scholar]
- Connan RM. Hyostrongylus rubidus: the size and structure of worm populations in adult pigs. Vet. Rec. 1971;89:186–191. doi: 10.1136/vr.89.7.186. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4 + lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- Desai ID, Garcia Tavares ML, Dutra de Oliveira BS, Douglas A, Duarte FA, Dutra de Oliveira JE. Food habits and nutritional status of agricultural migrant workers in Southern Brazil. Am. J. Clin. Nutr. 1980;33:702–714. doi: 10.1093/ajcn/33.3.702. [DOI] [PubMed] [Google Scholar]
- Doenhoff M, Musallam R, Bain J, McGregor A. Studies on the host–parasite relationship in Schistosoma mansoni-infected mice: the immunological dependence of parasite egg excretion. Immunology. 1978;35:771–778. [PMC free article] [PubMed] [Google Scholar]
- Enigk K, Stoye M. Studies of the pathway of infection of Ancylostoma caninum. Med. Klin. 1968;63:1012–1017. [PubMed] [Google Scholar]
- Eysker M. Experiments on inhibited development of Haemonchus contortus and Ostertagia circumcincta in sheep in The Netherlands. Res. Vet. Sci. 1981;30:62–65. [PubMed] [Google Scholar]
- Fernandez AS, Fiel CA, Steffan PE. Study on the inductive factors of hypobiosis of Ostertagia ostertagi in cattle. Vet. Parasitol. 1999;81:295–307. doi: 10.1016/s0304-4017(98)00252-0. [DOI] [PubMed] [Google Scholar]
- Fernando MA, Stockdale PH, Ashton GC. Factors contributing to the retardation of development of Obeliscoides cuniculi in rabbits. Parasitology. 1971;63:21–29. doi: 10.1017/s0031182000067366. [DOI] [PubMed] [Google Scholar]
- Gemmill AW, Viney ME, Read AF. Host immune status determines sexuality in a parasitic nematode. Evolution. 1997;51:393–401. doi: 10.1111/j.1558-5646.1997.tb02426.x. [DOI] [PubMed] [Google Scholar]
- Gibbs HC. Hypobiosis in parasitic nematodes—an update. Adv. Parasitol. 1986;25:129–174. [PubMed] [Google Scholar]
- Gibson TE. The effect of repeated anthelmintic treatment with phenothiazine on the faecal egg counts of housed horses with some observations on the life cycle of Trichonema spp. in the horse. J. Helminthol. 1953;27:29–40. [Google Scholar]
- Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect. Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Escobar N, Lewis E, Maizels RM. A novel member of the transforming growth factor-beta (TGF-beta) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp. Parasitol. 1998;88:200–209. doi: 10.1006/expr.1998.4248. [DOI] [PubMed] [Google Scholar]
- Gomez-Escobar N, van den Biggelaar A, Maizels R. A member of the TGF-beta receptor gene family in the parasitic nematode Brugia pahangi. Gene. 1997;199:101–109. doi: 10.1016/s0378-1119(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Gordon HML. Phenothiazine and oesophagostomiasis. Vet. Rec. 1949;61:509–510. [Google Scholar]
- Grusby MJ, Auchincloss H, Jr, Lee R, Johnson RS, Spencer JP, Zijlstra M, Jaenisch R, Papaioannou VE, Glimcher LH. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl Acad. Sci. U.S.A. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AH, Harkema R, Miller GC. Maternal transmission of Pharyngostomoides procyonis Harkema, 1942 (Trematoda: Diplostomatidae. J. Parasitol. 1967;53:1114–1115. [PubMed] [Google Scholar]
- Harrison RA, Doenhoff MJ. Retarded development of Schistosoma mansoni in immunosuppressed mice. Parasitology. 1983;86:429–438. doi: 10.1017/s0031182000050629. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Gemmill AW, Read AF, Viney ME. The control of morph development in the parasitic nematode Strongyloides ratti. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:2057–2063. doi: 10.1098/rspb.2000.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Developmental adaptations in Nematodes. In: Toft CA, editor. Parasitism—Conflict or Co-existence. Oxford: Oxford University Press; 1991. pp. 274–298. [Google Scholar]
- Horak IG. The similarity between arrested development in parasitic nematodes and diapause in insects. J. S. Afr. Vet. Assoc. 1981;52:299–303. [PubMed] [Google Scholar]
- Hotez P, Hawdon JM, Schad GA. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans Daf-c paradigm. Parasitol. Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Ikeme MM. Retarded metamorphosis in larvae of Ascaridia galli following repeated challenge of poultry with infective eggs. Vet. Rec. 1970;87:725–726. doi: 10.1136/vr.87.23.725. [DOI] [PubMed] [Google Scholar]
- Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. Evidence for immunefacilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am. J. Trop. Med. Hyg. 1997;56:515–521. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- Keiser J, N’Goran EK, Traore M, Lohourignon KL, Singer BH, Lengeler C, Tanner M, Utzinger J. Polyparasitism with Schistosoma mansoni, geohelminths, and intestinal protozoa in rural Cote d’Ivoire. J. Parasitol. 2002;88:461–466. doi: 10.1645/0022-3395(2002)088[0461:PWSMGA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Keusch GT, Migasena P. Biological implications of polyparasitism. Rev. Infect. Dis. 1982;4:880–882. doi: 10.1093/4.4.880. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Bundy DA, Selkirk ME, Smith DF, Anderson RM. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- Michel JF. Arrested development of nematodes and some related phenomena. Adv. Parasitol. 1974;12:279–366. doi: 10.1016/s0065-308x(08)60390-5. [DOI] [PubMed] [Google Scholar]
- Michel JF, Lancaster MB, Hong C. Observations on the inhibition of development of Cooperia oncophora in calves. Br. Vet. J. 1970;126:35–37. [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Moncol DJ, Batte EG. Transcolostral infection of newborn pigs with Strongyloides ransomi. Vet. Med. Small Anim. Clin. 1966;61:583–586. [PubMed] [Google Scholar]
- Olsen OW, Lyons ET. Life cycle of the hookworm Uncinaria lucasi Stiles, of northern fur seals, Callorhinus ursinus, on the Pribilof Islands in the Bering Sea. J. Parasitol. 1962;48 Suppl.:42–43. [Google Scholar]
- Olsen OW, Lyons ET. Life cycle of Uncinaria lucasi Stiles, 1901 (Nematoda: Ancylostomatidae) of fur seals, Callorhinus ursinus Linn., on the Pribilof Islands, Alaska. J. Parasitol. 1965;51:689–700. [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Albert PS. Genetic and environmental regulation of dauer larva development. In: Riddle D, Blumenthal T, Meyer B, Priess J, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 739–768. [PubMed] [Google Scholar]
- Rohde K. The origins of parasitism in the platyhelminthes. Int. J. Parasitol. 1994;24:1031–1053. doi: 10.1016/0020-7519(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Rossiter LW. The epizootiology of nematode parasites of sheep in the coastal of the Eastern Province. Onderstepoort J. Vet. Res. 1964;31:143–150. [Google Scholar]
- Roye O, Delacre M, Williams IR, Auriault C, Wolowczuk I. Cutaneous interleukin-7 transgenic mice display a propitious environment to Schistosoma mansoni infection. Parasite Immunol. 2001;23:133–140. doi: 10.1046/j.1365-3024.2001.00365.x. [DOI] [PubMed] [Google Scholar]
- Saule P, Adriaenssens E, Delacre M, Chassande O, Bossu M, Auriault C, Wolowczuk I. Early variations of host thyroxine and interleukin-7 favor Schistosoma mansoni development. J. Parasitol. 2002;88:849–855. doi: 10.1645/0022-3395(2002)088[0849:EVOHTA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Schad GA. Arrested development of Ancylostoma caninum in dogs: influence of photoperiod and temperature on induction of a potential to arrest. In: Meerovitch E, editor. Aspects of Parasitology, a Festschrift Dedicated to the Fiftieth Anniversary of the Institute of Parasitology. Montreal, Canada: McGill University; 1982. pp. 361–391. [Google Scholar]
- Schad GA, Chowdhury AB, Dean CG, Kochar VK, Nawalinski TA, Thomas J, Tonascia JA. Arrested development in human hookworm infections: an adaptation to a seasonally unfavorable external environment. Science. 1973;180:52–54. [PubMed] [Google Scholar]
- Schwartz B, Alicata JE, Lucker JT. Resistance of rats to superinfection with a nematode Nippostrongylus muris and an apparently similar resistance of horses to superinfection with nematodes. J. Wash. Acad. Sci. 1931;21:259–261. [Google Scholar]
- Shoop WL, Corkum KC. Transmammary infection of newborn by larval trematodes. Science. 1984;223:1082–1083. doi: 10.1126/science.6695195. [DOI] [PubMed] [Google Scholar]
- Shoop WL, Corkum KC. Maternal transmission by Alaria marcianae (Trematoda) and the concept of amphiparatenesis. J. Parasitol. 1987;73:110–115. [PubMed] [Google Scholar]
- Shoop WL, Michael BF, Eary CH, Haines HW. Transmammary transmission of Strongyloides stercoralis in dogs. J. Parasitol. 2002;88:536–539. doi: 10.1645/0022-3395(2002)088[0536:TTOSSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Smeal MG, Donald AD. Effects on inhibition of development of the transfer of Ostertagia ostertagi between geographical regions of Australia. Parasitology. 1981;82:389–399. doi: 10.1017/s0031182000066920. [DOI] [PubMed] [Google Scholar]
- Sommerville RI. The growth of Cooperia curticei (Giles, 1892) a nematode parasite of sheep. Parasitology. 1960;50:261–267. doi: 10.1017/s0031182000025348. [DOI] [PubMed] [Google Scholar]
- Sprent JFA. Observations on the development of Toxocara canis (Werner, 1782) in the dog. Parasitology. 1958;48:184–209. doi: 10.1017/s0031182000021168. [DOI] [PubMed] [Google Scholar]
- Sprent JFA. Post-parturient infection of the bitch with Toxocara canis. J. Parasitol. 1961;47:284. [Google Scholar]
- Stoye M, Krause J. Versuche zur reaktiverung inhibierter larven von Ancylostoma caninum: die wirkung von oestradiol und progesteron. Zentralbl. Veterinarmed. A. 1976;23:822–839. [PubMed] [Google Scholar]
- Thiong’o FW, Luoba A, Ouma JH. Intestinal helminths and schistosomiasis among school children in a rural district in Kenya. East Afr. Med. J. 2001;78:279–282. doi: 10.4314/eamj.v78i6.9017. [DOI] [PubMed] [Google Scholar]
- Viney ME. A genetic analysis of reproduction in Strongyloides ratti. Parasitology. 1994;109(Pt 4):511–515. doi: 10.1017/s0031182000080768. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab MF, Warren KS, Levy RP. Function of the thyroid and the host–parasite relation in murine schistosomiasis mansoni. J. Infect. Dis. 1971;124:161–171. doi: 10.1093/infdis/124.2.161. [DOI] [PubMed] [Google Scholar]
- Waller PJ, Thomas RJ. Arrested development of Nematodirus species in grazing lambs. Res. Vet. Sci. 1983;34:357–361. [PubMed] [Google Scholar]
- Warren EG, Needham DJ. On the presence of Neoascaris vitulorum in calves from New South Wales. Aust. Vet. J. 1969;45:22–23. doi: 10.1111/j.1751-0813.1969.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Weinmann CJ, Hunter GW. Studies on Schistosomiasis. XIV. Effects of cortisone upon the Schistosoma mansoni burden in mice. Exp. Parasitol. 1960;9:239–242. doi: 10.1016/0014-4894(60)90030-8. [DOI] [PubMed] [Google Scholar]
- Wolowczuk I, Delacre M, Roye O, Giannini SL, Auriault C. Interleukin-7 in the skin of Schistosoma mansoni-infected mice is associated with a decrease in interferon-gamma production and leads to an aggravation of the disease. Immunology. 1997;91:35–44. doi: 10.1046/j.1365-2567.1997.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolowczuk I, Nutten S, Roye O, Delacre M, Capron M, Murray RM, Trottein F, Auriault C. Infection of mice lacking interleukin-7 (IL-7) reveals an unexpected role for IL-7 in the development of the parasite Schistosoma mansoni. Infect. Immun. 1999;67:4183–4190. doi: 10.1128/iai.67.8.4183-4190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]