Abstract
The discovery that platelets possess cell membrane, cytoplasmic and secreted forms of the co-stimulatory molecule CD40 ligand (CD40L, also known as CD154) has led to a revolution in the view of this anucleate, differentiated cell fragment, previously thought only to be involved in blood clotting (hemostasis). During the last decade it has become clear that platelets function in innate and adaptive immunity and possess pro-inflammatory, as well as pro-thrombotic properties. They interact not only with other platelets and endothelial cells, but with lymphocytes, dendritic cells and structural cells such as fibroblasts. Soluble forms of CD40L (sCD40L) in the human circulation are almost entirely derived from platelets. Elevated levels of CD40L are associated with clinically important conditions, such as vascular disease, abnormal clotting (thrombosis), lung injury and autoimmune disease. Each year millions of platelet transfusions are given to patients that contain large amounts of sCD40L. sCD40L in the supernatant of stored platelets can induce cytokines, chemokines and lipid mediators by activating CD40 bearing cells. Increased levels of sCD40L in transfused blood are associated with transfusion related acute lung injury, a potentially fatal complication, as well as more common, milder transfusion reactions such as fever and rigors. These effects come under the rubric of transfusion immunomodulation, which postulates that transfusion recipient biology, particularly immune function, is dramatically altered by transfusion of stored allogeneic blood.
Keywords: platelet, CD40L, CD154, transfusion, immunity, inflammation
Introduction to Platelet CD40L
For over a century the platelet has been viewed as an anucleate cell fragment derived from the megakaryocyte, whose only function was to initiate hemostasis at the site of vascular injury. While there were some reports of platelets mediating cellular cytoxicity against pathogens, a major change in the way platelet biology was conceptualized occurred when Henn and colleagues [1]reported in 1998 that platelets contained and secreted CD40L, a costimulatory molecule previously thought mainly restricted to T lymphocytes. Platelets are now known as the main source of circulating soluble CD40L (formally called CD154), a member of the tumor necrosis family of cytokines. CD40L has long been appreciated as a T cell mediator responsible for immunoglobulin class switching and important for dendritic cell maturation. [2, 3] The importance of the CD40/CD40L receptor/ligand pair in transplantation of solid organs has been investigated as a key pathway for generating allograft rejection. [4, 5] The platelet is a vital link between clotting and inflammation, two processes now clearly known to be intimately connected. [6, 7] Figure 1 shows a conceptual model of the role of platelet CD40L in inflammation. In this review, we will briefly summarize CD40L as a platelet derived mediator and, in particular, its potential effects on patients following blood transfusion.
Figure 1. Role of Platelet CD40L in Inducing Inflammation.
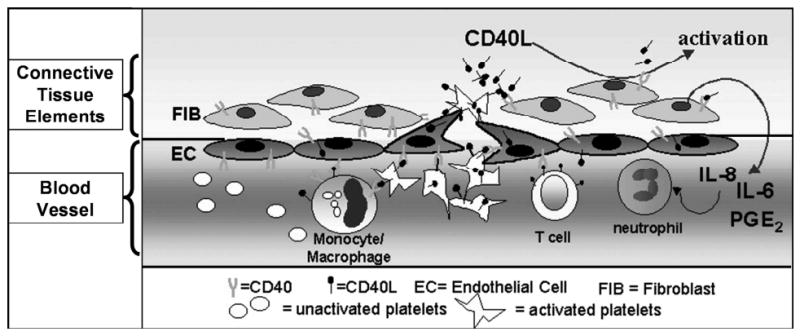
- Platelets become activated at the site of vessel injury
- Activated platelets express / secrete CD40L
- Platelet CD40L serves to trigger the onset of inflammation
sCD40L and Innate Immunity
CD40, the receptor for CD40L, is found on fibroblasts, [8] neutrophils, [9] endothelial cells[1] and keratinocytes, [10] as well as platelets themselves, [11] it appears the CD40:CD40L axis is involved in many essential cell signaling pathways.[12] Over time, additional mediators (e.g., IL-1B, IL-6, IL-8) [13] and receptors (e.g., Toll like receptors[TLR]) [14-16]have been described as part of the platelet's functional repertoire, suggesting its complex role in immunity and inflammation, as well as hemostasis. Platelet:neutrophil interactions have long been observed as part of various pathologic conditions in humans and experimental animals. It appears TLRs play a major role in this relationship. [17] sCD40L also is involved in platelet-mediated neutrophil activation, [9] and this relationship is reciprocal. [18]
CD40L and B and T Cell Biology
Ratliff and colleagues postulated that early in the immune response when the number of CD4+ T cells is small, platelet-derived sCD40L provides early signals to the B cell compartment, and demonstrated that sCD40L stimulation led to increased serum IgG levels and germinal center formation under conditions of modest or no T cell help. [19] This finding of platelet:T cell interaction may be clinically significant in that sCD40L from platelets enhances cytotoxic T cell activity and survival in a dose-dependent manner in a mouse model of listeria infection. [20]
sCD40L may also be involved in B cell production of anti-platelet autoantibodies by way of increased levels of sCD40L on platelets in vivo and stimulating autoantibody production. [21] Cognasse, Garraud and colleagues have demonstrated that purified sCD40L stimulates immunoglobulin production in purified human B cells in the absence of any additional cellular elements. [22, 23] Interestingly, this modulation of humoral immunity appears to be mediated by sCD40L in platelet microparticles. [24] The role of sCD40L in adaptive immunity has been recently reviewed. [25]
CD40L and Hemostasis/Thrombosis
Platelets play a pivotal role in the initiation of blood clotting. When blood clotting “goes bad” in deep vein or coronary artery thrombosis, or pulmonary embolus, platelets are thought to play an important pathologic role, although the evidence is much stronger for arterial than venous thromboses. [26] As an example, there is the success of anti-platelet drugs such as aspirin in partially preventing such events, particularly in vessels with high pressures (i.e., arteries). When platelets interact with endothelial cells, sCD40L release from platelets is associated with increased endothelial tissue factor and decreased thrombomodulin expression. [13] Mediators such as IL-8, MCP-1, adhesion molecules and metalloproteinases are upregulated by platelet sCD40L interaction with CD40 positive endothelial cells. [13] Platelet sCD40L clearly is a primary agonist as it binds to the major fibrinogen receptor on platelets (αIIbB3) and contributes to platelet activation and enchanced clot stability, creating an autocrine loop that facilitates hemostasis. [27, 28] Given that elevated levels of sCD40L are associated with increased risks of various thrombotic events, this activation of endothelial cells and platelets may represent a key pathologic mechanism in diseases such as myocardial infarction, stroke and similar conditions.
Further in vitro evidence for the importance of sCD40L-endothelial cell interactions in disease are recent in vitro findings that both cultured and tissue endothelial cells exhibit decreased endogenous nitric oxide synthesis and increased oxidative stress after exposure to high (1-5 μg/ml) concentrations of sCD40. [29-31] CD40 on endothelial cells is a key receptor facilitating vascular inflammation and early narrowing of arteries. [32]
That these interactions between platelets and endothelial cells, particularly through sCD40L and membrane CD40L, may have importance for pathophysiology is demonstrated by observations that sCD40L levels are independently associated with increased risk of death, myocardial infarction and congestive heart failure. [33, 34] Interestingly, atorvastatin, a drug employed for lowering blood cholesterol levels, but now known to have anti-inflammatory properties, decreases platelet CD40L levels in patients. [35] Statin treatment successfully reduces the incidence of both first time and recurrent coronary events, and it seems likely that treatments that interfere with the CD40L: CD40 pathway may be particularly effective in this regard. [33]
Finally, another disease characterized by platelet activation, thrombosis and chronic inflammation, sickle cell anemia, has been shown to be associated with dramatic increases of blood levels of sCD40L which correlates with disease activity. [36]
sCD40L and Leukemia Biology
There is preliminary evidence that platelet-derived sCD40L can act as a growth factor for and provide rescue from apoptosis in acute leukemia cells in vitro. [37] Exposure to sCD40L has a similar effect in the more slowly growing chronic lymphocytic leukemia cells, suggesting that this effect may not be restricted to myeloid or acute leukemia cells. [38] These pro-survival, pro-self-renewal effects of CD40L on hematologic malignancies may be of great interest and concern in patient care, because a frequent treatment modality in such patients is platelet transfusion, as described later in this review. One question which arises is whether infusion of stored platelet sCD40L, on an almost daily basis for weeks, influence treatment outcomes in human acute leukemia?
sCD40L and Platelet Transfusion
A growing body of data demonstrates that patients who receive blood transfusions experience altered immune function, including the long-appreciated alloimmunization to polymorphic red cell, white cell, platelet and plasma antigens. However, only in recent decades has it been appreciated that transfusion causes impaired host immunity against bacteria and cancer, and increased acceptance of solid organ transplants and fetuses as an allograft. [39] The concept that platelet transfusions immunomodulate the recipient has been a focus of our work for some time. Platelet transfusions are likely both pro-thrombotic[40, 41] and pro-inflammatory[42, 43] in the clinical setting. We and our collaborators have provided evidence that platelet transfusion-derived sCD40L plays a role in acute lung injury, [9] fever, [44-47] and perhaps even impairment of host versus leukemia immunity. [48, 49] It is entirely probable that other soluble and cell mediators play important roles in transfusion immunomodulation, but sCD40L is a key candidate molecule. [50, 51]
sCD40L Accumulates In Platelets During Storage For Transfusion
After the discovery of platelet CD40L by Henn and colleagues, we demonstrated that platelets collected and stored for transfusion under blood bank conditions from all individuals tested release sCD40L into the supernatant at biologically relevant (ng/ml) levels, as well as expressing significant increases in surface CD40L, an activation marker. (Figure 2). [44, 45, 47]
Figure 2. Platelets secrete soluble CD40L (sCD40L) during storage prior to transfusion.
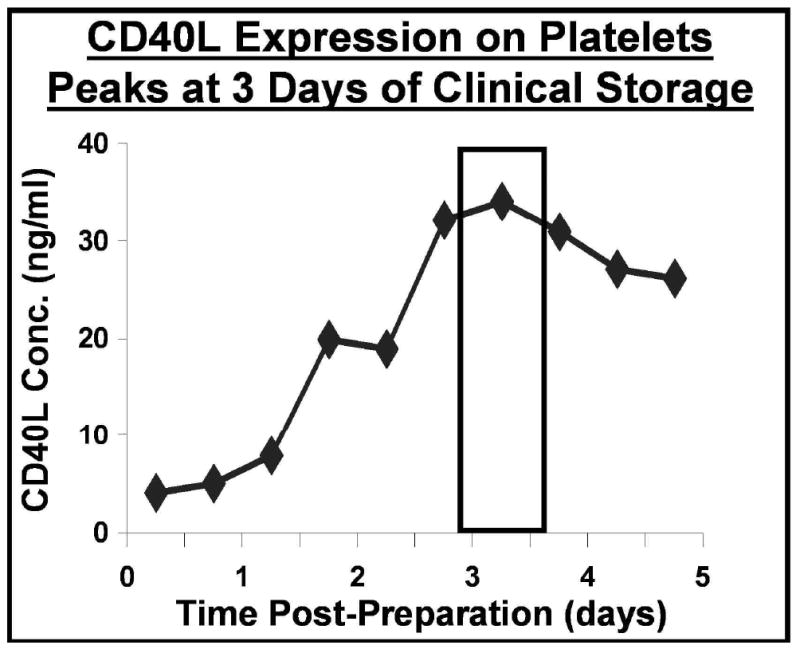
The concentration of sCD40L in the storage supernatant of washed platelet prepared for transfusion is greatest at 3 or 4 days of storage. Soluble CD40L was measured by standard sandwich ELISA.
These levels are similar to those seen in patients with highly active inflammatory disease, and suggest that sCD40L in transfused platelets was at biologically relevant concentrations. Platelet supernatant CD40L specifically stimulated upregulation of cyclo-oxygenase(Cox-2) and in vitro production of inflammatory mediators by human fibroblasts, including IL-6 and PGE2. (Figure 3) [44, 45, 47]
Figure 3. Platelet-derived CD40L induces Cox-2 expression in human lung fibroblasts, and induces the production of PGE2 and IL-6.
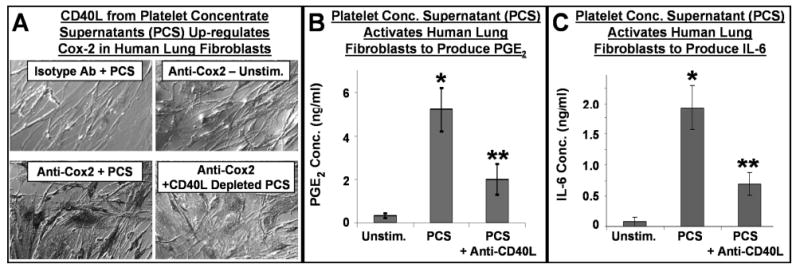
Panel A: Human lung fibroblasts were stimulated with medium (unstimulated), PCS (platelet concentrate supernatant) or CD40L-depleted PCS. After 24 hours, cells were stained with a Cox-2 specific antibody or an isotype control antibody. Unstimulated cells express low levels of Cox-2, while PCS strongly activated lung fibroblasts to up-regulate Cox-2 protein. The induction of Cox-2 was partly dependent on CD40L, as CD40L-depleted PCS had a significantly reduced capacity for Cox-2 induction. Panels B and C: Cell-free culture medium from similarly treated human lung fibroblast cultures was analyzed for PGE2 and IL-6 content. Unstimulated cells produced low levels of PGE2 and IL-6, while PCS caused great amounts of these mediators to be produced by the fibroblasts. These high levels of production were reduced when CD40L was neutralized in the PCS via an anti-CD40L antibody. Mean +/- SEM, n = 9 similar experiments, * p < 0.001 PCS vs. Unstim., ** p < 0.001 PCS + anti-CD40L vs. PCS alone.
sCD40L in Platelet Transfusion is a Candidate Mediator of Acute Lung Injury and other Inflammatory Conditions
One life-threatening complication of transfusion is transfusion-related acute lung injury (TRALI). TRALI is characterized clinically by a pulmonary vascular leak syndrome with inflammatory signs and symptoms, including fever and rigors. Once thought to be due only to infusion of anti-white cell antibodies and rare, other cofactors/mediators may also contribute to TRALI. [52] Milder forms of pulmonary dysfunction similar to TRALI in mechanism and clinical findings may be considerably more common than previously thought. We hypothesized that sCD40L was a key mediator because of its high concentration in transfused platelets, known potency as a stimulator of inflammatory reactions (including lung injury), and the wide range of cells that express its receptor, CD40. Some reports indicate that platelet transfusions are commonly involved in clinical cases of TRALI, and are associated with a higher rate of infection, stroke, myocardial infarction and respiratory failure.
In collaboration with Dr. Chris Silliman and his colleagues at the University of Colorado, we demonstrated that levels of sCD40L in platelet transfusions implicated in cases of transfusion acute lung injury were significantly higher than similar transfusions which did not cause such complications. (Figure 4A). [9] Finally, platelet-derived sCD40L is capable of activating neutrophils and injuring endothelial cells in vitro, [29-31] which is the postulated final common pathway of acute lung injury after transfusion. [9] sCD40L from platelets is implicated in a range of potentially serious complications of transfusion. [53]
Figure 4. CD40L concentration in platelet concentrates is associated with adverse clinical events following transfusion.
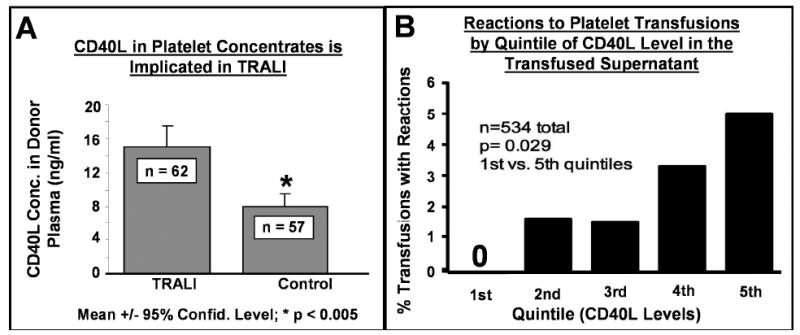
Panel A shows the mean levels of CD40L in platelet transfusions implicated in cases of transfusion related acute lung injury (TRALI) as opposed to non-implicated control platelet transfusions. CD40L levels were significantly higher in transfusions implicated in TRALI. Panel B displays the rate of transfusion reactions (fever/rigors) versus quintiles of CD40L levels in platelet transfusions. The rate of adverse reactions increases significantly from 0% to 5% as the levels of CD40L increase from the 1st to 5th quintile.
Recent data support a role for platelet sCD40L as a global mediator of endothelial cell dysfunction, particularly in coronary arteries. [29-31] This is of great interest because transfusion is associated with a significant increase in myocardial infarction[54] and thrombosis in epidemiologic studies. [41]
sCD40L in Platelet Transfusion is a Candidate Mediator of Fever and Rigors
We are particularly interested in the pro-inflammatory properties of sCD40L in platelet transfusions because one of the most common complications of platelet transfusion is fever with shaking chills (rigors). [55] While rarely life threatening, these reactions are clinically important as a source of patient discomfort, treatment delay and potential confusion with more serious septic complications frequently seen in patients who receive platelet transfusions. Figure 4B demonstrates that as sCD40L levels increase in stored platelets, the observed incidence of inflammatory responses after transfusion, such as fever and rigors, rises by an order of magnitude. This dramatic dose-response effect suggests that sCD40L and/or similar mediators are candidate causes of these reactions. [46] When the supernatant of transfused platelets is removed prior to infusion into the patient by simple saline washing, these complications are largely abrogated. [56]
Platelet Derived Soluble Mediators as Mediators of Leukemia Recurrence During Treatment of Acute Leukemia
Platelet derived CD40L has been shown by our group to induce the COX-2 enzyme and stimulate production of PGE2, the major mediator of fever in humans. [44, 45, 47] Stored platelet plasma supernatants at dilutions as high as 1/200 induce PGE2 in human lung fibroblasts and this activity can be partially or completely neutralized by anti-CD40L monoclonal antibody treatment of the platelet supernatant. Typically, 200-250 ml of plasma are infused with each platelet transfusion. Assuming a blood volume of 5,000 ml, this amount of plasma exceeds by a factor of 10 the concentration needed to provoke PGE2 production in vitro. Furthermore, such infusions are typically repeated every day or two for 2-3 weeks in patients with acute leukemia during induction therapy or stem cell transplantation.
It is possible that platelet-derived mediators such as sCD40L alter host defenses against cancer by causing immune-deviation towards type 2 immune function, with impaired cell-mediated immunity. [57, 58] These mediators may also serve as proliferation and survival promoting factors for leukemic cells. In acute leukemia, patients receiving saline washed transfusions which are largely depleted of sCD40L experience better survival than patients receiving unmodified transfusions(Figure 5). [48, 49] If our hypotheses concerning the effects of CD40L infusion and stored platelet transfusions on immune function and leukemia cell survival/growth are correct, reductions in morbidity and mortality may be possible for patients with acute leukemia and other hematologic malignancies employing relatively simple and inexpensive changes in transfusion practice, such as saline washing or withholding marginally indicated transfusions.
Figure 5. Washed transfusions are associated with improved survival in leukemia patients.
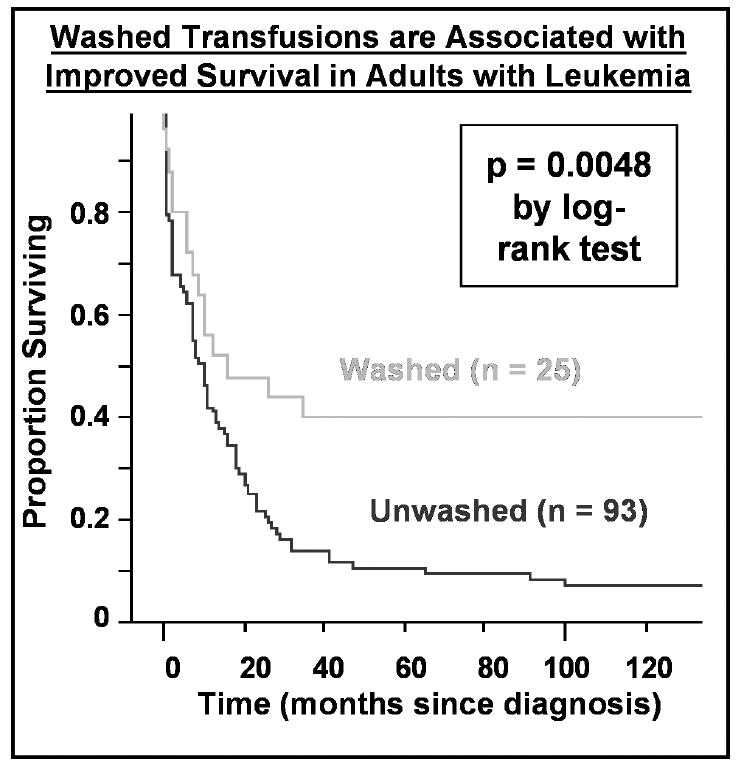
A Kaplan-Meier plot of survival of 118 consecutive adult patients (ages 18-80) treated with curative intent for acute leukemia is shown according to whether patients received washed versus unwashed transfusions during their entire course. Patients receiving washed transfusions had significantly better survival, even after adjusting for other prognostic factors by proportional hazards analysis.
Summary
The last decade has seen an exciting and striking growth in research into the platelet's role as a key cell involved in both innate and adaptive immunity. This has led to an understanding of the interaction and interdependence of inflammation and hemostatic mechanisms that halt bleeding after vascular injury, yet prevent thrombosis at sites distant from the injury. Clearly, evolution has fostered development of platelets as cells that prevent hemorrhagic death while delivering initial anti-microbial functions to the site of injury. CD40L and CD40, by their very presence on diverse cells of the peripheral blood, endothelium and structural cells, are important components in both hemostasis and host immunologic defense. Derangements in platelet, endothelial, white cell and organ-specific functions dramatically affect the CD40L/CD40 system, and are associated with alterations in hemostasis, anti-microbial defenses, auto-immunity and cancer biology. These insights have led to new strategies for altering the immunologic and hemostatic complications of platelet transfusions, such as removal of supernatant sCD40L and other mediators prior to transfusion. Further research into the platelet's role as an immune cell and the effects of storage prior to transfusion will likely generate new approaches to render platelet transfusion more effective and minimize deleterious effects of transfusion immunomodulation in patients.
Acknowledgments
This work is in part supported by NHLBI R21HL086367, ES01247, NHLBI R01HL078603, a James P. Wilmot Cancer Center Discovery Fund Seed Grant, Gambro BCT (now Caridian BCT), Inc., Lakewood, CO. We gratefully acknowledge contributions to the research described here by Julia Kaufman, Denise Ray, Stephen Pollock, Joanna Heal and Kelly Gettings.
References
- 1.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Kehry MR. CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J Immunol. 1996;156:2345–8. [PubMed] [Google Scholar]
- 4.Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006;116:769–74. doi: 10.1172/JCI27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000;165:4783–6. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- 6.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1321–4. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 7.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 8.Sempowski GD, Chess PR, Phipps RP. CD40 is a functional activation antigen and B7-independent T cell costimulatory molecule on normal human lung fibroblasts. J Immunol. 1997;158:4670–7. [PubMed] [Google Scholar]
- 9.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspari AA, Sempowski GD, Chess P, Gish J, Phipps RP. Human epidermal keratinocytes are induced to secrete interleukin-6 and co-stimulate T lymphocyte proliferation by a CD40-dependent mechanism. Eur J Immunol. 1996;26:1371–7. doi: 10.1002/eji.1830260629. [DOI] [PubMed] [Google Scholar]
- 11.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–8. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 12.Danese S, Fiocchi C. Platelet activation and the CD40/CD40 ligand pathway: mechanisms and implications for human disease. Crit Rev Immunol. 2005;25:103–21. doi: 10.1615/critrevimmunol.v25.i2.20. [DOI] [PubMed] [Google Scholar]
- 13.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–14. [PubMed] [Google Scholar]
- 14.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–8. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 15.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–23. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 16.Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K, Kawashima S, Yokoyama M. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113:379–85. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 18.Vanichakarn P, Blair P, Wu C, Freedman JE, Chakrabarti S. Neutrophil CD40 enhances platelet-mediated inflammation. Thromb Res. 2008;122:346–58. doi: 10.1016/j.thromres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005;78:80–4. doi: 10.1189/jlb.1104669. [DOI] [PubMed] [Google Scholar]
- 20.Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, Ratliff TL. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008;111:3684–91. doi: 10.1182/blood-2007-05-091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solanilla A, Pasquet JM, Viallard JF, Contin C, Grosset C, Dechanet-Merville J, Dupouy M, Landry M, Belloc F, Nurden P, Blanco P, Moreau JF, Pellegrin JL, Nurden AT, Ripoche J. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105:215–8. doi: 10.1182/blood-2003-07-2367. [DOI] [PubMed] [Google Scholar]
- 22.Cognasse F, Chavarin P, Acquart S, Sabido O, Beniguel L, Genin C, Richard Y, Garraud O. Differential downstream effects of CD40 ligation mediated by membrane or soluble CD40L and agonistic Ab: a study on purified human B cells. Int J Immunopathol Pharmacol. 2005;18:65–74. doi: 10.1177/039463200501800108. [DOI] [PubMed] [Google Scholar]
- 23.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogne M, Richard Y, Garraud O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35:1376–87. doi: 10.1016/j.exphem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–36. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprague DL, Sowa JM, Elzey BD, Ratliff TL. The role of platelet CD154 in the modulation in adaptive immunity. Immunol Res. 2007;39:185–93. doi: 10.1007/s12026-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 26.Francis CW, Kaplan KL. Principles of Antithrombotic Therapy. In: Lichtman MA, et al., editors. Williams Hematology. Seventh. New York: McGraw-Hill; 2006. pp. 283–300. [Google Scholar]
- 27.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247–52. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 28.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100:12367–71. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluvinet R, Olivar R, Krupinski J, Herrero-Fresneda I, Luque A, Torras J, Cruzado JM, Grinyo JM, Sumoy L, Aran JM. CD40: an upstream master switch for endothelial cell activation uncovered by RNAi-coupled transcriptional profiling. Blood. 2008;112:3624–37. doi: 10.1182/blood-2008-03-143305. [DOI] [PubMed] [Google Scholar]
- 30.Phipps RP. CD40: Lord of the endothelial cell. Blood. 2008;112:3531–2. doi: 10.1182/blood-2008-08-175034. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Chai H, Wang X, Jiang J, Jamaluddin MS, Liao D, Zhang Y, Wang H, Bharadwaj U, Zhang S, Li M, Lin P, Yao Q. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood. 2008;112:3205–16. doi: 10.1182/blood-2008-03-143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, Wijnands E, Cleutjens J, Zernecke A, Weber C, Ahonen CL, Benbow U, Newby AC, Noelle RJ, Daemen MJ, Lutgens E. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008;111:4596–604. doi: 10.1182/blood-2007-05-088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003;10:356–61. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Varo N, Vicent D, Libby P, Nuzzo R, Calle-Pascual AL, Bernal MR, Fernandez-Cruz A, Veves A, Jarolim P, Varo JJ, Goldfine A, Horton E, Schonbeck U. Elevated plasma levels of the atherogenic mediator soluble CD40 ligand in diabetic patients: a novel target of thiazolidinediones. Circulation. 2003;107:2664–9. doi: 10.1161/01.CIR.0000074043.46437.44. [DOI] [PubMed] [Google Scholar]
- 35.Pignatelli P, Sanguigni V, Lenti L, Loffredo L, Carnevale R, Sorge R, Violi F. Oxidative stress-mediated platelet CD40 ligand upregulation in patients with hypercholesterolemia: effect of atorvastatin. J Thromb Haemost. 2007;5:1170–8. doi: 10.1111/j.1538-7836.2007.02533.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee SP, Ataga KI, Orringer EP, Phillips DR, Parise LV. Biologically active CD40 ligand is elevated in sickle cell anemia: potential role for platelet-mediated inflammation. Arterioscler Thromb Vasc Biol. 2006;26:1626–31. doi: 10.1161/01.ATV.0000220374.00602.a2. [DOI] [PubMed] [Google Scholar]
- 37.Aldinucci D, Poletto D, Nanni P, Degan M, Rupolo M, Pinto A, Gattei V. CD40L induces proliferation, self-renewal, rescue from apoptosis, and production of cytokines by CD40-expressing AML blasts. Exp Hematol. 2002;30:1283–92. doi: 10.1016/s0301-472x(02)00921-9. [DOI] [PubMed] [Google Scholar]
- 38.Willimott S, Baou M, Naresh K, Wagner SD. CD154 induces a switch in pro-survival Bcl-2 family members in chronic lymphocytic leukaemia. Br J Haematol. 2007;138:721–32. doi: 10.1111/j.1365-2141.2007.06717.x. [DOI] [PubMed] [Google Scholar]
- 39.Blumberg N, Heal JM. Transfusion immunomodulation. In: Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD, editors. Blood Banking and Transfusion Medicine. 2nd. Philadelphia, PA: Churchill Livingstone Elsevier; 2007. pp. 701–12. [Google Scholar]
- 40.Cook D, Crowther M, Meade M, Rabbat C, Griffith L, Schiff D, Geerts W, Guyatt G. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33:1565–71. doi: 10.1097/01.ccm.0000171207.95319.b2. [DOI] [PubMed] [Google Scholar]
- 41.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai M, Lyman GH. Blood transfusions, thrombosis and mortality in hospitalized cancer Patients. Archives of Internal Medicine. 2008;168 doi: 10.1001/archinte.168.21.2377. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, Murkin J, Nadel A. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–8. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 43.Kenton AB, Hegemier S, Smith EO, O'Donovan DJ, Brandt ML, Cass DL, Helmrath MA, Washburn K, Weihe EK, Fernandes CJ. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol. 2005;25:173–7. doi: 10.1038/sj.jp.7211237. [DOI] [PubMed] [Google Scholar]
- 44.Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001;357:2023–4. doi: 10.1016/s0140-6736(00)05108-4. [DOI] [PubMed] [Google Scholar]
- 45.Blumberg N, Phipps RP, Kaufman J, Heal JM. The causes and treatment of reactions to platelet transfusions. Transfusion. 2003;43:291–2. doi: 10.1046/j.1537-2995.2003.t01-2-00362.x. author reply 2. [DOI] [PubMed] [Google Scholar]
- 46.Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–21. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–96. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 48.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blumberg N, Heal JM, Liesveld JL, Phillips GL, Rowe JM. Platelet transfusion and survival in adults with acute leukemia. Leukemia. 2008;22:631–5. doi: 10.1038/sj.leu.2404920. [DOI] [PubMed] [Google Scholar]
- 50.Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, Garraud O. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–9. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 51.Skripchenko A, Kurtz J, Moroff G, Wagner SJ. Platelet products prepared by different methods of sedimentation undergo platelet activation differently during storage. Transfusion. 2008;48:1469–77. doi: 10.1111/j.1537-2995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 52.Rogers MA, Blumberg N, Heal JM, Hicks GL., Jr Increased risk of infection and mortality in women after cardiac surgery related to allogeneic blood transfusion. J Womens Health (Larchmt) 2007;16:1412–20. doi: 10.1089/jwh.2007.0397. [DOI] [PubMed] [Google Scholar]
- 53.Aiboshi J, Moore EE, Ciesla CJ, Silliman CC. Blood transfusion and the two-insult model of post-injury multiple organ failure. Shock. 2001;15:302–6. doi: 10.1097/00024382-200115040-00009. [DOI] [PubMed] [Google Scholar]
- 54.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. Jama. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 55.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 56.Vo TD, Cowles J, Heal JM, Blumberg N. Platelet washing to prevent recurrent febrile reactions to leucocyte-reduced transfusions. Transfus Med. 2001;11:45–7. doi: 10.1046/j.1365-3148.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 57.Kirkley SA, Cowles J, Pellegrini VD, Jr, Harris CM, Boyd AD, Blumberg N. Cytokine secretion after allogeneic or autologous blood transfusion. Lancet. 1995;345:527. doi: 10.1016/s0140-6736(95)90627-4. [DOI] [PubMed] [Google Scholar]
- 58.Babcock GF, Alexander JW. The effects of blood transfusion on cytokine production by TH1 and TH2 lymphocytes in the mouse. Transplantation. 1996;61:465–8. doi: 10.1097/00007890-199602150-00026. [DOI] [PubMed] [Google Scholar]


