Abstract
Background
HLA antibody testing of previously transfused or pregnant donors may help reduce the risk of transfusion-related acute lung injury (TRALI). However, the prevalence of HLA antibodies in transfused donors has not been well characterized.
Methods
Transfusion and pregnancy history was obtained from consenting donors. HLA Class I & II antibody testing was performed by multi-antigen bead Luminex platform. Cut off values for class I & II antibodies used normalized background ratio of 10.8 and 6.9 respectively. Linear probability models were used to evaluate potential associations between HLA alloimmunization and donor characteristics.
Results
7,920 donors (2,086 males and 5,834 females) were tested. HLA antibody prevalence did not significantly differ between 895 transfused (1.7%) and 1138 non-transfused males (1.0%), [odds ratio (OR) 1.75; 95% CI 0.80, 3.82]. Prevalence in 45 transfused nulliparous females (4.4%, 95% CI 0.1%, 11.8%) was not statistically different from the 1.6% prevalence in 1732 non-transfused nulliparous females (odds ratio 2.94, 95% CI 0.68, 12.74). Transfused parous females had higher prevalence than non-transfused counterparts (p=0.004), odds ratio 1.39 (95% CI 1.07, 1.80). In a linear probability model, the estimated additive risk of transfusion-induced alloimmunization was only 0.8% (95% CI -0.2%, 1.8%), (p=0.10). Donor transfusion history showed that 58% of transfusions occurred >10 years previously.
Conclusion
Transfused volunteer blood donors do not appear to have a significantly higher prevalence of HLA antibodies than their non-transfused counterparts. Thus, in an effort to reduce TRALI risk, ascertaining past history of transfusion and testing these donors for HLA antibodies is not necessary.
Introduction
Transfusion-related acute lung injury (TRALI) appears to be mediated by donor leukocyte antibodies in approximately 80–90% of the cases. Among leukocyte antibodies, HLA Class I and HLA Class II antibodies are frequently implicated. Donor risk factors for HLA antibody formation include allo-exposure to white blood cells during pregnancy or from blood transfusion. Exposure by blood transfusion occurs from the presence of HLA antigens present on the transfused leukocytes. Many HLA antigens are known to be strong immunogens and therefore, alloantibody (anti-HLA) production in transfusion recipients is frequent as has been demonstrated in frequently transfused patients with hematologic malignancies. The sensitization rates in these patients can be reduced if they are transfused with leukocyte-reduced blood components. Despite this overall reduction, the rates of alloimmunization in different studies vary considerably and range from 7% to 44% among recipients of leukocyte-reduced blood transfusions and from 20% to 50% among control recipients of non-leukoreduced blood components.1 Other factors that influence the rate of HLA alloimmunization from transfusion include the number of units transfused,2 the underlying clinical condition resulting in transfusion,1 time since transfusion2 and the method used for detecting HLA antibodies.3–4
These variables are pertinent when one considers prevalence of HLA alloimmunization in previously transfused blood donors, who comprise 4.2% of the donor pool.5 Since blood donors are deferred for 12 months after transfusion, transient antibodies will no longer be detectable. Donors are generally younger than the typical patients who are transfused. Finally, blood donors, like other transfused individuals in the general population, are likely to be transfused with only red blood cells, and only once or twice in their lifetime.6
Potential TRALI risk reduction strategies include not collecting plasma or apheresis platelets from transfused donors by either deferring these donors or redirecting them to red blood cell donation. Knowing the proportion of apheresis donors who have ever been transfused can help estimate donor/donation loss were such policies adopted. Another possible strategy could involve HLA antibody testing of apheresis donors who have a history of transfusion, and deferral or redirection of those transfused donors who have HLA (and/or neutrophil) antibodies. In this regard, there are very limited published data that provide HLA antibody prevalence estimates in transfused donors and predict consequent donor/donation loss. One study from the UK showed HLA antibodies in 4 of 205 (2.0%, 95% CI 0.5%–4.9%) non-transfused and 1 of 48 (2.1%, 95% CI 0.1%–11.1%) transfused male donors.7 These authors concluded that previous transfusion history did not influence HLA antibody prevalence in eligible blood donors.
We report the results of a large study of HLA antibody reactivity in U.S. donors designed in part to define the relative prevalence of antibody positivity in transfused and non-transfused donors.
Materials and Methods
The Leukocyte Antibody Prevalence Study (LAPS) was conducted between December 2006 and May 2007 as a prospective cross-sectional multi-center study by the National Heart, Lung, and Blood Institute’s (NHLBI) Retrovirus Epidemiology Donor Study – II (REDS-II). Study participants were recruited from eligible volunteer blood donors at the six REDS-II blood centers participating in the study: American Red Cross New England Region (Dedham, MA); American Red Cross Southern Region (Douglasville, GA); Blood Center of Wisconsin (Milwaukee, WI); Blood Centers of the Pacific (San Francisco, CA); Hoxworth Blood Center/University of Cincinnati Academic Health Center (Cincinnati, OH); and the Institute for Transfusion Medicine (Pittsburgh, PA). Westat (Rockville, MD) serves as the coordinating center. Blood Systems Research Institute (San Francisco, CA) is the central laboratory. LAPS was approved by the institutional review board at each participating center and at the coordinating center.
Study Population and Enrollment
The targeted enrollment goal for LAPS was 7,900 donors - approximately 5,700 females, 1,100 transfused males and 1,100 non-transfused males. Females and non-transfused males were enrolled for the study without using any particular targeted enrollment strategies. Donors who were not eligible to donate blood due to a history of blood transfusion in the previous 12 months were not eligible for the study. In order to enroll a sufficient number of transfused males to meet the study objectives, this population group was specifically over-recruited through the use of various targeted enrollment strategies at the participating blood centers. These strategies included: 1) identifying donors who indicated a past history of transfusion on the REDS-II supplemental questionnaire administered at time of donation, setting aside their collected tubes of blood, and then contacting the donor post-donation to get consent and questionnaire information; 2) identifying donors scheduled to donate who had previously indicated a history of transfusion and sending study recruiters to those drives to recruit them; and 3) letter recruitment – identifying transfused male donors in the center’s database, contacting them by mail, getting their consent by mail, and having them enroll in the study at the time of their next donation.
Donor Questionnaire
Each donor who consented to be in the study completed a short questionnaire inquiring into demographics, transfusion history and pregnancy history. The transfusion history questions were the following: “Have you ever received someone else’s blood?” and if yes, the month and year of the last transfusion episodes, up to two prior transfusions. Pregnancy history was elicited using six validated questions from the National Health and Nutrition Examination Survey (NHANES) addressing the number, outcome, and date of the last pregnancy including live and still births, miscarriages, terminations, or tubal pregnancies.
Sample Collection
A blood sample for HLA antibody testing was obtained at the time of donation, aliquoted and stored frozen at −70° C for subsequent batch testing. A written consent was obtained from each participating donor in the study authorizing leukocyte antibody testing, and retention of specimens in a study repository for future relevant testing including genetic testing to understand the basis for alloimmunization.
HLA Class I and Class II antibody testing
EDTA plasma samples were thawed for testing at the Central Laboratory and screened for HLA antibodies using One Lambda (Canoga Park, CA) Lab Screen LSM12 (LabScreen Mixed, Lot 13) multi-antigen bead kits according to manufacturer’s instructions. For Class I, the assay included six fluorescence tagged beads that were coated with approximately 30 antigens per bead. Similarly, 3 such beads for Class II antibody detection were included in the assay with each bead containing approximately 20 antigens. Reaction mixture consisting of 5 µl of beads and 20 µl of EDTA plasma was incubated at room temperature for 30 minutes. Beads were washed by centrifugation and were incubated with phycoerythrin (PE)-conjugated anti-human IgG for 30 minutes at room temperature. After two additional washes, fluorescence intensity was measured in a luminometer (Luminex, Austin, TX). As previously reported, normalized background (NBG) ratios for positive test interpretations for Class I and II assays were derived by testing the 1138 non-transfused male blood donors in LAPS.8 Fluorescence values obtained with these samples were log transformed and the assay cutoffs for positivity were established as those NBG ratios that exceeded the mean plus 3 standard deviations.8 For Class I, the cutoff NBG ratio was >10.8 and for Class II, it was >6.9.
Statistics
Fisher exact tests were performed to assess associations between two variables (e.g. transfusion history and HLA alloimmunization). Unadjusted odds ratios (with 95% confidence intervals) were calculated to compare transfused subgroups with non-transfused subgroups. To develop a multivariable model (predictive of HLA alloimmunization) a logistic regression model was considered. However, multivariable effects of interest are likely additive risks, rather than multiplicative risks as inferred by a logistic model. That is, for example, a transfusion likely causes an incremental increase in HLA alloimmunization. Hence, linear probability models were used to evaluate potential associations of donor demographics, pregnancy history, and transfusion history, on the presence or absence of HLA antibodies.9
Results
Of 8,171 donors who consented to participate in LAPS, 7,920 (97%) had valid HLA antibody test results. Of the 2,086 males with valid test results, 895 were transfused and 1,138 not transfused (transfusion history was missing for 53). Of the 5,834 females with valid test results, 1,816 had never been pregnant while 3,992 had had a previous pregnancy (pregnancy or transfusion history information was missing for 181). The prevalence of HLA antibody and associated 95% confidence intervals (CI) in different donor subgroups are shown in Table-1. The unadjusted odds ratios (and 95% CI) between subgroup pairs were as follows: transfused males v. non-transfused males odds ratio 1.75 (0.80, 3.82), transfused nulliparous females v. non-transfused nulliparous females odds ratio 2.94 (0.68, 12.74), and transfused parous females v. non-transfused parous females odds ratio 1.39 (1.07, 1.80). As previously reported, pregnancy history was strongly associated with HLA antibody prevalence.8 Adjusted logistic regression models revealed that the apparent increase in HLA antibody prevalence in transfused women with a previous history of pregnancy (as compared to their non-transfused counterpart) was primarily due to the larger number of pregnancies observed in the transfused group.8
Table-1.
HLA antibody prevalence in transfused and non-transfused subgroups of blood donors.
| Percentage (95% CI) |
p-value* | |||||
|---|---|---|---|---|---|---|
| Class I | Class II | Class I + II | any antibody | |||
| Males | Transfused (N=895) |
0.8% (0.3%, 1.6%) |
0.8% (0.3%, 1.6%) |
0.1% (0.0%, 0.6%) |
1.7% (0.9%, 2.7%) |
0.54 |
| Non- transfused (N=1138) |
0.4% (0.1%, 1.0%) |
0.4% (0.1%, 1.0%) |
0.1% (0.0%, 0.5%) |
1.0% (0.5%, 1.7%) |
||
| Nulliparous Females |
Transfused (N=45) |
2.2% (0.1%, 6.5%) |
2.2% (0.1%, 6.5%) |
0.0% (0.0%, 3.2%) |
4.4% (0.1%,11.8%) |
0.18 |
| Non- transfused (N=1732) |
0.8% (0.4%, 1.4%) |
0.7% (0.4%, 1.2%) |
0.1% (0.0%, 0.3%) |
1.6% (1.0%, 2.3%) |
||
| Parous Females |
Transfused (N=299) |
6.7% (3.9%, 9.5%) |
12.0% (8.6%,16.3%) |
11.7% (8.1%,15.4%) |
30.4% (25.3%, 36.0%) |
0.004 |
| Non- transfused N=3598) |
7.9% (7.0%, 8.8%) |
9.6% (8.6%,10.6%) |
6.5% (5.7%, 7.4%) |
24.0% (22.6%, 25.4%) |
||
Fisher exact test of association.
To better evaluate the effect of pregnancy and transfusion on HLA alloimmunization, a linear probability model was developed. This model provides information about additive risks for HLA antibody due to gender, transfusion and pregnancies. These additive risks are shown in Table-2. The intercept of the model (1.6%, Table-2) represents the HLA antibody prevalence in non-transfused nulliparous females.
Table-2.
Linear Probability Model for HLA antibody prevalence***
| Additive risk for HLA alloimmunization (95% CI) |
p-value | ||
|---|---|---|---|
| Intercept | 1.6% (1.0%, 2.2%) | ||
| Transfusion history | Non-transfused Transfused |
0.0% 0.8% (−0.2%, 1.8%) |
0.10 |
| Gender | Female Male |
0.0% −0.6% (−1.4%, 0.2%) |
0.12 |
| Deliveries* | Zero deliveries One delivery Two deliveries Three deliveries ≥4 deliveries |
0.0% 14.5% (11.7%, 17.3%) 21.9% (19.7%, 24.0%) 29.0% (25.9%, 32.0%) 32.4% (28.0, 36.8%) |
<0.0001 |
| Lost pregnancies** | 0 lost pregnancies 1 lost pregnancy ≥2 lost pregnancies |
0.0% −0.4% (−1.7%, 0.9%) 4.5% (1.3%, 7.7%) |
0.02 |
All deliveries include live as well as still births
Pregnancies that were medically or spontaneously terminated
Missing data as follows: Delivery information (N=21); Lost pregnancy information (N=17), and Transfusion history (N=188)
Probability of HLA antibody prevalence = intercept + transfusion effect + gender effect + delivery effect + lost pregnancy effect
For example, transfused males have predicted probability of HLA antibodies = 1.6% + 0.8% + -0.6% + 0.0% + 0.0% = 1.8%
Additive risk related to gender
As shown in Table-2, the prevalence of HLA antibody in males was lower by -0.6% (95% CI: -1.4%, 0.2%) compared to the rate of 1.6% in non-transfused nulliparous females. However, this difference was not statistically significant (p=0.12).
Additive risk related to deliveries
The model in Table-2 also showed that each delivery (up to 4) resulted in a higher likelihood of being positive for HLA antibodies (p<0.0001). For instance, females with one delivery had additive risk of 14.5% for HLA antibody. Hence, a non-transfused female with one delivery and no lost pregnancies would have a predicted probability of HLA antibodies equal to 16.1% (1.6% +14.5%). Each additional pregnancy statistically increases the likelihood of being positive for HLA antibodies (analysis not shown though is evidenced by confidence intervals in Table 2).
Additive risk related to lost pregnancies
The model also examined the additive risk for one or more lost pregnancies. As seen in Table-2, while one pregnancy loss did not appear to be associated with an increase in HLA antibody positivity, 2 or more early pregnancy losses were (4.5% additional risk, p=0.02).
Additive risk related to transfusion history
As seen in Table-2, after adjusting for gender and pregnancy history (number of deliveries and lost pregnancies), the additive risk of a past transfusion was small, 0.8%, and not statistically significant (p=0.10, 95% CI -0.2%, 1.8%). Time since last transfusion was also not statistically significant (p=0.25), whereas time since last pregnancy was statistically significant (p=0.001) (data not shown). Time since last pregnancy was not included in the presented model as it would not have substantively altered the findings but would have complicated the model (data not shown).
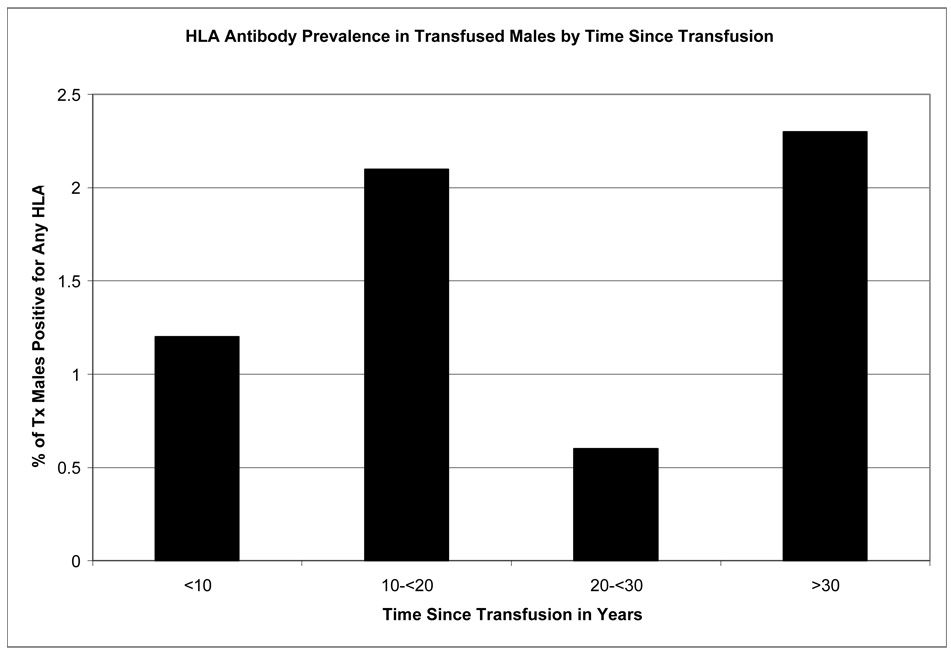
Time since last transfusion for male subgroup
Time intervals since last reported transfusion in male donors and corresponding HLA antibody prevalence data are shown in Figure-1 (female donors were not included for this analysis, since the dominating risk factor among females for HLA alloimmunization appears to be pregnancy history). The HLA antibody prevalence estimates were 1.2% (3/241), 2.1% (3/144), 0.6% (1/154), and 2.3% (7/30) for male donors whose last transfusion was <10, 10–20, 20–30, and 30+ years prior to the tested donations, respectively. Time since last transfusion was not significantly associated with HLA antibody prevalence (p=0.54). Most donors had a remote transfusion history, as 58% (328/569) had 10+ years since their last transfusion.
Figure 1.
Prevalence of HLA antibodies in transfused males
Discussion
Our study demonstrates that the prevalence of persistent HLA antibodies detectable in previously transfused male donors is low and statistically comparable to the level in non-transfused male donors. This is also the case for non-transfused and transfused nulliparous female donors and in those with one lost pregnancy. These results suggest that there is minimal value in screening blood from transfused males or females in the absence of additional sources of alloexposure, in particular a history of more than one lost pregnancy.
The prevalence of HLA antibody reactivity in previously pregnant transfused and non-transfused females (30% and 24% respectively) was much higher than in males or non-parous females, highlighting the importance of pregnancy as the major alloimmunization factor among donors. Interestingly, a single lost pregnancy did not stimulate detectable HLA antibody whereas two lost pregnancies resulted in an increase in HLA antibody prevalence. Thus the first lost pregnancy may be sufficient only to act as a sensitizing event whereas a second lost pregnancy may be capable of stimulating an alloimmune response. Further studies would be needed to corroborate these observations and to explore their underlying biology.
In an analysis controlling for pregnancy history, the association between transfusion history and HLA antibody reactivity was small (additive risk of 0.8%, with upper confidence limit of 1.8%) and non-significant, suggesting that past transfusion does not have a marked independent effect on HLA antibody prevalence. The upper confidence limit is suggestive that the transfusion effect, if it exists, is less than 2%.
Our study included a large number of transfused males to better assess the association between transfusion and HLA antibody prevalence. Although our study wasn’t powered to detect very small differences in prevalence between transfused and non-transfused males, the percentages of donors with HLA antibody are so low that even if a larger study with more statistical power showed a significant difference, this would likely be of little clinical importance in terms of driving a TRALI policy recommendation for deferral or testing of previously transfused donors.
Previous studies have established that transfusions stimulate HLA antibody formation. The reason that an increased HLA antibody prevalence was not associated with transfusions in this study is likely due to the fact that the donors enrolled in LAPS had a remote history of blood transfusion. Due to donor eligibility requirements, all transfused donors in this study received their transfusion a minimum of 12 months previously. In fact, based on questionnaire data, 58% of our donors reported that their most recent transfusions occurred more than 10 years previously. Although previous studies have documented that 4.2% of donors and 4.9% of donations have a previous history of transfusion,5 we are unaware of any previous study that has documented the timing of past transfusions. In particular, we have shown that our blood donors gave a history indicative that they had received transfusions in the remote past. One limitation of our data is the possibility that some donors may not be able to recall a history of previous blood transfusion. For 2005–2006, the National Health and Nutrition Examination Survey (NHANES) reported that 81 of 8378 (0.97%) individuals didn’t know whether they had ever received blood transfusion. Blood donation data compiled for the REDS-II during 2006–2008 has shown that for 97,681 of 3,637,936 (2.69%) donation visits, the donor indicated that they were not sure or didn’t know if they had ever received transfusion. The percent of LAPS enrolled donors who answered “don’t know” to the question of ever received transfusion was also low (2.3%). Thus, the percent of individuals in both the general and blood donor populations who are unable to recall a history of previous transfusion is low (1–3%). Nonetheless, self-reported transfusion history may underestimate the frequency of previous transfusion. Our observations that the HLA alloimmunization rate difference was minimal among transfused and non-transfused donors, there was low frequency of inability to recall the transfusion history, and the donors were transfused remotely suggest that under-reporting of transfusion history does not impact the conclusions of our study.
The 1–2% prevalence of HLA antibodies observed in our study for male donors was much lower than the 45% and 63% estimates reported in two recent studies.10–11 In the first study, HLA antibodies were reported in 25 of 55 (45%) male donors.10 These authors did not indicate transfusion history, but it is likely that only a small percentage of these donors had previously been transfused. While these authors used the same HLA antibody testing method that we used (One Lambda screening assay on a Luminex platform), they chose the manufacturer’s suggested assay cutoff (NBG ratio of 2.2), which was lower than the assay cutoffs used in our study. The manufacturer’s suggested cutoffs have been previously used for screening of organ recipients to maximize sensitivity of the test to prevent graft rejection. Applying such low cutoffs to the blood donor population may result in the identification of reactivity to HLA antigens stimulated by mechanisms other than transfusion or pregnancy (see below). In the second study, 267 of 424 (63%) non-transfused healthy male subjects had HLA antibodies when screened by a different assay (One Lambda single antigen assay on a Luminex platform).11 These authors also used a low cutoff (>1000 fluorescence intensity) for this assay. They postulated that the high HLA antibody prevalence detected in non-alloexposed males may be due to exposure to microbial antigens that are cross reactive with specific HLA epitopes (i.e., heterophile antibodies). To some extent, false positives may also explain the high antibody rates (45 to 63%) seen in non-transfused males, a population that is unlikely to produce allo-antibodies. In fact, if the fluorescence intensity threshold is lowered to 500, then the prevalence of antibodies goes up from 63% to more than 80%.11 It is unlikely that more than 80% of non-transfused males are sensitized to HLA antigens and that they produce persistent allo-antibodies. Specimens showing antibodies by a sensitive assay when tested by another flow-based method do not confirm in 20% of the cases.10 These findings indicate that the reactivity seen with the sensitive assay may represent false positives. Similarly, for organ transplants, the clinical relevance of antibodies detected by very sensitive assays has been questioned because excellent long-term kidney graft survival has been seen in many patients with donor-specific antibodies detectable in Luminex assays.12 Although low assay cut offs might permit detection of heterophile naturally occurring antibodies, clinical significance of such antibodies is currently unknown, both with respect to donor health and potential to induce TRALI. Based on these considerations, we believe that deriving the assay cutoff from the distribution of results in non-alloexposed male donors, as we have done, is appropriate for blood donor screening in order to avoid false positive results and/or the detection of low-titer or heterophile antibodies.
One additional recent study found that none of the 229 male donors (uncharacterized for transfusion history) had detectable HLA antibodies when tested using an ELISA method.13 Therefore, HLA antibody prevalence in non-alloexposed blood donors is clearly dependent upon the testing methodology (ELISA versus flow cytometry) used to detect the antibody, as well as the assay cutoff chosen in the flow cytometry methods.
Based on our data, we conclude that HLA antibody screening of male donors (whether or not they have a history of transfusion), nulliparous female donors who have a history of transfusion or females with a history of one lost pregnancy is not necessary as a risk reduction strategy for TRALI. This conclusion could be modified in the future if clinical data show that low-level HLA antibody reactivity found only with very sensitive assays is clinically important in TRALI causation.
Acknowledgement
This work was supported by NHLBI contracts N01-HB-47168, -47169, -47170, -47171, -47172, -47175 and -57181.
The Retrovirus Epidemiology Donor Study (REDS)- II is presently the responsibility of the following persons:
Blood Centers:
-
American Red Cross Blood Services, New England Region
R. Cable, J. Rios, R. Benjamin
-
American Red Cross Blood Services, Southern Region / Emory University
C.D. Hillyer, K.L. Hillyer, J.D. Roback
-
Blood Center of Wisconsin
J. Gottschall, A.E. Mast
-
Hoxworth Blood Center, University of Cincinnati Academic Health Center
R.A. Sacher, S.L. Wilkinson, P.M. Carey
-
Regents of the University of California/Blood centers of the Pacific/BSRI
E.L. Murphy, M.P. Busch, B. Custer
-
The Institute for Transfusion Medicine (ITxM)/LifeSource Blood Services
D. Triulzi, R. Kakaiya, J. Kiss
Central Laboratory:
Blood Systems Research Institute: M.P. Busch, P. Norris
Coordinating Center:
Westat, Inc.: Jane Schulman, M. King
National Heart, Lung, and Blood Institute, NIH:
G.J. Nemo
Steering Committee Chairman:
R.Y. Dodd
Footnotes
Conflict of Interest: None known
References
- 1.Vamvakas EC. Meta-analysis of randomized controlled trials of the efficacy of white cell reduction in preventing HLA-alloimmunization and refractoriness to random-donor platelet transfusions. Trans Med Rev. 1998;12:258–270. doi: 10.1016/s0887-7963(98)80002-3. [DOI] [PubMed] [Google Scholar]
- 2.Gleichmann H, Breninger J. Over 95& sensitization against allogeneic leukocytes following single massive blood transfusion. Vox Sang. 1975;28:66–73. doi: 10.1111/j.1423-0410.1975.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 3.Lubenko A, Rodi KM. The detection of enzyme-linked immunoabsorbent assays of non-complement-fixing HLA antibodies in transfusion medicine. Transfusion. 1998;38:41–44. doi: 10.1046/j.1537-2995.1998.38198141496.x. [DOI] [PubMed] [Google Scholar]
- 4.Gebel H, Pollack MS, Bray RA. In: The HLA system. In: Technical Manual. 16th Edition. Roback JD, et al., editors. AABB; 2008. p. 558. [Google Scholar]
- 5.Wang B, Higgins MJ, Kleinman S, Schreiber GB, Murphy EL, Glynn SA, Wright DJ, Nass CC, Chang D, Busch MP. Comparison of demographic and donation profiles and transfusion-transmissible disease markers and risk rates in previously transfused and nontransfused blood donors. Transfusion. 2004;44:1243–1251. doi: 10.1111/j.1537-2995.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 6.Vamvakas EC. Universal leukoreduction is a major advancement in transfusion therapy. Con. Trans Alter Trans Med. 2004;6:63–64. [Google Scholar]
- 7.MacLennon S, Lucas G, Brown C, et al. Prevalence of HLA and HNA antibodies in donors: correlation with pregnancy and transfusion history. Vox Sanguinis. 2004;87 Suppl3:S4. [Google Scholar]
- 8.Triulzi DJ, Kleinman S, Kakaiya R, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: Implications for a Transfusion Related Acute Lung Injury (TRALI) risk reduction strategy. Transfusion. 2009;49:1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agresti A. An introduction to categorical data analysis. Wiley series in probability and mathematical statistics. Hoboken, NJ: Wiley-Interscience; 2007. [Google Scholar]
- 10.Fadeyi E, Adams S, Peterson B, Hackett J, Byrne P, Klein HG, Marincola FM, Leitman SE, Stroncek DE. Analysis of a high-throughput HLA antibody screening assay for use with platelet donors. Transfusion. 2008;48:1174–1179. doi: 10.1111/j.1537-2995.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, Lee JH, El-Awar N, Alberu J. “ Natural ” human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 12.Claas FJH. HLA antibody testing: a tool to facilitate not to prevent organ transplantation. Internat J Immunogenetics. 2008;35:275–277. doi: 10.1111/j.1744-313X.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reil A, Keller-Stanislawski B, Gunay S, Bux EJ. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–317. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]