Abstract
Objective
To investigate the effects of epigallocatechin gallate (EGCG), an extract of green tea on cultured human leiomyoma cells (HuLM).
Design
Laboratory study.
Setting
University hospitals.
Patients(s)
Not applicable.
Interventions(s)
Not applicable.
Main Outcome Measure(s)
HuLM cells were treated with various EGCG concentrations. Cell proliferation was assayed using Hoechst 33258 dye and apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Total RNA was isolated and gene expression profiling was performed on 84 key genes related to 18 different signal transduction pathways. The protein levels of PCNA, CDK4, BCL-2 and BAX were examined by Western blot analysis.
Result(s)
HuLM cells treated with EGCG showed a dose- and time-dependent inhibition of cell proliferation. TUNEL staining indicated a significant increase in apoptosis in HuLM cells treated with 100 µM of EGCG compared to untreated control (p<0.05). Gene expression profiling indicated that EGCG treatment upregulated representative genes from the TGF-β and stress pathways, while inhibiting the survival pathway and NFκB-dependent inflammatory pathway. Western blotting analysis confirmed that EGCG at ≥50µM significantly decreased the expression of PCNA, CDK4 and BCL-2 as well as increased the expression of the proapoptotic BAX in a dose-dependent manner (p<0.05).
Conclusion(s)
EGCG inhibits the proliferation of HuLM cells and induces apoptosis. These results suggest that EGCG may be a potential anti-uterine fibroid agent acting through multiple signal transduction pathways.
Keywords: Epigallocatechin gallate, Leiomyoma, Proliferation, Apoptosis
INTRODUCTION
Uterine fibroids (leiomyomas) of the human myometrium are a major women’s health problem. They are clinically apparent in at least 25% of those of reproductive age (1, 2). These benign tumors cause considerable morbidity, including prolonged or heavy menstrual bleeding, and in some cases, reproductive dysfunction. Higher incidence of symptomatic leiomyomas in African American women compared to white women, have been reported by us and others (3–5). Uterine fibroids are the leading cause of hysterectomy in premenopausal women, because other non-surgical treatment modalities to reduce fibroid-associated symptoms are scarce. Side-effects limit the long-term use of effective medications such as GnRh agonists (6). An orally administered compound that can effectively and safely reduce or eliminate fibroid-associated symptoms will be a welcome addition and will have a major favorable impact on women’s health.
Tea is the most popular beverage in the world and consists of three major commercial types: green, black and oolong tea. Polyphenols, the majority of chemical composition of tea, show diverse chemical and biological activities, including modulation of key enzymes such as mitogen-activated protein kinases and protein kinase C (7). These compounds also regulate expressions of cyclins, oncogenes, and tumor suppressor genes at the DNA, RNA, and protein levels (8, 9).
Epigallocatechin gallate (EGCG), the principal catechin, comprises >40% of the total polyphenolic mixture of green tea catechins and represents 150 mg in a single brewed cup of green tea (9). Catechines are a group of bioflavonoids that exhibit antioxidant and anti-inflammatory capacity. Chemically, catechines are polyhydroxylated with water soluble characteristics (10). EGCG exhibits various biological activities including potent antioxidant and anti-inflammation capacity (11). Previous studies have shown that EGCG inhibited the growth of various human cancer cells such as epidermoid carcinoma cells (12), hepatoma cells (13), prostate carcinoma cells (14, 15), breast cancer cells (16) and myeloid leukeamic cells (17). In this study, we wanted to evaluate the effect and potential mechanisms of EGCG action on human leiomyoma (HuLM) cells.
MATERIAL AND METHODS
Smooth muscle cell basal medium were purchased from Lonza (Walkersville, MD, USA). EGCG was from Sigma (St. Louis, MO). RNease kit was purchased from Qiagen (Qiagen, Valencia, CA). RT first strand kit, superarray RTqPCR master mix and human signal pathway finder were purchased from SuperArray Bioscience Corporation (Frederick, MD). All other chemicals and biochemicals were of the highest quality available from commercial resources.
Cell Culture
The human uterine leiomyoma (HuLM) cell line was a gift from Dr. D. Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, NC) (18, 19). These cells are a representative cell model of HuLM. and have no phenotypic alteration from immortalized and parental leiomyoma, and myometrial cells.
These HuLM cells express both estrogen receptors and progesterone receptors, and are negative for mutant p53 protein as well. This cell line has been used extensively in various HuLM research by our group and others (20–22). HuLM cells were cultured in SmBM medium supplied with 5%FBS, 0.1% insulin, 0.2% hFGF-B, 0.1% GA-1000 and 0.1% hEGF (Lonza, Walkersville, MD, USA). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
EGCG Dosage Experiments
EGCG dissolved in distilled water and filtered through 0.22µm filter as a 10mM stock solution and stored at −20°C. For cell proliferation assay, the cells were plated at a density of 2×105cells/well in 6-well plates and grown under conditions mentioned above. The monolayer cultures at approximately 70% confluence were treated with various concentrations (0, 0.1, 1.0, 10, 50, 100 and 200µM) of EGCG for 7 days. Culture media were changed every other day. For western blotting assay, HuLM cells were seeded in a range of 1×106 cells/100mm dish. After 24 h the cells were treated with EGCG at concentrations indicated above. Following 48 h EGCG treatment, the cells were lysed with RIPA buffer (Sigma, St. Louis, MO). The concentrations of proteins were determined using BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL).
HuLM Cell Morphological Evaluations
Cell morphological evaluation was performed under a Nikon Eclipse TS-100 phase-contrast microscope when cells reached 70% confluence following EGCG addition at various (ascending) concentrations. Photographs were taken at desired time interval.
Cell Proliferation Assay
To evaluate cell proliferation, DNA content was measured using the fluorometric method as we have described previously (21). Briefly, HuLM cells (5×103 cells/well) were plated in six-well plates. The cells were allowed to attach overnight at 37°C, and then the cells were treated with desired concentration of EGCG. On day 1, 3, 5, and 7 after EGCG treatment, medium was aspirated and rinsed twice with ice cold PBS. One milliliter of distilled water was added into each well and stored at −20°C to allow the cell lysis. Cell lysates were collected and stained with Hoechst 33258 dye solution (1 µg/ml, Sigma, USA). The emission spectrum of the mixture was determined for an excitation at 365 nm by measuring fluorescence emission of 458 nm using plate reader. The standard curve was determined using a known concentration of calf thymus DNA (Sigma, St. Louis, MO).
TUNEL Assay
EGCG Treated HuLM Cells were stained for TUNEL to detect apoptosis. HuLM cells were seeded onto BioCoat CultureSlide (BD Bioscience, San Jose, CA) and treated with 100 µM of EGCG or vehicle for 48 h. This dose of EGCG was selected based on our preliminary cell morphology and dose range evaluations as well as literature on prior studies with cancer cells (17, 18). Apoptosis in HuLM cells was determined by the TUNEL technique using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI). The slides were fixed in 4% formaldehyde (methanol-free) solution. Slides were then incubated in equilibration buffer followed by rTdT incubation buffer containing nucleotide mix and rTdT enzyme at 37°C for 1 h. The reaction was terminated with 2× SSC buffer. Positive control reaction was performed using 10 units/ml DNase I (Sigma) treated slides; the negative control reaction was done by omitting the rTdT enzyme. The apoptotic nuclei were stained by 1 µg/ml of propidium iodide solution (Sigma). Slides were examined under an Eclipse TE2000-S, fluorescence microscope (Nikon, Melville, NY). The number of positive cells (green fluorescence) in four fields of each slide was counted with 200× magnification. Images were captured and merged using Nikon Advanced Research Imaging Software available at the Meharry Morphology Core (supported by NIH grant U54NS041071).
RNA Isolation and RT Profiler PCR Array
To explore the expression of representative genes under EGCG treatment, HuLM cells were treated with either 100µM EGCG or vehicle (control) for 48 h. The 100µM EGCG was selected based on our dose-range observation of morphological changes and antiproliferative effects with HuLM cells. Total RNA was isolated from treated and control cells and used for RT Profiler PCR Array. Cultured cells were lysed in buffer containing β-mercaptoethanol and processed for RNA extraction using the RNAeasy column. One microgram of total RNA was used for reverse transcription using the RT first strand kit. Gene expression profiling was performed using commercial cDNA arrays from SuperArray (Cat No.: PAHS-014A, SuperArray, Inc., Frederick, MD) that contains 84 key genes representative of 18 different signal transduction pathways. The PCR reactions were carried out in a final volume of 50µl. The standard cycling condition was 50°C for 2 minutes, 90°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Fold units were calculated dividing the expression fold changes of the candidate genes by the expression fold changes of the reference genes, using the comparative threshold cycle (Ct) method (23). Using cut-off criteria, a 2.0 fold induction or repression in expression were considered to be of biological importance.
Western Blotting for PCNA, CDK4, BCL2 and BAX
PCNA, CDK4, BCL2 and BAX protein levels were detected by western blot analysis. Equivalent amounts of protein extracts, from HuLM cells treated with various dosage of EGCG were separated by NuPAGE Novex 10% Bis-Tris Gel (Invitrogen Life Technologies, Carlsbad, CA) under a reducing condition using 200V for 50 min, as we have described earlier (20). The proteins were then electrophoretically transferred onto PVDF membranes (Millipore Corp., Billerica, MA) using the XCell II Blot Module (Invitrogen Life Technologies, Carlsbad, CA). After blocking nonspecific binding sites by incubation for 1 h with PBS containing 5% fat-free milk and 0.1% Tween 20, the membranes were incubated with the primary antibodies overnight at 4°C. Immunological detection was performed using the following primary antibodies human proliferation cell nuclear antigen (PCNA) (1:500 dilution), BCL2 (1:200 dilution), Bax (1:500 dilution) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and Cdk4 (1:1000 dilution, Sigma, St. Louis, MO). The membranes were then incubated for 1 h with horseradish peroxidase conjugated secondary antibodies diluted 1:5000 with blocking buffer. The antigen-antibody complexes were detected with the ECL chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ, USA). The membranes were reprobed with a monoclonal antibody raised against beta-actin (diluted1:5000, Sigma, St. Louis, MO) as an internal control for protein loading and normalization between samples. Films exposed to blots were scanned and optical densities of the band signals were quantified.
Statistical Analysis
All data were expressed as the mean ± SD of the values obtained from three replicates. Statistic significance was determined using ANOVA. A difference with p< 0.05 was concerned statistically significant.
RESULTS
Effects of EGCG on Cell Morphology in HuLM Cells
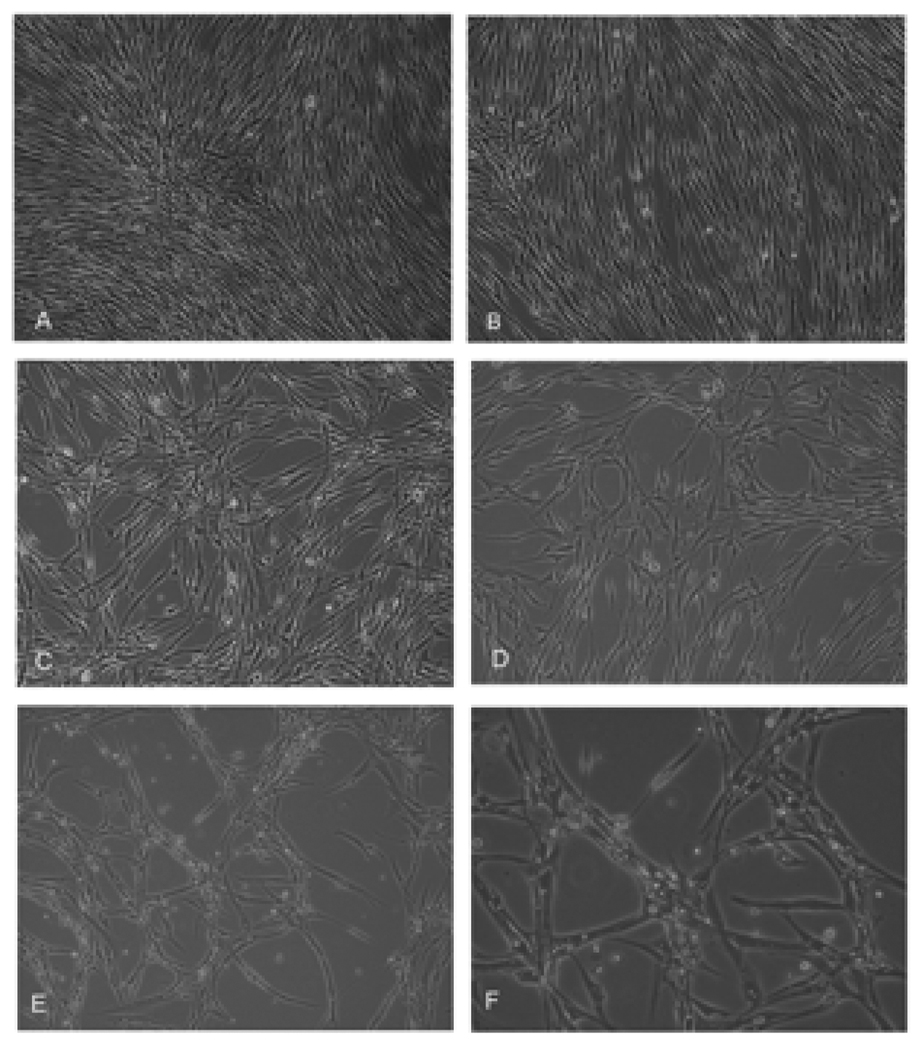
The general morphology of HuLM cells incubated with varied concentration of EGCG is shown in Figure 1. HuLM cells displayed fibroblast-like morphology and appeared healthy in complete medium. The figure shows that the cells were well spread, and there was no distinct change in morphology relative to control cells even after 48 h of incubation with 10 µM EGCG (Fig. 1a, b). However, obvious changes occurred when HuLM cells were treated with ≥50 µM EGCG. Within 48 h of exposure to EGCG at concentrations of ≥50 µM, the cells became less confluent, with visible apoptotic vesicles. (Fig. 1c–f).
Figure 1.
Effect of EGCG on morphology of HuLM cells. HuLM cells were treated with EGCG concentrations at 0µM(a), 10 µM(b), 50µM(c), 100µM(d) and 200µM(e, f) for 48h. Pictures were taken using a phase-contrast microscope at 100× (a–e) or 200 × (f) magnifications. The data shown here are representative of experiments repeated twice.
Antiproliferative Effects of EGCG on HuLM cells
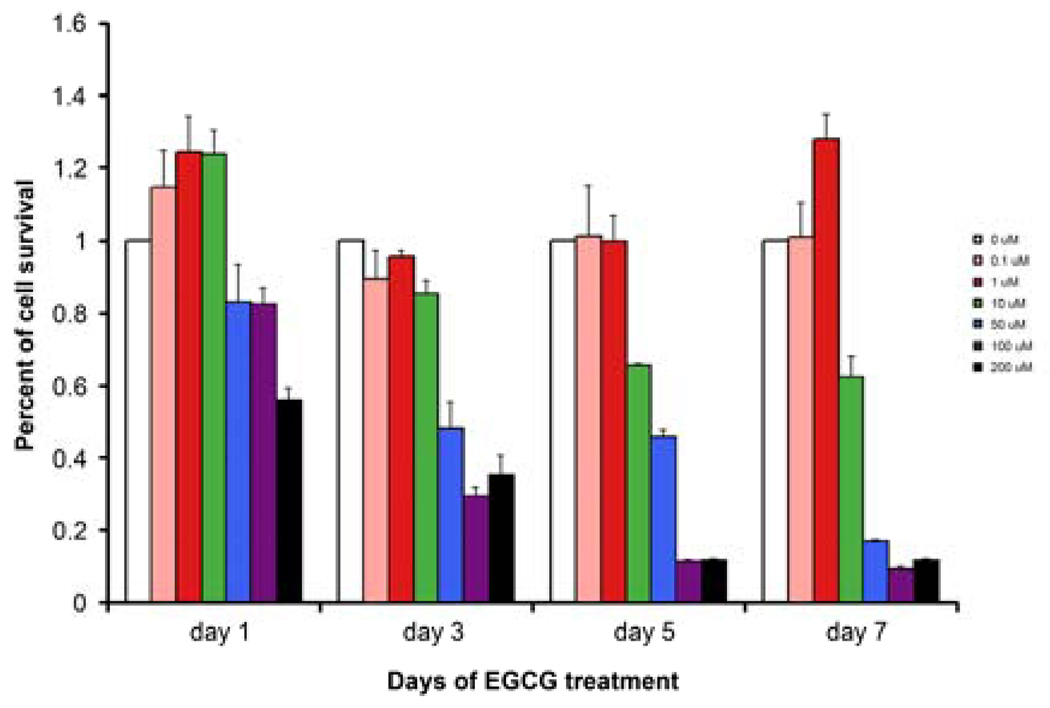
There was a gradual decrease in the DNA content throughout the 7-days culture period for leiomyoma cells treated with ≥ 10µM EGCG. The inhibitory effects on HuLM cells were detected at EGCG concentrations of 50 µM and above, by day-3 post treatment while the lower 10 µM EGCG dosage required 5 day treatment to exhibit similar inhibition of proliferation. At 200µM EGCG significantly reduced cell proliferation by 45% after 24 h of treatment, compared with untreated control (p<0.05). The inhibitory effect of ≥ 50µM EGCG on or after 3 days, and 10 µM EGCG after 5 days was statistically significant (p<0.05) (Fig. 2).
Figure 2.
EGCG inhibits the proliferation of HuLM cells. HuLM cells were treated with EGCG (0, 0.1, 1, 10, 50, 100 and 200 uM) for up to 7 days. The cells were lysed and DNA content was measured with Hoechst 33258 dye solution. The results are presented as the percent changes from control (0µM EGCG). The inhibitory effect of ≥ 50µM EGCG and above was statistically significant (p<0.05).
Proapoptosis effect of EGCG on HuLM Cells
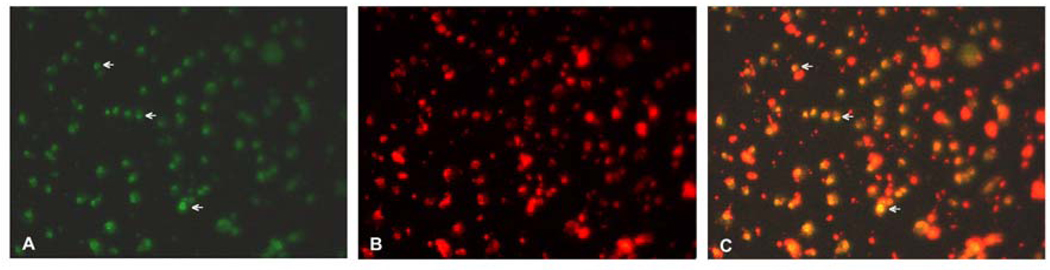
The inhibitory effect of EGCG on HuLM cells via induction of apoptosis was confirmed by TUNEL staining (Fig. 3). Examination under microscope showed some apoptotic nuclear condensation with green fluoresence, while no membrane blebbing visible. The number of apoptotic cells was about 54.8 % ± 15.4% after 100 µM EGCG treatment and 17.1% ± 9% positive cells per field in untreated control (p<0.05).
Figure 3.
EGCG-mediated apoptosis in HuLM cells assessed by TUNEL staining (green fluorescence- panel A). Arrows point to representative apoptotic HuLM cells treated with 100µM EGCG (A). Nuclei were stained with propidium iodide (B-red fluorescence). Images in A and B are super imposed in panel C (note the apoptotic nuclei appear yellow). Magnification 200×.
EGCG Modulate Gene Expression through Multiple Signaling Pathways
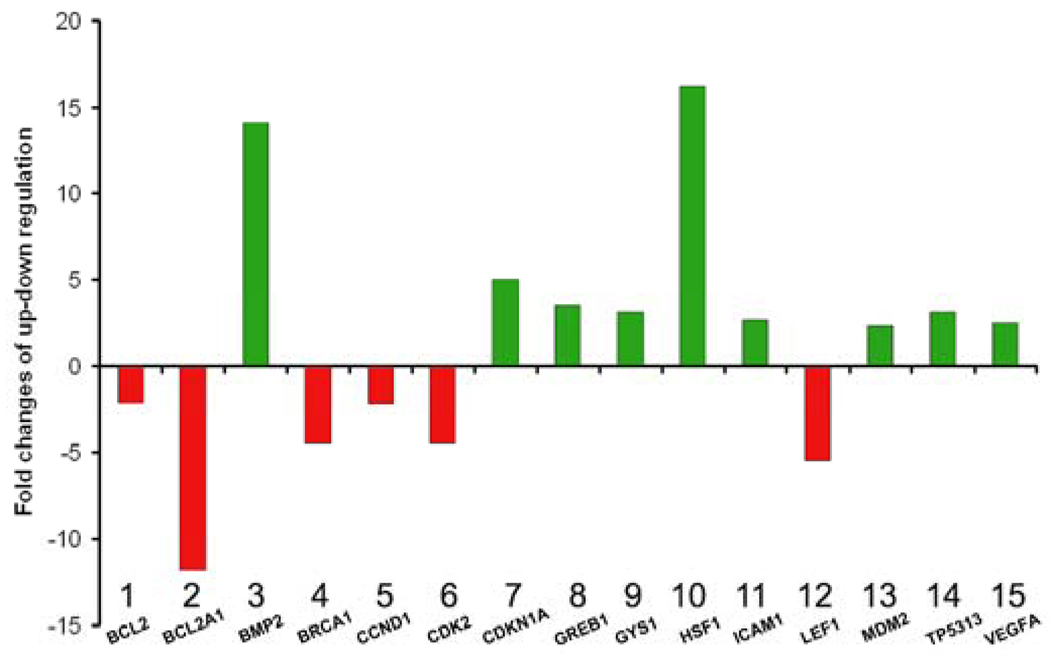
EGCG significantly altered the expression of multitude of genes related to several cellular pathways including the survival pathway, stress pathway, NFκB pathway, phospholipase C pathway and p53 pathway. Figure 4, gives the normalized data, for the genes that showed minimum two fold change of up- or down-regulation. It is apparent that the EGCG modulated the expression of at least fifteen genes, from a total of 84 tested, which belong to multiple signal pathways. EGCG inhibited the expression of BCL2, BCL2A1 and Cyclin D1, while enhancing the expression of the genes encoding Cyclin Dependent Kinase Inhibitors (CDKN1A, CDKN2B), MDM2 and tumor protein p53 inducible protein 3 (TP53I3). EGCG dramatically enhanced expression of bone morphogenetic protein 2 (BMP2) and heat shock factor 1 (HSF1) at 14.1- and 16.2- fold higher than control, respectively. Intriguingly, EGCG altered the expression of several individual genes within the Wnt pathway, i.e., increased the expression of vascular endothelial growth factor A (VEGFA), while decreasing the expression of cyclin D1 and lymphoid enhancer-binding factor 1 (LEF1).
Figure 4.
Effect of EGCG treatment on the expression of genes related to multiple signal transduction pathways in HuLM cells. Expression levels in HuLM cells treated with 100µM EGCG were normalized against untreated cells. Only genes with expression fold change of ≥2 (green bars) or ≤2 (red bars) are depicted.
EGCG treatment induces pro-apoptosis, and inhibits proliferation-associated genes in HuLM Cells
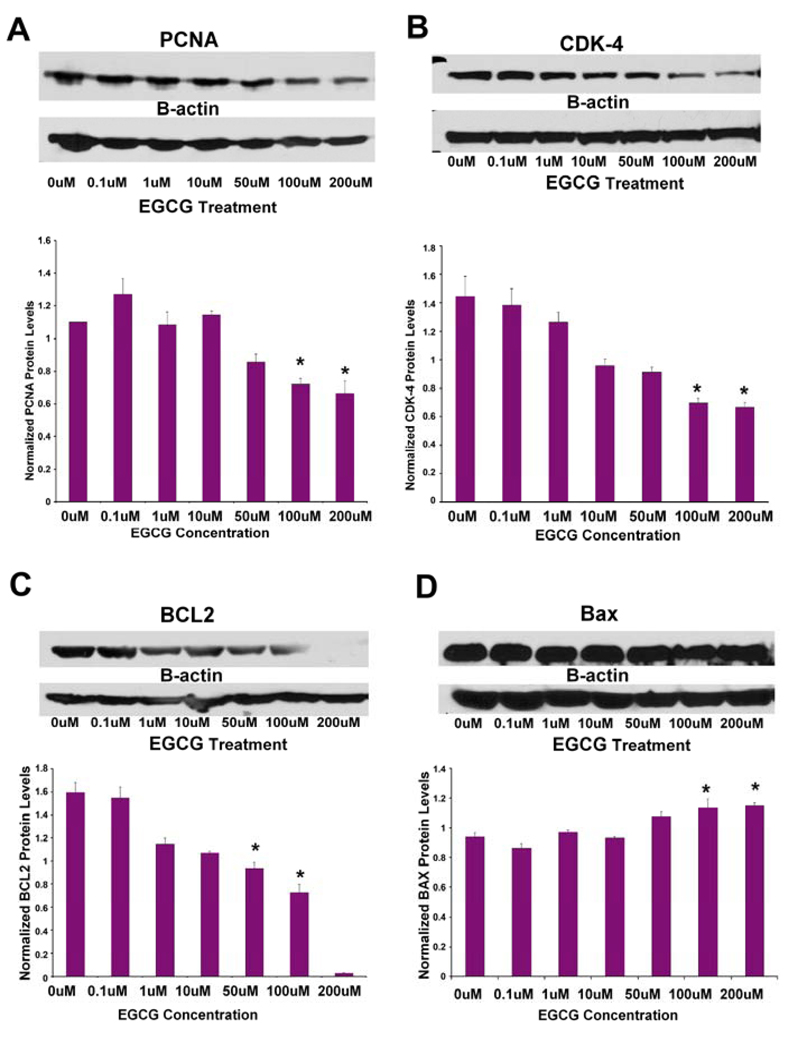
To further confirm the effects of EGCG on signal transduction, HuLM cells were cultured in the presence or absence of the corresponding concentrations of EGCG for 48 h (Fig. 5) and were assessed by western blotting, for PCNA, Cdk4, Bcl-2 and Bax expression as representatives of cell proliferation, cell cycling, and apoptosis, respectively. EGCG at concentration of 50µM or higher significantly decreased the expression of PCNA (Fig. 5A), Cdk4 (Fig. 5B), and Bcl-2 (Fig. 5C), in comparison to untreated control culture (p<0.05). Additionally, EGCG increased the expression of the proapoptotic Bax protein at 100 µM and 200 µM, compared with untreated control culture (p<0.05, Fig. 5D).
Figure 5.
Western blot analysis detecting the effects of various EGCG concentrations on pro-apoptosis and proliferation-associated genes in HuLM cells. EGCG, at 50µM or higher concentration significantly decreased the expression of PCNA (A), Cdk4 (B), and Bcl-2 (C), in comparison to untreated control culture (p<0.05). EGCG increased the expression of the proapoptotic Bax protein at 100 µM and 200 µM, compared with untreated control culture (p<0.05) (D). Representative western blots of PCNA, Cdk4, Bcl-2, Bax are shown with β-actin probed for normalization. Data are representatives of two independent experiments. Representative pictures of PCNA, Cdk4, Bcl-2, Bax and β-actin. β-actin was used as loading control. *Indicates significant difference from the vehicle-treated control (p<0.05). Data are representatives of two independent experiments.
DISCUSSION
Uterine fibroids are the most common benign tumor of the female genital tract. Recent longitudinal studies have estimated that the lifetime risk of fibroids in a woman over the age of 45 years is more than 60% (24). There are currently no effective, long-term orally administered drug therapies for leiomyomas (2).
A number of recent studies have found that EGCG could inhibit proliferation of several cancer cells (12–16, 25). In the present study, HuLM cell line is used to evaluate the antitumor effects of EGCG. We have shown that antiproliferative effects of EGCG were both time- and dose-dependent. EGCG at 50µM and above significantly inhibited the growth of HuLM cells as early as 72h of incubation, while there was no significant difference in either the morphology or proliferation at 1µM and lower concentration at same time points. The inhibitory effect of 10 µM EGCG on HLM cells was not significant until 5 days post-treatment. However these levels are within physiologically achievable range (26)
The signal transduction pathway by which EGCG exerts cell cycle arrest and induction of apoptosis remains to be clarified. Several mechanisms of cell-cycle arrest by EGCG have been postulated (8). The d-type cyclins, through the interaction with CDKs-forming cyclin d1-CDK4/6 complexes, are mainly responsible for driving the cell cycle from G1 to S phase (12). In the present study, we demonstrate a significant decrease in the expression of CDK4 and PCNA in EGCG treated HuLM cells.
The induction of apoptosis by 100 µM EGCG treatment on HuLM cells was revealed by TUNEL assay. It is consistent with the changes in BCL2 at RNA and protein levels. The BCL family includes proapoptotic members such as BAX and antiapoptotic members such BCL-2. Antiapoptotic BCL-2 members act as repressors of apoptosis by blocking the release of cytochrome-c, whereas proapoptotic members act as promoters. BCL2 is over-expressed in many tumors, including leiomyoma, that cause resistance to chemotherapeutic drugs and radiation therapy, while decreasing BCL2 expression may promote apoptotic responses to anticancer drugs (27). These effects are more dependent on the balance between BCL2 and BAX than on BCL2 quantity alone (28). We found that the level of BCL2 protein was significantly down-regulated while BAX was up-regulated in a dose-dependent manner, when HuLM cells were incubated with ascending concentration of EGCG. Quantitative RT-PCR results showed that BCL2 and BCL2A1 mRNA was significantly down regulated after EGCG treatment. These changes contributed synchronously to the EGCG-induced apoptosis in HuLM cells. Additionally, EGCG-treated HuLM cells exhibited increased expression of several genes that represent p53 pathway such as BAX, p21, transformed 3T3 cell double minute 2 (MDM2) and TP53I3. As a transcription factor, p53 regulates downstream genes important in cell cycle arrest, DNA repair, and apoptosis. Loss of p53 in many cancers leads to genomic instability, impaired cell cycle regulation, and inhibition of apoptosis. (29).
Studies have shown that EGCG induces apoptosis and growth inhibition of cancer cells, but not normal cells (31, 32). EGCG was shown to reduce NFκB levels in human epidermoid carcinoma; cells at a much lower dose than an equivalent inhibition in normal human epidermal keratinocytes (32). The mechanism of these different effects underscores a therapeutic window demonstrated by EGCG, where the inhibitory effects were only observed in tumor cells. Our results also showed that the expression of bcl2A1, a key factor in NFκB pathway, was reduced 11.8-fold in 100µM EGCG treated huLM cells, compared to untreated control.
Of 18 pathways, we found the expression of 15 genes that represent 10 pathways changed significantly (Figure 4), while remaining 8 pathways did not show significant changes in this study. Impressively, the expression of BMP2 gene in EGCG-treated HuLM cells in our study was 14 fold more than untreated control. BMPs are members of the transforming growth factor-beta superfamily which regulate cell differentiation, proliferation and apoptosis. The BMP-signaling network plays a pivotal role during embryogenesis and tumorigenesis. Prior studies found that BMP2 acts as a tumor suppressor, promoting apoptosis in human primary colonic epithelial cells (33). Another report demonstrated that the effect of BMP signaling and growth suppression in cancer cells was facilitated by CDKN1A. Additionally, loss of BMP2 is related to progression towards aggressive phenotype of prostate cancer (34).
HSF1, which regulates transcription of heat-shock genes induced by stress, was 16 fold higher in EGCG-treated HuLM cells than the untreated control. HSF1 is increasingly implicated in cancer, and earlier studies have found that over-expressing a constitutively activated form of HSF1 could sensitize HeLa cells to Fas-mediated killing (35). The implication is that up-regulation of the expression of HSF1 in tumor cells, either through pharmacologic or gene-therapy approaches, could sensitize tumors to the killing effects of cancer therapies operating through the Fas receptor. Interestingly, HSF1 binds to the corepressor metastasis-associated protein 1 (MTA1) and participates in the repression of estrogen-dependent transcription in breast carcinoma cells (36). This repression effect of HSF1 may conceivably contribute to the role of EGCG in the treatment of leiomyoma, which is another example of estrogen-dependent tumors (2).
Although higher concentration of EGCG displayed more profound antiproliferation effects on HuLM cells, it is impractical to expect such high plasma levels of EGCG to be achieved in vivo. The fact that a significant inhibitory effect on HuLM cells was detected at doses of EGCG as low as 10µM is encouraging. Such levels are achievable in plasma and tissue by regular consumption of brewed green tea or EGCG supplement (9, 26). Theoretically, the maximum blood concentration of EGCG may reach 60 µM after drinking a cup of tea (9). A previous study, however, reported that peak plasma EGCG levels of 200–400 ng/ml (0.4–0.8 µM) can be achieved after the oral administration of 800 mg EGCG (equivalent to 3–4 cups of green tea) daily in healthy individuals (37). The reason could be due to the instability of EGCG and metabolic transformation such as methylation (38). A wide range of EGCG doses (0.5–10mM) have shown antitumor effects in animal studies (39). Besides inhibiting tumor cells directly, EGCG at lower concentration may trigger other multiple pathways to suppress tumor growth indirectly. We have recently confirmed such observation in a nude mouse model of uterine fibroids treated daily with EGCG added to drinking water (unpublished data).
In this report we demonstrate that the antiproliferative effect of 10 µM EGCG on HuLM cells occurred after 5 days which suggests that such intervention in humans might be more suited for regular use over a prolonged period of time. Thus, such intervention would be an option more appropriate for slowly growing benign tumors such as uterine fibroids, where a decrease in tumor burden but not necessarily complete eradication is sufficient to produce significant clinical improvement.
Based on the down-regulation of BCL2 and up-regulation of BAX at both protein and mRNA levels, we suggest that the altered expression of BCL2 family members, could be key in the EGCG-mediated induction of apoptosis HuLM cells. Down-regulation of CDK4 protein may also contribute to the cell cycle arrest effects of EGCG, because down-regulation of both cyclin D1 mRNA and cdk4 protein could lower the kinase activities associated with cyclin Dl-CDK4 complexes, leading to cell-cycle arrest. This was evident by the significant reduction of cyclin-dependent kinase 2 (CDK2), and increase in cyclin-dependent kinase inhibitor 1A (CDKN1A, p21) at mRNA levels after EGCG treatment.
In conclusion, this current study demonstrate for the first time a growth inhibition and apoptosis induction effect of EGCG in HuLM cells, and suggests that such effects are mediated through the modulation of multiple cellular signaling pathways. Pending further in vivo evaluation, this nutritional supplement can potentially be used as a simplified oral alternative for the prevention or treatment for uterine fibroids.
Acknowledgments
Financial Support: NIH/NICHD 1 R01 HD046228-01 to AA and RCMI grant G12 RR03032
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capsule
The major green tea component, epigallocatechin gallate, acts via multiple signal transduction pathways to inhibit proliferation and induce apoptosis in cultured human leiomyoma cells.
REFERENCES
- 1.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J. Clin Pathol. 1990;94(4):435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 2.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 3.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig. 2006;13(2):136–144. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Othman EE, Al-Hendy A. Molecular genetics and racial disparities of uterine leiomyomas. Best Pract Res Clin Obstet Gynecol. 2008;22(4):589–601. doi: 10.1016/j.bpobgyn.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreyko JL, Marshall LA, Dumesic DA, Jaffe RB. Therapeutic uses of gonadotropin-releasing hormone analogs. Obstet Gynecol Surv. 1987;42:1–21. [PubMed] [Google Scholar]
- 7.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51(1):116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6(5):389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 9.Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58(6):911. doi: 10.1016/s0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 10.Chung JE, Kurisawa M, Kim YJ, Uyama H, Kobayashi S. Amplification of antioxidant activity of catechin by polycondensation with acetaldehyde. Biomacromolecules. 2004 Jan–Feb;5(1):113–118. doi: 10.1021/bm0342436. [DOI] [PubMed] [Google Scholar]
- 11.Mukhtar H, Ahmad N. Green tea in chemoprevention of cancer. Toxicol Sci. 1999;52:111–117. doi: 10.1093/toxsci/52.2.111. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275:328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst (Bethesda) 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Zhao DY, Elliott S, Zhao W, Curiel TJ, Beckman BS, et al. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int J Oncol. 2007;31(4):705–711. [PubMed] [Google Scholar]
- 17.Nakazato T, Ito K, Miyakawa Y, Kinjo K, Yamada T, Hozumi N, et al. A green tea component rapidly induces apoptosis of myeloid leukeamic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica. 2005;90(3):317–325. [PubMed] [Google Scholar]
- 18.Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82(6):719–728. doi: 10.1097/01.lab.0000017499.51216.3e. [DOI] [PubMed] [Google Scholar]
- 19.Varella-Garcia M, Chen L, Zheng X, Yu L, Dixon D. Karyotypic characteristics of human uterine leiomyoma and myometrial cell lines following telomerase induction. Cancer Genet Cytogenet. 2006;170(1):71–75. doi: 10.1016/j.cancergencyto.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Salama SA, Kamel M, Christman G, Wang HQ, Fouad HM, Al-Hendy A. Gene therapy of uterine leiomyoma: adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells in vitro and in a nude mouse model. Gynecol Obstet Invest. 2007;63(2):61–70. doi: 10.1159/000095627. [DOI] [PubMed] [Google Scholar]
- 21.Salama SA, Nasr AB, Dubey RK, Al-Hendy A. Estrogen metabolite 2-methoxyestradiol induces apoptosis and inhibits cell proliferation and collagen production in rat and human leiomyoma cells: a potential medicinal treatment for uterine fibroids. J Soc Gynecol Investig. 2006 Dec;13(8):542–550. doi: 10.1016/j.jsgi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Veeriah S, Kautenburger T, Habermann N, Sauer J, Dietrich H, Will F, et al. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol Carcinog. 2006;45(3):164–174. doi: 10.1002/mc.20158. [DOI] [PubMed] [Google Scholar]
- 23.Okolo S. Incidence, etiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Masuda M, Suzui M, Weinstein B. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 25.Chu KO, Wang CC, Chu CY, Choy KW, Pang CP, Rogers MS. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007;22(1):280–287. doi: 10.1093/humrep/del353. [DOI] [PubMed] [Google Scholar]
- 26.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benchimol S. p53-dependent pathways of apoptosis. Cell Death Differ. 2001;8:1049–1051. doi: 10.1038/sj.cdd.4400918. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado V, Melendez-Zajgla J, Ortega A. Modulation of NF-kappa B, and Bcl-2 in apoptosis induced by cisplatin in HeLa cells. Mut Res. 1997;381:67–75. doi: 10.1016/s0027-5107(97)00150-4. [DOI] [PubMed] [Google Scholar]
- 29.Kuhnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M, et al. NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275:6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea pigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Letters. 1998;129:173–179. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376(2):338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 32.Beck SE, Jung BH, Fiorino A, Gomez J, Rosario ED, Cabrera BL, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J. Physiol Gastrointest Liver Physiol. 2006;291(1):G135–G145. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19(7):1465–1472. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horvath LG, Henshall SM, Kench JG, Turner JJ, Golovsky D, Brenner PC, et al. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate. 2004;59(3):234–242. doi: 10.1002/pros.10361. [DOI] [PubMed] [Google Scholar]
- 35.Xia W, Voellmy R, Spector NL. Sensitization of tumor cells to fas killing through overexpression of heat-shock transcription factor 1. J Cell Physiol. 2000;183(3):425–431. doi: 10.1002/(SICI)1097-4652(200006)183:3<425::AID-JCP16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Khaleque MA, Bharti A, Gong J, Gray PJ, Sachdev V, Ciocca DR, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27(13):1886–1893. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- 37.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001;10:53–58. [PubMed] [Google Scholar]
- 38.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31(5):572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 39.Fassina G, Venè R, Morini M, Minghelli S, Benelli R, Noonan DM, et al. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10(14):4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]