Abstract
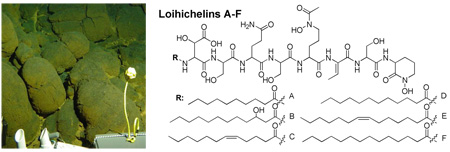
A suite of amphiphilic siderophores, loihichelins A-F, were isolated from cultures of the marine bacterium Halomonas sp. LOB-5. This heterotrophic Mn(II)-oxidizing bacterium was recently isolated from the partially weathered surfaces of submarine glassy pillow basalts and associated hydrothermal flocs of iron oxides collected from the southern rift zone of Loihi Seamount east of Hawai’i. The loihichelins contain a hydrophilic head group consisting of an octapeptide comprised of D-threo-β-hydroxyaspartic acid, D-serine, L-glutamine, L-serine, L-N(δ)-acetyl-N(δ)-hydroxy ornithine, dehydroamino-2-butyric acid, D-serine and cyclic N(δ)-hydroxy-D-ornithine, appended by one of a series of fatty acids ranging from decanoic acid to tetradecanoic acid. The structure of loihichelin C was determined by a combination of amino acid and fatty acid analyses, tandem mass spectrometry and NMR spectroscopy. The structures of the other loihichelins were inferred from the amino acid and fatty acid analyses, and tandem mass spectrometry. The role of these siderophores in sequestering Fe(III) released during basaltic rock weathering, as well as their potential role in the promotion of Mn(II) and Fe(II) oxidation, is of considerable interest.
The basaltic rocks continuously produced at mid-ocean ridge spreading centers and along the flanks of submarine volcanoes are prone to extensive dissolution and oxidation during reaction with seawater. Recently, significant attention has focused on the energy released during basalt weathering and the potential to support chemolithoautotrophic bacteria that use reduced Fe, Mn or S as an energy source for growth in oxygenated seawater.1 Over time, extensive weathering crusts containing abundant Fe(III)- and Mn(IV)-oxide phases, as well as clays and zeolite minerals, develop on the basalt surfaces. Intriguingly, all of these secondary minerals are intimately associated with extensive biofilms and surprisingly diverse microbial consortia.2,3 Although the metabolic capabilities of most of the organisms are unknown, numerous bacteria with the functional capability of Fe(II) and Mn(II) oxidation have been detected and directly isolated from basalt surfaces recovered from submarine sites such as Loihi Seamount, Vailulu’u Seamount, Juan de Fuca Ridge and the East Pacific Rise.2, 4–7
Loihi Seamount, located 30 km offshore of the big island of Hawai’i, is an active submarine volcano dominated by Fe(II)-rich hydrothermal fluids.8 Recently a novel class of obligate Fe(II)-oxidizing bacteria have been isolated from Fe-oxide mats in the hydrothermal pit crater,9,10 and numerous heterotrophs have also been demonstrated to also oxidize both Fe(II) and Mn(II).2 Several of these heterotrophs, particularly Halomonas and Marinobacter sp., produce copious amounts of siderophores, as determined by plate-based Fe(III)-CAS assays11 on a glycerol-based medium. One of the Loihi siderophore-producing isolates, Halomonas LOB-5, was chosen for study because it is closely related to numerous facultative heterotrophic, halotolerant Halomonas strains isolated from low-temperature hydrothermal vents and sulfide rocks (e.g. references 12 and 13) and it is >99% similar to several environmental clone sequences obtained from weathered basalt surfaces at the East Pacific Rise.3 Thus Halomonas LOB-5 is an environmentally-relevant and a broadly distributed strain that may be a good model organism for investigating metal-cycling in oligotrophic, rock-dominated environments on the seafloor.
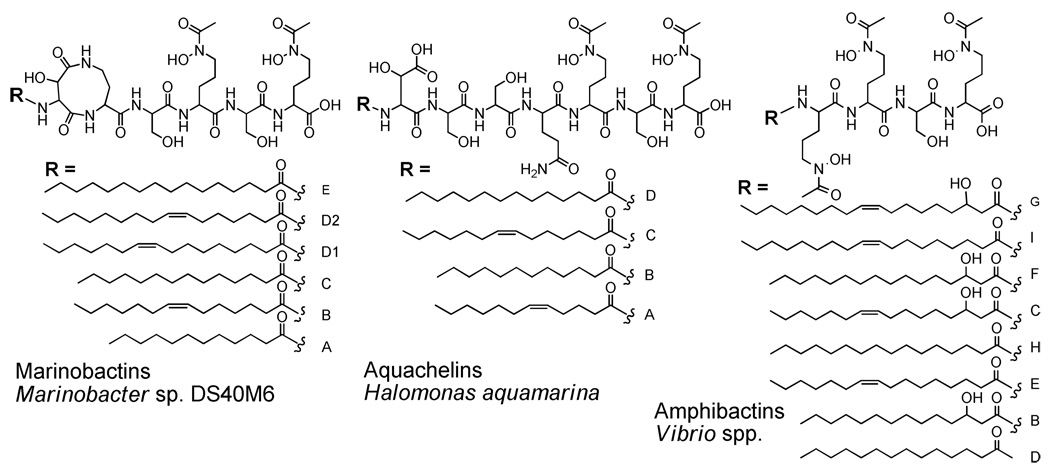
Little is known about the growth requirements of Halomonas LOB-5; however, most bacteria require Fe as an essential nutrient, regardless of whether they use Fe for energy generation. To acquire Fe, bacteria growing aerobically often produce siderophores, low molecular weight compounds that coordinate Fe(III) with high affinity. Relatively few siderophore structures from marine bacteria have been characterized, while hundreds of siderophore structures from terrestrial bacteria are known. A distinctive characteristic of marine bacteria that is emerging is the production of suites of amphiphilic siderophores composed of a peptidic head-group appended by one of a range of fatty acids. For example, the marinobactins, aquachelins and amphibactins14,15 are all families of amphiphilic peptide siderophores (Figure 1). These siderophores differ in the number and identity of the amino acid residues in the peptide head group, and within one family, the chain length of the fatty acid appendage also varies. As a result, each of these siderophores varies in its degree of amphiphilicity. One amino acid that is often present in the marine peptidic siderophores is β-hydroxyaspartic acid,16 which is photoreactive when coordinated to Fe(III).17 Thus ferric complexes of the aquachelins and marinobactins undergo a photoreduction of Fe(III) and photooxidation of the siderophore ligand.17
Figure 1.
Structures of previously characterized marine amphiphilic peptide siderophores.
Characterization of the siderophores produced by bacteria involved in basalt weathering and oxidation of Fe(II) and Mn(II) is a topic of much current interest, due to the potential role that siderophores may play in controlling the rates and mechanisms of metal oxidation or complexation. We report herein isolation and structure determination of a new suite of amphiphilic peptide siderophores, the loihichelins A-F, produced by the basalt-weathering bacterium Halomonas strain LOB-5.
Results
Halomonas LOB-5 produces a suite of six siderophores, named loihichelins A-F. High-resolution electrospray mass spectrometric data for loihichelins A through F are summarized in Table 1. Amino acid analysis18 of the acid hydrolyzed loihichelin C established the presence of D-threo-β- hydroxyl-aspartic acid, D-ornithine, L-ornithine, L-serine, two D-serines, and L-glutamic acid.
Table 1.
Exact mass and fatty acid analysis for loihichelins A-F.
| Siderophore | Exact mass | Molecular formula | Δ ppm | Fatty acid |
|---|---|---|---|---|
| Loihichelin A | 1082.4982 | C44H73N11O19Na | −3.99 | C10:0 |
| Loihichelin B | 1126.5244 | C46H77N11O20Na | −6.9 | C12:0−OH |
| Loihichelin C | 1108.5138 | C46H75N11O19Na | 8.0 | C12:1 |
| Loihichelin D | 1110.5295 | C46H77N11O19Na | −9.6 | C12:0 |
| Loihichelin E | 1136.5451 | C48H79N11O19Na | −8.5 | C14:1 |
| Loihichelin F | 1138.5608 | C46H81N11O19Na | 9.6 | C14:0 |
GC-MS analysis of the fatty acid methylester of each loihichelin revealed the presence of the fatty acids C10:0, C12:0-OH, C12:1, C12:0, C14:1, and C14:0, as confirmed by comparison to authentic fatty acid methyl ester standards (Supelco) (Table 1). The hydroxyl group in the fatty acid of loihichelin B, C12:0-OH, was determined to be at carbon 3, given that a major fragment in the GC-MS spectrum had a mass of m/z 103, characteristic of the 3-hydroxyalkanoate methyl ester.19 The position of the double bond in the fatty acids of loihichelins C and E, C12:1 and C14:1, respectively, were determined by ozonolysis. In both cases, 1, 1-dimethoxy-heptane (m/z of 159), indicative of the double bond at the position ω7c, was detected. Therefore loihichelins C and E contain cis-7-dodecenoic fatty acid and cis-7-tetradecenoic, respectively.
The variation in the fatty acid appendages is also reflected in the mass spectrometry data of loihichelins A-F, consistent with mass differences that have been observed in other families of amphiphilic siderophore composed of different fatty acid appendages.14,15
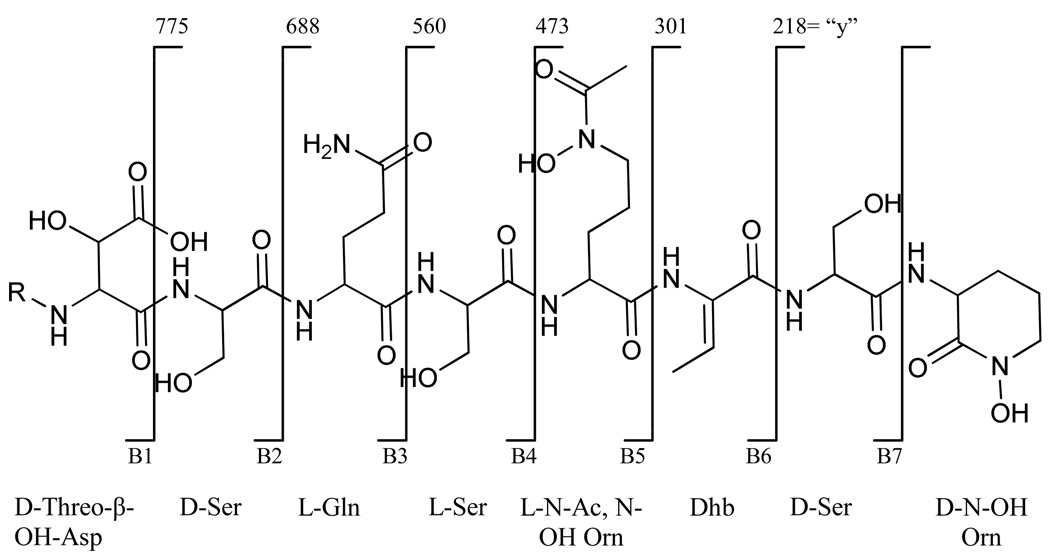
Tandem mass spectrometry data indicates the connectivity of the amino acids in the peptidic headgroup of the loihichelins. The “y”20 fragmentation of each loihichelin demonstrates a high degree of similarity. The observed fragmentation pattern in the tandem mass spectra can be attributed to the sequential loss of 130, 87, 83, 172, 87, 128, and 87 mass units corresponding to the amino acids, cyclic N(δ)-hydroxy-ornithine, serine, dehydroamino-2-butyric acid, N(δ)-acetyl N(δ)-hydroxy-ornithine, serine, glutamine and serine, from the carboxylate terminus.
The positions of the D and L amino acids in the peptide sequence were determined through partial hydrolysis of the siderophore. After separation of these peptide fragments, they were fully hydrolyzed, then derivatized to form the pentafluoropropyl isopropyl ester of the amino acids before chiral GC-MS analysis, as described in the Experimental Section. By comparison of the overlap of D and L amino acids within each peptide fragment, it was possible to determine the placement of each D and L ornithine and serine (Figure 2).
Figure 2.
Observed “y” and “b” fragment analysis of the loihichelins A-F. R represents the different fatty acid tail appendages of loihichelins A-F. The “y” [M+2H]+ fragments are the same for each loihichelin. The “b” fragments are summarized in Table 2.
The “y” and the “b” fragments for the six loihichelins are summarized in Figure 2 and Table 2.
Table 2.
The “b” fragment m/z values observed by tandem mass spectrometry of the loihichelins.
| “b” | Loihichelin | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| B1 | 286 | 330 | 312 | 314 | 340 | 342 |
| B2 | 373 | 417 | 399 | 401 | 427 | 429 |
| B3 | 501 | 545 | 527 | 529 | 555 | 557 |
| B4 | 588 | 632 | 614 | 616 | 642 | 644 |
| B5 | 760 | 804 | 786 | 788 | 814 | 816 |
| B6 | 843 | 887 | 869 | 871 | 897 | 899 |
| B7 | 930 | 974 | 956 | 958 | 984 | 986 |
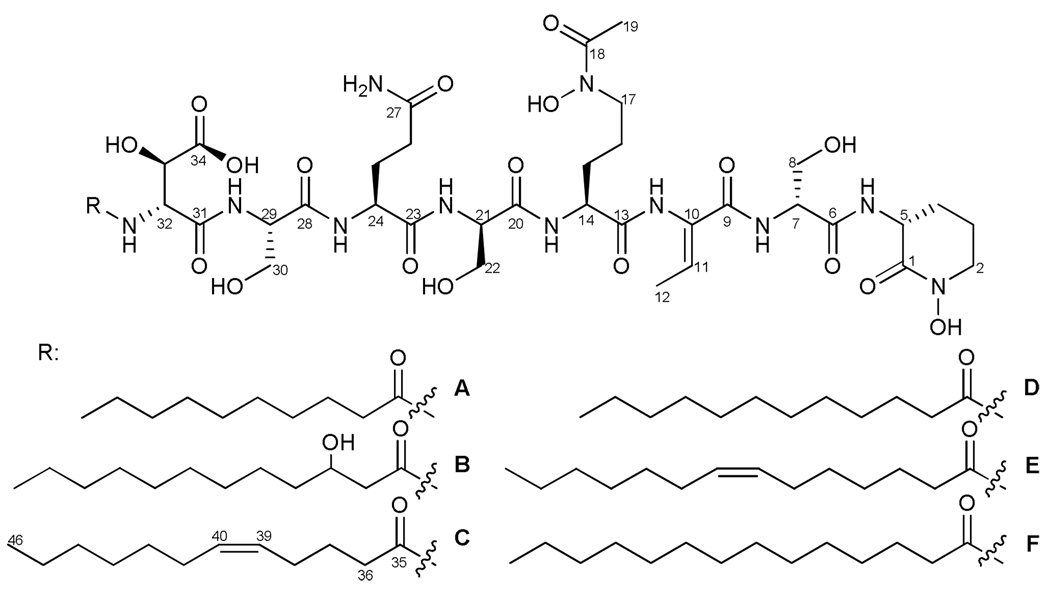
NMR of loihichelin C was used for final structure elucidation and confirmed the amino acid sequence obtained for the tandem mass spectrometry data. The 1H and 13C chemical shift assignments are summarized in Figure 3 and Table 3. The structures of the other loihichelins were inferred from the tandem mass spectrometry and the amino acid and fatty acid analyses.
Figure 3.
Loihichelins A-F, the suite of siderophores produced by Halomonas LOB-5.
Table 3.
1H (600 MHz) and 13C NMR (125 MHz) data for loihichelin C (8 mgs) in d6- DMSO
| δc | δH (J in Hz) | HMBC | |
|---|---|---|---|
| Cyclic, N-OH Ornithine | |||
| C1 | 170.4 | N/A | |
| C2 | 51.2 | 3.51, m | 1, 3, 4 |
| C3 | 27.9 | 1.73, m | 2, 4 |
| C4 | 31.3 | 2.08, m | 3, 5 |
| C5 | 52.5 | 4.24, m | 1, 3, 4, 6 |
| Nl | 7.82, d (7.2) | 6 | |
| Serine | |||
| C6 | 169.6 | N/A | |
| C7 | 55.7 | 4.34, m | 6, 8, 9 |
| C8 | 61.7 | 3.58, m | 7 |
| N2 | 7.53, d (7.8) | 7, 9 | |
| Dehydroamino-2-butyric acid | |||
| C9 | 164.0 | N/A | |
| C10 | 130.4 | N/A | 11, 12 |
| C11 | 127.9 | 6.37, dd (7.2, 6.6) | 9, 10, 12 |
| C12 | 13.0 | 1.62, d (7.2) | 10, 11 |
| N3 | 7.25, s | 13 | |
| N-acetyl, N-OH Ornithine | |||
| C13 | 164.5 | N/A | |
| C14 | 55.7 | 4.33, m | 13, 15, 16, 17, 20 |
| C15 | 27.5 | 1.68, m | 14, 16, 17 |
| C16 | 20.3 | 1.92, m | 14, 15, 17 |
| C17 | 46.7 | 3.49, m | 14, 15, 16 |
| C18 | 174.1 | N/A | |
| C19 | 20.1 | 1.92, m | 18 |
| N4 | 7.93, d (7.2) | 14, 20 | |
| Serine | |||
| C20 | 169.9 | N/A | |
| C21 | 55.0 | 4.35, m | 20, 22, 23 |
| C22 | 61.7 | 3.62, m | 21 |
| N5 | 7.99, d (7.8) | 21, 23 | |
| Glutamine | |||
| C23 | 171.3 | N/A | |
| C24 | 53.3 | 4.28, m | 23, 25, 27 |
| C25 | 26.7 | 1.60, m | 24, 27 |
| C26 | 51.2 | 3.47, m | 25 |
| C27 | 171.1 | N/A | |
| N6 | 8.25, d (6.6) | 24, 28 | |
| Serine | |||
| C28 | 170.6 | N/A | |
| C29 | 53.3 | 4.32, m | 30, 28 |
| C30 | 61.5 | 3.55, m | 29 |
| N7 | 8.05, d (7.8) | 29, 30, 31 | |
| β-OH Aspartic acid | |||
| C31 | 169.6 | N/A | |
| C32 | 55.6 | 4.80, d (2.4) | 31, 33, 34, 35 |
| C33 | 70.5 | 4.50, d (2.4) | 31, 32, 34 |
| C34 | 173.0 | N/A | |
| N8 | 7.92, d (8.4) | 31, 32, 33, 35 | |
| Fatty acid tail | |||
| C35 | 172.6 | N/A | |
| C36 | 34.9 | 2.19, t (7.8) | 35, 37, 38 |
| C37 | 25.5 | 1.50, t (7.8) | 36, 38 |
| C38 | 28.5 | 1.56, m | 37, 39 |
| C39 | 129.1 | 5.36, dt (12, 6.6) | 38, 40, 41 |
| C40 | 130.1 | 5.33, dt (12, 6.6) | 39, 41, 42 |
| C41 | 26.3 | 2.00, m | 40, 42 |
| C42 | 29.2 | 1.29, m | 41, 43 |
| C43 | 31.2 | 1.24, m | 42, 44, 45 |
| C44 | 28.4 | 1.24, m | 45, 46 |
| C45 | 20.1 | 1.25, m | 46, 44, 43 |
| C46 | 14.0 | 0.85, t (6.6) | 43, 44, 45 |
a. HMBC correlations are from proton(s) state to the indicated carbon
The 1H-NMR spectrum reveals the presence of eight α-amide protons (δH 7.25 to δH 8.25), seven Cα protons (δH 4.24 to δH4.80) and three vinyl protons (δH 5.34 to δH 6.37). The proton resonances between 0.85 – 2.19 ppm are primarily attributed to methylene protons of the fatty acid appendage. The 13C-NMR spectrum shows the presence of twelve carbonyl carbon signals (δc 163.6 to δc 174.1), four unsaturated carbon atoms (carbons involved in a double bond) (δc 127.9 to δc 130.4) and seven Cα carbon signals (δc 52.5 to δc 55.7). The resonance at 70.5 ppm is indicative of the β carbon of β-hydroxy aspartic acid, which was also observed in other β-hydroxy-aspartic-acid-containing siderophores.21
The results from the APT-NMR spectrum revealed three anti-phase and one in-phase carbon signals between 125 ppm and 135 ppm, consistent with the presence of four double bond carbons, one of which is a quaternary carbon. Two of the three anti-phase carbons reside in the fatty acid chain (C39 δc 129.1 and C40 δc 130.1). The small coupling constants of 12 Hz between the vinyl protons are indicative of a cis configuration of the double bond.22 The remaining, anti-phase carbon and the quaternary carbon signals (C10 δc 130.4 and C11 δc 127.9) arise from the unusual amino acid dehydroamino-2-butyric acid, which is consistent with the loss of 83 mass units in the tandem mass spectrometry data (i.e. m/z difference between “b6” to “b5” and “y3” to “y2”). The presence of a quaternary carbon signal also agrees with the finding that only seven Cα proton and carbon signals are detected, despite the presence of eight amino acids. The APT spectrum also reveals three methyl groups (C12 δc 13.0, C46 δc 14.0 and C19 δc 20.1), corresponding to the methyl groups of the dehydroamino-2-butyric acid moiety, the fatty acid appendage and the N(δ)-acetyl, N(δ)-hydroxy ornithine moiety.
The two-bond HMBC correlation from the amide NHs of cyclic N(δ)-hydroxy-ornithine, serine, dehydroamino-2-butyric acid, N(δ)-acetyl-N(δ)-hydroxy-ornithine, serine, glutamine and serine to the carbonyl carbons of serine, dehydroamino-2 butyric acid, N(δ)-acetyl-N(δ)-hydroxyornithine, serine, glutamine, serine and β-hydroxy aspartic acid confirmed the sequence of the eight amino acids. The nOe between the C11 proton (δ 6.37) and the amide N2 proton (δ 7.53) establishes the E geometry for dehydroaminobutyric acid residue (Figure S8 in Supporting Information).
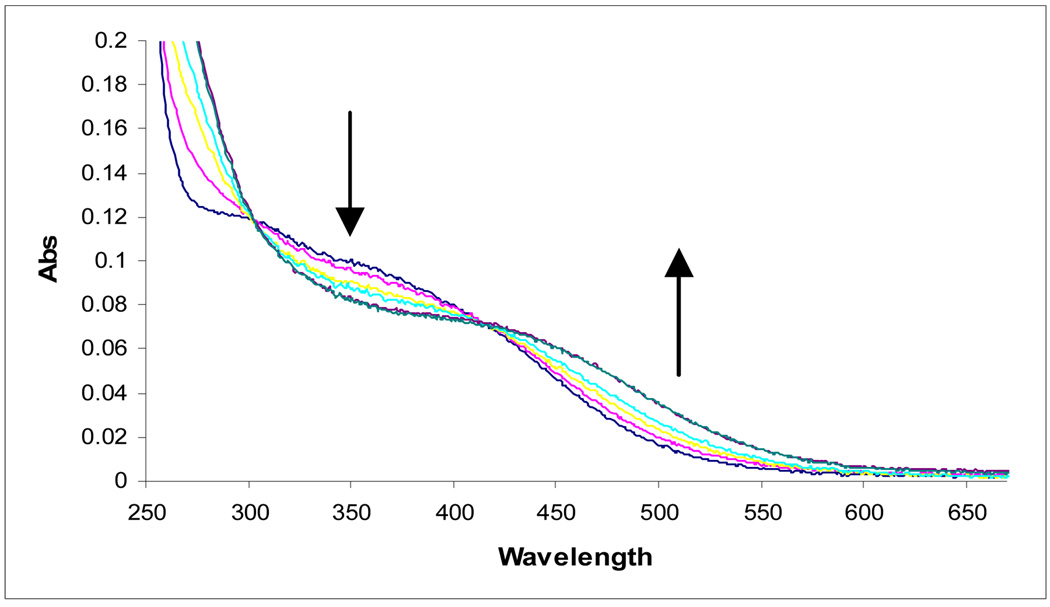
The UV-visible spectrum of Fe(III)-loihichelin D shows the characteristic charge-transfer bands from β-hydroxyaspartic acid-to-Fe(III) at ~300 nm and the hydroxamate-to-Fe(III)-charge transfer band at ∼400 nm, as expected for these siderophores. UV photolysis of the Fe(III)-loihichelin D complex shows that these siderophores are also photoreactive, as monitored by changes in the UV-vis spectra with loss characteristic loss of the electronic transition in the near-ultraviolet (~ 300 nm), corresponding to the charge-transfer band from β-hydroxyaspartate to Fe(III) (Figure 4). RP-HPLC also shows the disappearance of the Fe(III)-loihichelin D peak and the appearance of multiple new peaks (data not shown). Further structural characterization of loihichelin photoproducts is in progress.
Figure 4.
Continuous irradiation of ca. 30 µM Fe(III)-loihichelin D in water (pH 7.6) with a 200 W mercury arc lamp. UV-visible scans were taken at 0, 5, 10, 15, 45 and 60 minutes. (The scans taken at 45 and 60 min are overlapping). During photolysis the absorbance decreases in the UV region and increases in the visible (~425 nm-600 nm).
Discussion
We report a new suite of amphiphilic peptide siderophores produced by Halomonas LOB-5, a metal-oxidizing heterotrophic bacterium commonly associated with seafloor rocks. Compared to other families of amphiphilic peptide siderophores, such as the marinobactins, aquachelins and amphibactins, the loihichelins would be expected to be the most hydrophilic of these siderophores because of their longer peptide headgroup (an octapeptide) and relatively short fatty acid appendages (C10–C14), whereas the amphibactins would be the most hydrophobic, with only four amino acids in the head group and longer fatty acids (C14–C18). The loihichelins are also distinguished from the other amphiphilic siderophores by the presence of cyclic N(δ)-hydroxy ornithine and dehydroamino 2-butyric acid. Cyclized ornithine has previously been observed in other siderophore structures (e.g., pseudobactins 23,24) as well as cyclized lysine (e.g., mycobactins,25 formobactins,26 and nocobactins27). Dehydroamino 2-butyric acid is an unusual amino acid, yet it has been identified in other natural products, including the antifungal glycosylated lipopeptide, hassallidin A.28 A curious feature of the loihichelins pertains to the presence of β-hydroxyaspartic acid, which when coordinated to Fe(III) is photoreactive. However Halomonas LOB-5 was isolated from materials collected at −1714 meters, a depth to which sunlight would not penetrate. Thus β-hydroxyaspartic acid clearly provides one bidentate coordination site for Fe(III), with the other four oxygen ligands coming from the two hydroxamates, however, photoreactivity of the Fe(III)-loihichelins is not likely to play a significant role in Fe uptake.
The production of amphiphilic siderophores by a bacterium that colonizes these basalts raises a number of questions. Do the loihichelins play a role in the bacterial oxidation of Fe(II) or Mn(II), since Halomonas LOB-5 is potentially a key player in the oxidation of both metals at this site? For example, do the loihichelins only serve the purpose of acquisition of Fe as a trace nutrient or are they required for energy generation? Or once Fe(II) is oxidized, do the loihichelins assist in the stripping of Fe(III) from the surface of the basaltic rock, either to acquire Fe(III) or to enhance the continued solubilization of Fe(II) from the underlying silicate matrix? Another possibility is that siderophore-assisted oxidation of Mn(II) and perhaps Fe(II) is significant given the enhanced oxidation of Mn(II) by dioxygen observed in the presence of the siderophores desferrioxamines B and G29, 30 and pyoverdine.31 Experiments are currently in progress to investigate the role of the loihichelins in Mn(II) and Fe(II) oxidation as well as to probe the interactions of the loihichelins with basaltic rock.
Experimental Section
General Experimental Procedures
1H, 1H -1H gCOSY, 1H-13C HMBC, 1H -1H TOCSY and 1H -13C HSQC were carried out at 25°C on a 600 MHz Varian Unity Inova instrument with standard pulse sequences. 13C and APT spectra were recorded at 25°C on a 500 MHz Varian Unity Inova instrument with standard pulse sequences. Electrospray ionization mass spectrometry (ESI-MS) and tandem mass spectrometry using argon as the collision gas were determined on a Micromass Q-TOF2 (Waters Corp.). The chiral amino acid analysis was determined using a Varian Saturn 2100T GC/MS fitted with an Alltech Chirasil-Val® capillary column.
Isolation and Characterization of Halomonas LOB-52
Halomonas LOB-5 was isolated from the partially weathered surface of submarine glassy pillow basalts collected from Marker 17 (depth of 1714 m) at Loihi Seamount (accession #DQ412065). Closely related Halomonas isolates have been obtained from hydrothermal Fe-oxide mats inside and outside the active pit crater as well. The isolation procedure relied upon aseptically collecting rocks and mats via Pisces submersible, subsampling Fe-oxide rich materials, and enriching for metal-oxidizing bacteria by using a microaerophilic, bicarbonate-buffered artificial seawater medium amended with Fe(II), Mn(II) and Na-acetate (see “X media”)2. Numerous serial transfers, dilutions and plating resulted in the isolation of pure colonies of this Halomonas strain. Subsequently, it was determined that Halomonas LOB-5 can grow lithotrophically on Fe(II) alone, with CO2 as a sole carbon source, or heterotrophically with micromolar concentrations Na-acetate, while oxidizing Fe(II) or Mn(II). Fortunately, Halomonas LOB-5 can grow to much higher optical density in nutrient rich media (e.g. see ASG-Fe below), enabling experiments to isolate the siderophores produced.
Siderophore Isolation and Purification
The siderophores were isolated and purified as previously described.14 Halomonas strain LOB-5 was cultured in an artificial seawater medium (ASG-Fe) containing: 2 L doubly deionized water (Barnstead Nanopure II), 2.0 g NH4Cl, 20 g casamino acids, 2.0 g glycerophosphate, 24.7 g MgS04 · 7 H2O, 2.9 g CaCl2 · 2 H2O, 35.1 g NaCl, 1.5 g KCl, and 6 mL glycerol. Before inoculation, 20 mL filter-sterilized 1.0 M HEPES buffer (pH 7.4), 4 mL filter-sterilized 1.0 M NaHC03, and 20 mL filter-sterilized vitamin stock solution were added to the medium. The vitamin stock solution contained 40 mg biotin, 4 mg niacin, 2 mg thiamin, 4 mg p-aminobenzoic acid, 2 mg calcium pantothenic acid, 20 mg pyridoxine hydrochloride, 2 mg cyanocobalamin, 4 mg riboflavin, and 4 mg folic acid in 200 mL of doubly deionized water. The cultures were grown for approximately 2 to 4 days on a rotary shaker (200 rpm).
Cultures were harvested by centrifugation at 6000 rpm for 30 minutes at 4°C. Siderophores were initially purified from the cell-free supernatant by solid phase extraction using Amberlite XAD-2 resin (Aldrich), rinsed sequentially with doubly deionized water, 20% methanol and eluted with 100% methanol. Siderophore-containing fractions were pooled and concentrated via rotary vacuum evaporation. The concentrated solution was further purified by reversed-phase high-pressure liquid chromatography (RP-HPLC) using a preparative Vydac C4 column (250 mm length × 22 mm diameter). Compounds were eluted with a linear gradient from 80% solvent A (0.05% trifluoroacetic acid in doubly deionized water), 20% B (0.05% trifluoroacetic acid in 19.95% water and 80% acetonitrile) to 100% B over 45 minutes. The eluent was monitored at 215 nm and peaks were collected by hand (see Figure S1 in Supporting Information for the HPLC chromatogram of the loihichelins). Collected fractions were concentrated under reduced pressure and lyophilized. Purified loihichelin C from three 2-L cultures were pooled for nmr analyses. Loihichelins A-F were all pale yellow powders; HRMS is listed in Table 1; the nmr of loihichelin C resonance is listed in Table 3.
Amino Acid Analysis
Amino acid analysis of the siderophores was performed using Marfey’s reagent.18 First, the siderophore was hydrolyzed in 55% hydroiodic acid at 110 °C for 24 hr. The samples were then derivatized with the Marfey’s reagent (l-fluoro-2, 4-dinitrophenyl-5-L-alanine amide; 1% w/v in acetone) and resolved by R-P HPLC using a 250 mm × 4.6 mm ID YMC ODS-AQ C18 analytical column (Waters) by monitoring the eluent at 340 nm. A linear gradient from 90% solvent A (50mM triethylamine phosphate (pH 3.0)) and 10% B (CH3CN) to 40% B over 45 minutes was used. Identification of the D- and L- amino acids was accomplished by comparison with external amino acid standards and co-injection with authentic standards (Sigma-Aldrich).
To determine the placement of the D- and L-Orn and Ser in the peptide, ca. 3 mg of loihichelin B were partially hydrolyzed in 200 µL of 6N HCl for 20 min at 110°C. The peptide fragment mixture was derivatized with dimethylaminoazobenzene isothiocyanate (DABITC)32 and separated on a reserve phase C-18 (YMC ODS-AQ) HPLC column. Each peptide fraction was then fully hydrolyzed in 200 µL of 6N HCl for 24 hours at 110°C. The dried sample was derivatized to form the pentafluoropropionyl isopropyl esters of the amino acids and analyzed directly by chiral GC-MS using a Chirasil-Val capillary column.
Fatty Acid Analysis
Fatty acid moieties were identified by generating corresponding fatty acid methyl esters through hydrolysis with 3 N methanolic HCl (Sigma) for 3 hr at 110 °C. The fatty acid methyl esters were extracted into hexanes and analyzed by GC-MS. Identification of the methyl esters was accomplished by comparison with external methyl ester standards (Supelco). The position of unsaturation in the fatty acid tail moiety was determined by ozonolysis.
Supplementary Material
ACKNOWLEDGMENT
Funding from NIH GM38130 (AB), The Center for Environmental BioInorganic Chemistry (CEBIC), an NSF Environmental Molecular Science Institute CHE-0221978 (AB) and NSF grants OCE-0433692 (BT and AT) and MCB-0348668 (BT) is gratefully acknowledged. We thank Dr. J. Pavlovich (mass spectrometry), and Drs. Hongjun Zhou and Ata Shirazi (NMR) for technical assistance and Prof. R. Dahlquist for helpful discussions.
Footnotes
Supporting Information Available: RP-HPLC, 1H NMR, 13C NMR, APT NMR, HSQC, HMBC and COSY. This information is available via the Internet http://pubs.acs.org.
References
- 1.Bach W, Edwards KJ. Geochim. Cosmochim. Acta. 2003:3871–3887. [Google Scholar]
- 2.Templeton AS, Staudigel H, Tebo BM. Geomicrobiol. J. 2005;22:127–139. [Google Scholar]
- 3.Santelli C, Orcutt B, Banning E, Bach W, Moyer C, Sogin M, Staudigel H, Edwards K. Nature. 2008;453:653–659. doi: 10.1038/nature06899. [DOI] [PubMed] [Google Scholar]
- 4.Staudigel H, Hart SR, Pile A, Bailey BE, Baker ET, Brook S, Connelly DP, Hauck L, German CR, Hudson L, Jones D, Kopper AAP, Konter J, Lee R, Pietsch TW, Tebo BM, Templeton AS, Zierenberg R, Young CM. Proc. Natl. Acad. Sci. USA. 2006;103:6448–6453. doi: 10.1073/pnas.0600830103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards KJ, Rogers DR, Wirsen CO, McCollom TM. App. Environ. Microbiol. 2003;69:2906–2913. doi: 10.1128/AEM.69.5.2906-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey B, Orcutt B, Templeton A, Banning E, Moyer C, Edwards C, Staudigel H, Tebo BM. Geomicrobiol. J. 2008 (in review) [Google Scholar]
- 7.Sudek L, Bailey BE, Templeton AS, Davis R, Staudigel H, Tebo BM. Geomicrobiol. J. 2008 (in review) [Google Scholar]
- 8.Karl DM, McMurtry GM, Malahoff A, Garcia MO. Nature. 1988;335:533–535. [Google Scholar]
- 9.Emerson D, Moyer CL. App. Environ. Microbiol. 2002;68:3085–3093. doi: 10.1128/AEM.68.6.3085-3093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emerson D, Rentz JA, Liburn TG, Davis RE, Aldrich H, et al. PloS ONE. 2007:667–675. doi: 10.1371/journal.pone.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwyn B, Neilands JB. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 12.Kaye JZ, Baross JA. FEMS Microbiol. Ecol. 2000;32:249–260. doi: 10.1111/j.1574-6941.2000.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 13.Rogers DR, Santelli CM, Edwards KJ. Geobiol. 2003;1:109–117. [Google Scholar]
- 14.Martinez JS, Zhang GP, Holt PD, Jung H-T, Carrano CJ, Haygood MG, Butler A. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- 15.Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Butler A. Proc. Natl. Acad. Sci., USA. 2003;100:3754–3759. doi: 10.1073/pnas.0637444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler A. BioMetals. 2005;18:369–374. doi: 10.1007/s10534-005-3711-0. [DOI] [PubMed] [Google Scholar]
- 17.Barbeau K, Rue EL, Bruland KW, Butler A. Nature. 2001;413:409–413. doi: 10.1038/35096545. [DOI] [PubMed] [Google Scholar]
- 18.Marfey P. Carlsberg Res. Commun. 1984;49:591–596. [Google Scholar]
- 19.Huijberts GNM, de Rijk TC, de Waard P, Eggink G. J. Bacteriol. 1994;176:1661–1666. doi: 10.1128/jb.176.6.1661-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papayannopoulos IA. Mass Spectrom. Rev. 1995;14:49–73. [Google Scholar]
- 21.Reid R, Live DH, Faulkner DJ, Butler A. Nature. 1993;366:455–458. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- 22.Frost DJ, Gunstone FD. Chem. Phys. Lipids. 1975;15:53–85. doi: 10.1016/0009-3084(75)90032-8. [DOI] [PubMed] [Google Scholar]
- 23.Teintze M, Leong J, Hossain MB, Barners CL, van den Helm D. Phytopathology. 1981;71:908–908. [Google Scholar]
- 24.Teintze M, Leong J. Biochemistry. 1981;20:6446–6457. doi: 10.1021/bi00525a025. [DOI] [PubMed] [Google Scholar]
- 25.Snow GA. Biochem J. 1965;97:166–175. doi: 10.1042/bj0970166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami Y, Kato S, Nakajima M, Kawai H, ShinYa K, Seto H. J. Antibiot. 1996;49:839–845. doi: 10.7164/antibiotics.49.839. [DOI] [PubMed] [Google Scholar]
- 27.Ratledge C, Snow GA. Biochem J. 1973;139:407–413. doi: 10.1042/bj1390407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuhof T, Schmieder P, Preussel K, Dieckmann R, Pham H, Bartl F, von Döhren H. J. Natl. Prod. 2005;68:695–700. doi: 10.1021/np049671r. [DOI] [PubMed] [Google Scholar]
- 29.Duckworth OW, Sposito G. Environ. Sci. Technol. 2005a;39:6037–6044. doi: 10.1021/es050275k. [DOI] [PubMed] [Google Scholar]
- 30.Duckworth OW, Sposito G. Environ. Sci. Technol. 2005b;39:6045–6051. doi: 10.1021/es050276c. [DOI] [PubMed] [Google Scholar]
- 31.Parker DL, Morita T, Mozafarzadeh ML, Verity R, McCarthy JK, Tebo BM. Geochim. Cosmochim. Ac. 2007;71:5672–5683. [Google Scholar]
- 32.Chang J-Y. Biochem. J. 1981;199:537–545. doi: 10.1042/bj1990537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.