Abstract
Whereas activation of metabotropic glutamate receptors (mGluRs) modulates synaptic transmission, the roles of mGluRs in GABAergic transmission in the entorhinal cortex (EC) are elusive. Here, we examined the effects of mGluRs on GABAergic transmission onto the principal neurons in the superficial layers of the EC. Bath application of DHPG, a selective group I mGluR agonist increased the frequency and amplitude of spontaneous IPSCs (sIPSCs) whereas application of DCG-IV, an agonist for group II mGluRs or L-AP4, an agonist for group III mGluRs failed to change significantly sIPSC frequency and amplitude. Bath application DHPG failed to change significantly the frequency and amplitude of miniature IPSCs (mIPSCs) recorded in the presence of tetradotoxin but significantly reduced the amplitude of IPSCs evoked by extracellular field stimulation or in synaptically connected interneuron-pyramidal neuron pairs in layer III of the EC. DHPG increased the frequency but reduced the amplitude of APs recorded from entorhinal interneurons. Bath application of DHPG generated membrane depolarization and increased the input resistance of GABAergic interneurons. DHPG-mediated depolarization of GABAergic interneurons was mediated by inhibition of background K+ channels which are insensitive to extracellular Cs+, TEA, 4-AP and Ba2+. DHPG-induced facilitation of sIPSCs was mediated by mGluR5 and required the function of Gαq but was independent of phospholipase C activity. Elevation of synaptic glutamate concentration by bath application of glutamate transporter inhibitors significantly increased sIPSC frequency and amplitude demonstrating a physiological role of mGluRs in GABAergic transmission. Our results provide a cellular and molecular mechanism to explain the physiological and pathological roles of mGluRs in the EC.
Keywords: GABAA receptor, hippocampus, synapse, synaptic transmission, patch-clamp
Introduction
Glutamate is a major excitatory neurotransmitter in the brain where it interacts with ionotropic (NMDA, AMPA and kainate) and metabotropic glutamate receptors (mGluRs). Whereas ionotropic glutamate receptors mediate the fast excitatory synaptic transmission, mGluRs usually play a modulatory role in the brain. mGluRs are G protein-coupled receptors that are linked to various intracellular second messenger cascades. Group I mGluRs (mGluR1 and mGluR5) are coupled to Gq/11 (Ritzen et al., 2005). Activation of these receptors results in activation of phospholipase C (PLC) leading to an increase in intracellular Ca2+ release and activation of protein kinase C (PKC) (Ritzen et al., 2005). Group II (mGluR2 and mGluR3) and group III (mGluR4, mGluR6–8) mGluRs are coupled to Gi/o proteins. These two groups of receptors are negatively linked to adenylate cyclase leading to a reduction in the intracellular level of cyclic AMP and an inhibition of PKA (Ritzen et al., 2005).
The entorhinal cortex (EC) mediates the majority of connections between the hippocampus and other cortical areas (Witter et al., 1989; Witter et al., 2000a; Witter et al., 2000b). Sensory inputs converge onto the superficial layers (layers II–III) of the EC (Burwell, 2000) which give rise to dense projections to the hippocampus; the axons of the stellate neurons in layer II of the EC form the perforant path that innervates the dentate gyrus and CA3 (Steward and Scoville, 1976) whereas those of the pyramidal neurons in layer III form the temporoammonic pathway that synapses onto the distal dendrites of pyramidal neurons in CA1 and the subiculum (Steward and Scoville, 1976; Witter et al., 2000a; Witter et al., 2000b). Furthermore, neurons in the deep layers of the EC (layers V–VI) relay a large portion of hippocampal output projections back to the superficial layers of the EC (Dolorfo and Amaral, 1998a; Dolorfo and Amaral, 1998b; Kohler, 1986; van Haeften et al., 2003) and to other cortical areas (Witter et al., 1989). The EC is part of a network that is closely related to the consolidation and recall of memories (Haist et al., 2001; Squire et al., 2004), Alzheimer's disease (Hyman et al., 1984; Kotzbauer et al., 2001), schizophrenia (Arnold et al., 1991; Falkai et al., 1988; Joyal et al., 2002; Prasad et al., 2004) and temporal lobe epilepsy (Avoli et al., 2002; Spencer and Spencer, 1994).
In the EC, activation of group I/II mGluRs reduced the amplitude of pharmacologically isolated AMPA- and NMDA-receptor-mediated EPSPs/EPSCs in the EC (Iserhot et al., 2004). Furthermore, activation of group III mGluRs enhanced the frequency of spontaneous excitatory currents, but depressed the amplitude of evoked excitatory postsynaptic currents (Woodhall et al., 2007) and spontaneous GABA release in layer V of the EC (Woodhall et al., 2001). However, a complete spectrum for the effects of the three groups of the mGluRs on synaptic transmission, especially on GABAergic transmission, has not been established. Furthermore, the cellular and molecular mechanisms governing mGluR-mediated modulation of synaptic transmission in the EC have not been determined. Here we examined the effects of mGluRs on GABAergic transmission in the EC. Our results demonstrate that activation of group I mGluRs exerts diverse effects on GABAergic transmission whereas group II and III mGluRs have no effects.
Methods
Slice preparation
Horizontal brain slices (400 μm) including the EC, subiculum and hippocampus were cut using a vibrating blade microtome (VT1000S; Leica, Wetzlar, Germany) from 13- to 20-day-old Sprague Dawley rats as described previously (Deng and Lei, 2006; Deng and Lei, 2007; Deng and Lei, 2008). After being deeply anesthetized with isoflurane, rats were decapitated and their brains were dissected out in ice-cold saline solution that contained (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2 (pH 7.4). Slices were initially incubated in the above solution at 35°C for 40 min for recovery and then kept at room temperature (∼23°C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of spontaneous, miniature and evoked IPSCs from the principal neurons in the superficial layers of the EC
Whole-cell patch-clamp recordings using two Multiclamp 700B amplifiers (Molecular Devices, Sunnyvale, CA) in voltage-clamp mode were made from the principal neurons in layer II/III of the EC visually identified with infrared video microscopy (BX51WI; Olympus, Tokyo, Japan) and differential interference contrast optics (Deng et al., 2006; Deng et al., 2007; Lei et al., 2007; Xiao et al., 2009). All the recordings were conducted at room temperature (∼23°C). The recording electrodes were filled with the following solution (in mM): caesium gluconate 100, EGTA 0.6, MgCl2 5, NaCl 8, ATP2Na 2, GTPNa 0.3, HEPES 40 and QX-314 1 (pH 7.3). The extracellular solution contained (in mM) NaCl 130, NaHCO3 24, KCl 3.5, NaH2PO4 1.25, MgCl2 1.5, CaCl2 2.5 and glucose 10, saturated with 95% O2 and 5% CO2 (pH 7.4). To record GABAA receptor-mediated spontaneous IPSCs (sIPSCs), the external solution was supplemented with dl-2-amino-5-phosphonovaleric acid (dl-APV) (100 μM) and 6,7-dinitroquinoxaline-2,3(1H, 4H)-dione (DNQX) (10 μM) to block NMDA and AMPA receptor-mediated responses, respectively. sIPSCs were recorded at a holding potential of +30 mV (Deng and Lei, 2006; Deng and Lei, 2008; Deng et al., 2006). Under these conditions, the recorded inhibitory currents were completely blocked by bicuculline methobromide (10 μM), confirming that they were mediated by GABAA receptors. Miniature IPSCs (mIPSCs) were recorded by including TTX (1 μM) in the above external solution to block action potential (AP)-dependent responses. Evoked IPSCs (eIPSCs) were recorded from stellate and pyramidal neurons in the EC using the same internal and external solution at a holding potential of +30 mV by placing a stimulation electrode 100 μm from the recorded cell in layer III. Synaptic responses were evoked at 0.2 Hz by low-intensity stimulation (80-100 μsec duration; 10-40 μA intensity) via a constant-current isolation unit (A360; World Precision Instrument, Sarasota, FL) connected to a patch electrode filled with oxygenated extracellular solution. Series resistance was rigorously monitored by the delivery of a -5 mV voltage step after each evoked current. Experiments were discontinued if the series resistance changed by >10%. Data were filtered at 2 kHz, digitized at 10 kHz and acquired on-line using pCLAMP 9 (Clampex) software (Molecular Devices). The recorded sIPSCs and mIPSCs were subsequently analyzed by Mini Analysis 6.0.1 (Synaptosoft Inc., Decatur, GA, USA). Each detected event was inspected visually to exclude obvious artifacts before analysis. The threshold for detection was set to 3 times the standard deviation of the noise as recorded in an event-free stretch of data (Clements and Bekkers, 1997). Mean amplitude, frequency, cumulative amplitude and frequency histograms were calculated by this program. To avoid potential desensitization induced by repeated bath applications of DHPG, a group I mGluR agonist, one slice was limited to only one application of DHPG.
Recordings from layer III GABAergic interneurons
AP firing was recorded from interneurons in layer III of the EC with the intracellular solution containing (in mM) 130 K+-gluconate, 0.5 EGTA, 2 MgCl2, 5 NaCl, 2 ATP2Na, 0.4 GTPNa and 10 HEPES (pH 7.4). Usually for most of the cells a positive current injection was needed to bring the membrane potential to ∼ -45 mV to induce AP firing. DHPG was applied after AP firing had been stable for 5∼10 min. The frequency of the APs was calculated by Mini Analysis 6.0.1.
Holding currents (HCs) at -60 mV were recorded in the extracellular solution supplemented with TTX (1 μM) to block potential indirect effects of DHPG on synaptic transmission. The intracellular solution was the preceding K+-containing solution. HCs were recorded every 3 s and then averaged per minute. We subtracted the average of the HCs recorded for the last minute prior to the application of DHPG from those recorded at different time points to zero the basal level of the HCs for better comparison.
Voltage-current curves were constructed from interneurons in layer III of the EC. K+-gluconate-containing internal solution was used and the external solution contained (in μM) 1 TTX, 100 CdCl2, 10 DNQX, 50 dl-APV and 10 bicuculline. Voltage-current relationship was obtained by using a ramp protocol from -140 mV to -60 mV at a rate of 0.1 mV/ms. We compared the voltage-current curves recorded prior to and during the application of DHPG for 3-6 min.
Dual electrodes recordings from synaptically connected interneuron and pyramidal neuron pairs in the EC
The electrode sealed to the interneuron contained the above K+-gluconate intracellular solution and the electrode sealed to the pyramidal neuron was filled with the preceding Cs+-containing intracellular solution except that Cs+-gluconate was replaced with the same concentration of CsCl. Interneurons were held in current-clamp mode and stimulated at a frequency of 0.3 Hz by brief current pulses (duration 10 msec, amplitude 0.2-0.25 nA) to initiate APs unless specified. Pyramidal neurons were held in voltage-clamp mode (holding potential -60 mV). The recorded currents were completely blocked by application of bicuculline (10 μM) indicating that they were mediated by GABAA receptors.
Data analysis
Data are presented as the means ± S.E.M. DHPG concentration-response curve was fit by Hill equation: I = Imax × {1/[1 + (EC50/[ligand])n]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Student's paired or unpaired t test or analysis of variance (ANOVA) was used for statistical analysis as appropriate; P values are reported throughout the text and significance was set as P<0.05. For sIPSC or mIPSC cumulative probability plots, events recorded 5 min prior to and 5 min after reaching the maximal effect of DHPG were selected. Same bin size (25 ms for frequency and 2 pA for amplitude) was used to analyze data from control and DHPG treatment. Kolmogorov-Smirnoff test was used to assess the significance of the cumulative probability plots. N number in the text represents the cells examined.
Chemicals
(RS)-3,5-dihydroxyphenylglycine (DHPG), (2S, 2′ R, 3′ R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV), L-(+)-2-amino-4-phosphonobutyric acid (L-AP4), (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), (S)-4-carboxyphenylglycine (4-CPG), 2-methyl-6-(phenylethynyl)pyridine (MPEP), (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), dl-threo-β-benzyloxyaspartic acid (TBOA), dihydrokainic acid (DHK) and U73122 were purchased from TOCRIS (Ellisville, MO). GDP-β-S, GTP-γ-S, anti-Gαq and anti-Gβ were from BIOMOL (Plymouth Meeting, PA). 1-O-Octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine (edelfosine) was purchased from Calbiochem (Darmstadt, Germany). Other chemicals were products of Sigma-Aldrich (St. Louis, MO).
Results
Activation of group I not group II and III mGluRs increases sIPSC frequency and amplitude in the superficial layers of the EC
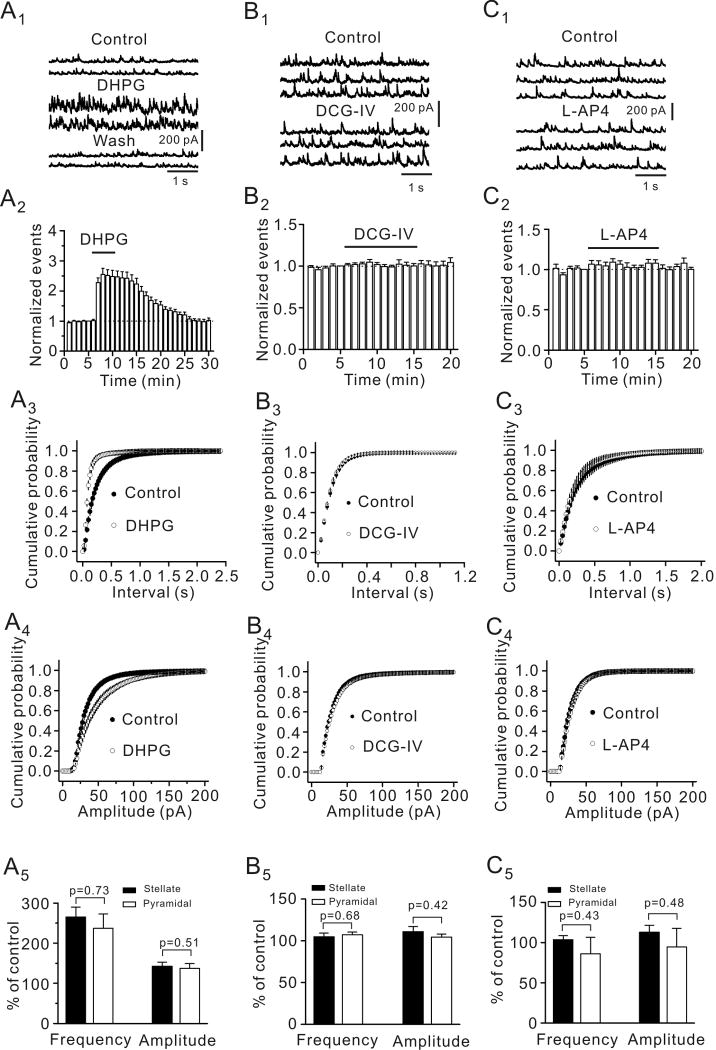
We first examined the roles of group I, II and III mGluRs in GABAergic transmission in the EC by recording GABAA receptor-mediated spontaneous IPSCs (sIPSCs) from the principal (stellate and pyramidal) neurons in the superficial layers (layer II/III) of the EC. We identified these two types of neurons by their morphology and location because previous studies have demonstrated that these neurons can be reliably differentiated by these two criteria (Deng and Lei, 2007; Lei et al., 2007). Stellate neurons are located in layer II or the border of layer II and III and they have larger and polygonal soma with variable number of main dendrites radiating out from the cell body, but are devoid of a clearly dominant dendrite. Pyramidal neurons have a pyramidal or elongated soma with dendrites orientated in a bidirectional way; an apical dendrite running to the surface of the cortex and basal dendrites extending towards the deeper layers. Application of DHPG (10 μM), a group I mGluR agonist, significantly increased sIPSC frequency (254±20% of control, p<0.001, Fig. 1A1-A3) and amplitude (141±7% of control, p<0.001, Fig. 1A4) in 21 cells examined suggesting that activation of group I mGluRs facilitates GABAergic transmission in the EC. Maximal effect of DHPG occurred in 3-6 min after the beginning of its application and was used for statistical comparison thereafter. Of the 21 cells recorded, 13 cells were stellate neurons and 8 cells were pyramidal neurons identified by their morphology and location (Deng and Lei, 2007; Deng et al., 2007). There were no significant differences for DHPG-mediated increases in sIPSC frequency (p=0.73) and amplitude (p=0.51) between stellate and pyramidal neurons (Fig. 1A5) suggesting that DHPG-mediated modulation of GABAergic transmission is not cell-specific in the EC.
Fig. 1.
Activation of group I (A1-A5) not group II (B1-B5) and group III (C1-C5) mGluRs increases the frequency and amplitude of sIPSCs recorded from principal (stellate and pyramidal) neurons in the EC. A1, sIPSCs recorded from a stellate neuron before, during and after the application of the group I mGluR agonist, DHPG (10 μM). A2, DHPG increased sIPSC frequency (n=21, p<0.001, two-way ANOVA). A3, Cumulative probability of sIPSC frequency before and during the application of DHPG (n=21, p<0.001, Kolmogorov-Smirnov test). A4, Cumulative probability of sIPSC amplitude before and during the application of DHPG (n=21, p=0.001, Kolmogorov-Smirnov test). A5, DHPG increased sIPSC frequency and amplitude in stellate (n=13) and pyramidal (n=8) neurons equally (unpaired t-test). B1-B5, Application of the group II mGluR agonist, DCG-IV (10 μM) failed to change significantly sIPSC frequency and amplitude (n=10). The figure was arranged in the same way as A1-A5 and the same kinds of statistical analysis methods were used. C1-C5, Application of group III mGluR agonist, L-AP4 (10 μM) failed to change sIPSC frequency and amplitude (n=10). The figure was arranged in the same fashion and the same kinds of statistical analysis methods were used.
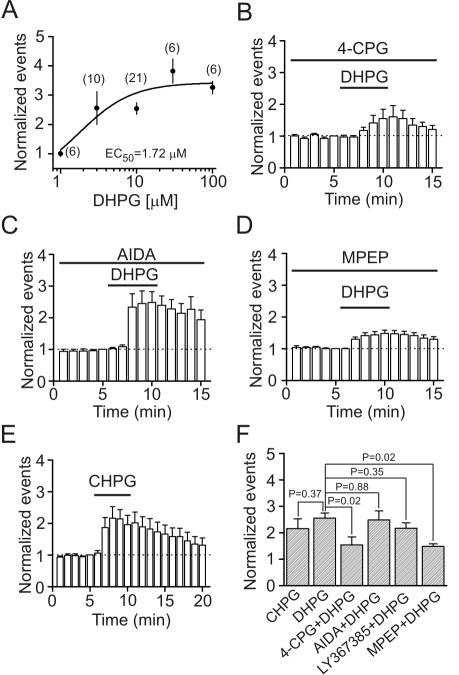
By contrast, application of DCG-IV (10 μM), a group II mGluR agonist, did not alter sIPSC frequency (106±3% of control, p=0.93, Fig. 1B1-1B3) and amplitude (108±4% of control, p=0.95, Fig. 1B4) in 10 cells examined. Of the 10 cells, 5 cells were stellate neurons and 5 cells were pyramidal neurons and neither cell type showed any responses to DCG-IV (Fig. 1B5). Similarly, application of L-AP4 (10 μM), a group III mGluR agonist, failed to change sIPSC frequency (95±10% of control, n=10, p=0.63, Fig. 1C1-1C3) and amplitude (104±12% of control, n=10, p=0.76, Fig. 1C4) in stellate neurons or pyramidal neurons (Fig. 1C5). These results together demonstrate that activation of group I not group II and III mGluRs facilitates GABAergic transmission in the superficial layers of the EC. We thereby further examined the mechanisms whereby group I mGluRs increase GABAergic transmission by recording from both stellate and pyramidal neurons in the EC. The EC50 value of DHPG was measured to be 1.72 μM (Fig. 2A).
Fig. 2.
DHPG increases sIPSCs via activation of mGluR5 receptors. A, Concentration-response curve for DHPG-induced increases in sIPSC frequency. Numbers in the parentheses are the numbers of cells recorded at each concentration. B, Pretretment of slices with and continuous bath application of 4-CPG (50 μM) significantly reduced DHPG-induced increases in sIPSC frequency (n=6, p=0.02 vs. DHPG alone, unpaired t-test). C, Pretreatment of slices with and continuous bath application of AIDA (200 μM), a mGluR1 antagonist, did not significantly alter DHPG-induced increases in sIPSC frequency (n=6, p=0.88 vs. DHPG alone, unpaired t-test). D, Pretreatment of slices with and continuous bath application of MPEP (10 μM), a mGluR5 antagonist, significantly reduced DHPG-induced increases in sIPSC frequency (n=10, p=0.02 vs. DHPG alone, unpaired t-test). E, Bath application of CHPG (100 μM), a mGluR5 agonist, significantly increased sIPSC frequency (n=6, p<0.001, two-way ANOVA). F, Summarized data for the effects of group I mGluR antagonists on DHPG-induced increases in sIPSC frequency (unpaired t-test vs. DHPG alone).
Involvement of mGluR5
Group I mGluRs include mGluR1 and mGluR5 (Ritzen et al., 2005). We next examined the roles of these two receptors in DHPG-induced increases in sIPSCs. Pretreatment of slices with and continuous bath application of 4-CPG (50 μM), a competitive group I mGluR antagonist for both mGluR1 and mGluR5, significantly reduced DHPG-induced increases in sIPSC frequency (155±30% of control, n=6, p=0.02, Fig. 2B and 2F) and amplitude (108±6% of control, n=6, p=0.03) indicating that the effect of DHPG was mediated by activation of group I mGluRs. However, application of AIDA (200 μM), a selective mGluR1 antagonist, in the same fashion did not significantly change DHPG-induced increases in sIPSC frequency (248±34% of control, n=6, p=0.88, Fig. 2C and 2F) and amplitude (158±11% of control, n=6, p=0.23). Similarly, application of LY367385 (30 μM), another selective mGluR1 antagonist, failed to significantly alter DHPG-induced increases in sIPSC frequency (217±20% of control, n=6, p=0.35, Fig. 2F) and amplitude (146±6% of control, n=6, p=0.7). However, application of MPEP (10 μM), a selective mGluR5 antagonist, significantly diminished DHPG-induced increases in sIPSC frequency (148±10% of control, n=10, p=0.02, Fig. 2D and 2F) and amplitude (112±6% of control, n=10, p=0.02) suggesting that DHPG increases sIPSCs via activation of mGluR5. We further verified the role of mGluR5 by application of CHPG, a selective mGluR5 agonist. Bath application of CHPG (100 μM) significantly increased sIPSC frequency (216±36% of control, n=6, p<0.001, Fig. 2E and 2F) and amplitude (146±7% of control, n=6, p=0.001). Taken together, these data indicate that DHPG increases sIPSCs via activation of mGluR5 in the EC.
DHPG does not modulate mIPSC frequency and remarkably inhibits the amplitudes of IPSCs evoked by extracellular field stimulation and depolarization of interneurons in synaptically connected interneuron-pyramidal neuron pairs
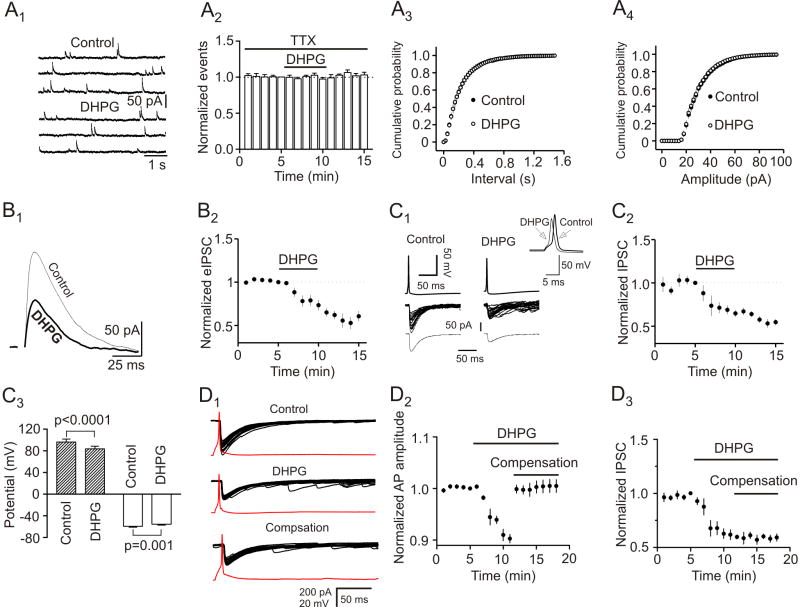
We next examined the effects of DHPG on mIPSCs recorded in the presence of TTX (1 μM). Application of DHPG (10 μM) failed to change either the frequency (102±2% of control, n=6, p=0.54, Fig. 3A1-3A3) or the amplitude (98±1% of control, n=6, p=0.28, Fig. 3A4) of mIPSCs suggesting that DHPG modulates presynaptic GABA release without affecting postsynaptic GABAA receptors.
Fig. 3.
DHPG has no effects on mIPSCs but remarkably reduced the amplitude of eIPSCs by extracellular field stimulation and depolarization of GABAergic interneurons in synaptically connected interneuron and pyramidal neuron pairs. A1, mIPSCs recorded from a neuron before and during the application of DHPG (10 μM) in the presence of TTX (1 μM). A2, Pooled time course of mIPSC frequency (n=6, p=0.54, two-way ANOVA). A3, Cumulative probability of mIPSC frequency before and during the application of DHPG (n=6, p=0.66, Kolmogorov-Smirnov test). A4, Cumulative probability of mIPSC amplitude before and during the application of DHPG (n=6, p=0.79, Kolmogorov-Smirnov test). B1, Application of DHPG (10 μM) decreased the amplitude of IPSCs evoked by extracellular filed stimulation from a principal neuron. Current traces were averaged from 10 traces before and during application of DHPG. B2, Summarized data for DHPG-induced inhibition of eIPSCs (n=7, p<0.001, two-way ANOVA). C1, Upper panel: Averaged trace from 20 individual APs prior to (left) and during (right) the application of DHPG (10 μM) recorded from a presynaptic interneuron. Inset shows the overlay of the two APs. Note that application of DHPG slightly depolarized the resting membrane potential and reduced the amplitude of APs. Middle panel: Superimposed IPSCs evoked by individual APs before (left) and during (right) the application of DHPG. Note that application of DHPG reduced the amplitude of the IPSCs and increased the frequency of sIPSCs. Bottom panel: Averaged IPSC before (left) and during (right) the application of DHPG. C2, Summarized time course of the IPSCs from 4 interneuron-pyramidal neuron pairs (p=0.004. two-way ANOVA). C3, Summarized AP amplitudes (gray bars, paired t-test) and resting membrane potentials (white bars, paired t-test) recorded from interneurons before and during the application of DHPG (n=4 pairs). D1, Averaged AP (from 20 traces, red) recorded from an interneuron and the corresponding IPSCs recorded from a principal neuron before (upper panel) and during (middle panel) the application of DHPG. Low panel shows the averaged AP and the corresponding IPSCs when a negative current (-21 pA) was injected to the interneuron to compensate DHPG-induced membrane depolarization. Note that IPSCs did not return to the control level after compensation of DHPG-induced membrane depolarization. D2, DHPG reduced the amplitude of APs recorded from presynaptic interneurons and injection of negative currents to compensate DHPG-induced depolarization returned the amplitude of AP to the initial level before application of DHPG (n=4 pairs, p=0.73 vs. baseline, paired t-test). D3, DHPG reduced IPSCs simultaneously recorded from postsynaptic principal neurons and injection of negative currents to compensate DHPG-induced depolarization in presynaptic interneurons failed to change significantly IPSC amplitudes (n=4 pairs, p=0.001 vs. baseline, paired t-test).
We then examined the effects of DHPG on eIPSCs by placing a stimulation electrode in layer III (∼200 μm from the recorded cell) to stimulate GABAergic inputs. Application of DHPG (10 μM) did not increase but instead remarkably reduced the amplitude of eIPSCs (73±6% of control, n=7, p<0.001, Fig. 3B1 and 3B2). This phenomenon (increases in sIPSCs but reduction in eIPSCs) has also been observed for kainate receptors (Frerking et al., 1998) and norepinephrine (Madison and Nicoll, 1988) in the hippocampus and 5-HT in the EC (Deng and Lei, 2008).
We then used dual electrodes and recorded simultaneously presynaptic APs and postsynaptic IPSCs in synaptically connected interneuron and pyramidal neuron pairs in layer III of the EC. The electrode sealed to presynaptic interneuron contained K+-gluconate whereas that sealed to postsynaptic pyramidal neuron contained CsCl. Postsynaptic pyramidal neurons were held at − 60 mV. Interneurons were differentiated from pyramidal neurons in layer III by their smaller sizes and fast firing spikes and pronounced afterhyperpolarization. As demonstrated previously (Deng and Lei, 2008), there are two types of interneurons in layer III of the EC. Type I interneurons showed little voltage sag in response to hyperpolarizing current injection with no rebound burst firing whereas type II interneurons displayed prominent voltage sag in response to hyperpolarizing current injection with rebound burst firing (Deng and Lei, 2008). Under these conditions, bath application of DHPG (10 μM) significantly reduced the amplitudes of IPSCs (65±4% of control, n=4 pairs, p=0.004, Fig. 3C1 and 3C2). The amplitudes of the APs simultaneously recorded from the presynaptic interneurons were significantly reduced (control: 96.2±5.3 mV, DHPG: 83.5±4.9 mV, n=4, p<0.0001, Fig. 3C1 and 3C3). Furthermore, application of DHPG induced a depolarizing resting membrane potential of presynaptic interneurons (control: -59.3±1.8 mV, DHPG: -55.5±1.5 mV, n=4, p=0.001, Fig. 3C1 and 3C3).
We next tested whether DHPG-induced reduction in the amplitude of APs was responsible for its inhibitory effect on eIPSCs. As demonstrated below (Fig. 4C and 4D), DHPG increased the input resistance of interneurons by ∼26% and increases in input resistance would change the threshold of AP generation. We therefore injected a suprathreshold current that was ∼ 30% higher than the threshold current to induce an AP in presynaptic interneuron to overcome the effects of increased input resistance on AP generation. In another 4 interneuron-pyramidal neuron pairs, application of DHPG (10 μM) also induced membrane depolarization (control: -61.6±0.5 mV, DHPG: -57.8±1.0 mV, n=4, p=0.02) and reduced AP amplitude (90.3±0.8% of control, n=4, p=0.001, Fig. 3D2) recorded from presynaptic interneurons and inhibited the amplitude of IPSCs (61.7±4.7% of control, n=4, p=0.004, Fig. 3D1 and D3) recorded from pyramidal neurons. We then injected a negative current via the recording pipette sealed to the interneuron to bring the membrane potential of the interneuron back to the initial level before the application of DHPG. In this condition, the amplitude of the AP returned to the initial level before DHPG application (100.5±1.2% of control, n=4, p=0.73, Fig. 3D2) whereas the amplitude of IPSCs simultaneously recorded from the postsynaptic pyramidal neurons did not recover (59.1±3.3% of control, n=4, p=0.001, Fig. 3D1 and 3D3). Together, these results suggest that a reduction of presynaptic AP amplitude may not, at least not only, be responsible for DHPG-mediated depression of eIPSCs in the EC.
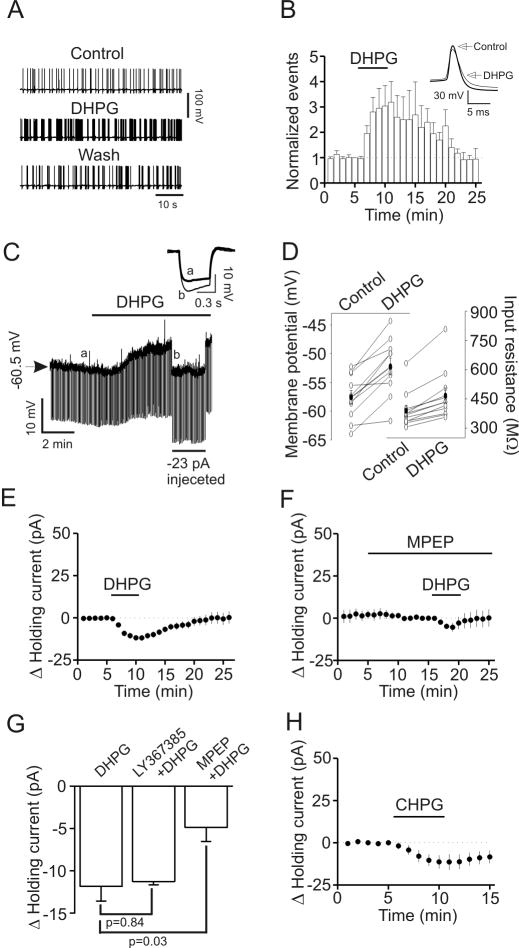
Fig. 4.
DHPG increases the excitability of GABAergic interneurons in the EC. A, APs recorded from an interneuron prior to, during and after application of DHPG (10 μM). B, Pooled time course of AP firing frequency before, during and after application of DHPG (10 μM, n=8). Note that DHPG significantly increased the firing frequency of APs in interneurons (p=0.04, two-way ANOVA). Inset shows the averaged AP before and during the application of DHPG. Note that application of DHPG induced depolarization and reduced AP amplitude. C, Application of DHPG (10 μM) generated membrane depolarization and increased input resistance in a layer III interneuron of the EC. Resting membrane potential was recorded in current-clamp mode and a hyperpolarizing current (-50 pA, 500 ms) was injected every 5 s to measure the input resistance. Note that DHPG generated depolarization and increased input resistance. To exclude the influence of DHPG-induced membrane depolarization on input resistance, a constant negative current (-23 pA indicated by the horizontal bar) was injected briefly to bring the membrane potential back to the initial level. Under these conditions, the voltage responses induced by the hyperpolarizing current (-50 pA, 500 ms) injection were still larger compared with control suggesting that DHPG-induced increases in input resistance are not secondary to its effect on membrane depolarization. Inset is the voltage traces taken before (a) and during (b) the application of DHPG when a constant negative current was injected. D, Summarized data for DHPG-induced depolarization (left, n=11, p=0.001, paired t-test) and increase in input resistance (right, n=11, p=0.001, paired t-test). Solid circles represent average values. E, Application of DHPG (10 μM) induced an inward HC in interneurons (n=11, p<0.001, two-way ANOVA). F, Application of MPEP, a mGluR5 antagonist, significantly reduced DHPG-induced increases in inward HCs (n=5, p=0.03 vs, DHPG alone, unpaired t-test). G, Summarized data showing that application of LY367385, a mGluR1 antagonist, did not change but application of MPEP significantly reduced DHPG-induced increases in inward HCs (unpaired t-test). H, Bath application of CHPG, a mGluR5 agonist, induced an inward HC (n=5, p<0.001, two-way ANOVA).
DHPG increases the excitability of GABAergic interneurons in the EC
The above results demonstrate that DHPG has opposite effects on sIPSCs and eIPSCs but does not modulate mIPSCs. Because both sIPSCs and eIPSCs are AP-dependent whereas mIPSCs are not, we examined the effects of DHPG on AP firing from identified interneurons in layer III of the EC. Bath application of DHPG (10 μM) significantly increased the frequency (303±80% of control, n=8, p=0.04, Fig. 4A, 4B) but reduced the amplitudes (93±2% of control, n=8, p=0.004, Fig. 4A and 4B) of APs. Among the 8 interneurons recorded, there were 3 Type I and 5 Type II interneurons. Because each interneuron responded to DHPG, we pooled the data obtained from the Type I and Type II interneurons for the rest of the experiments.
We then recorded DHPG-induced changes in resting membrane potentials in current-clamp mode from layer III interneurons in the extracellular solution supplemented with TTX (0.5 μM) to block potential indirect effects from synaptic transmission. A negative current (-50 pA for 500 ms) was injected every 5 s to assess the changes of input resistance induced by DHPG (Fig. 4C). Under these circumstances, application of DHPG (10 μM) generated membrane depolarization (control: -57.6±1.1 mV, DHPG: -52.2±1.5 mV, n=11, p=0.001, Fig. 4C and 4D) and increased input resistance (control: 389±28 MΩ, DHPG: 467±39 MΩ, n=11, p<0.001, Fig. 4C and 4D). The DHPG-induced increase in input resistance was not secondary to its effect on membrane depolarization because in 3 of the 11 cells we injected a negative current to bring the membrane potential back to the initial level, but the input resistance was still higher than control during the period of current injection (control: 358±12 MΩ, DHPG: 452±27 MΩ, n=3, p=0.04, Fig. 4C). These results suggest that DHPG reduces membrane conductance.
We also examined DHPG-induced depolarization by recording HCs at -60 mV in voltage-clamp mode in the extracellular solution supplemented with TTX (1 μM). Application of DHPG (10 μM) induced an inward HC (-11.8±1.7 pA, n=11, p<0.001, Fig. 4E). DHPG-induced increases in inward HCs were inhibited when MPEP (10 μM), a selective mGluR5 inhibitor, was applied (-4.8±1.6 pA, n=5, p=0.03 vs. DHPG alone, Fig. 4F and 4G) whereas application of LY367385 (30 μM), a selective mGluR1 inhibitor, failed to change DHPG-induced increases in inward HCs significantly (-11.3±0.4 pA, n=5, p=0.84 vs. DHPG alone, Fig. 4G). Furthermore, application of CHPG (100 μM), a selective mGluR5 agonist induced an inward HC (-13.9±3.9 pA, n=5, p<0.001, Fig. 4H). Together, these data indicate that DHPG depolarizes entorhinal interneurons via activation of mGluR5 receptors.
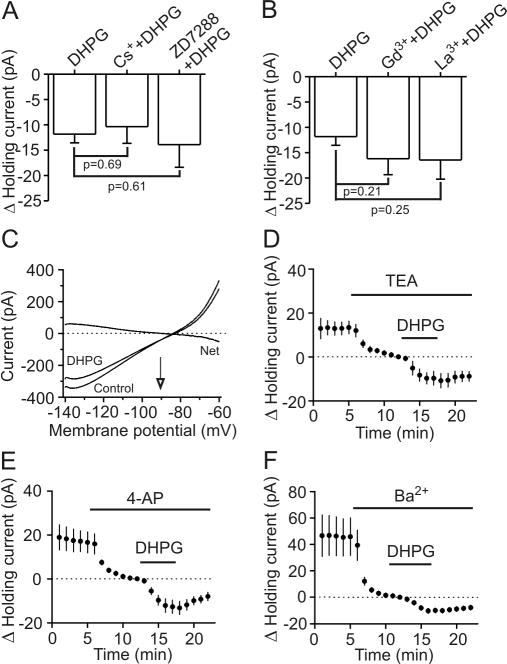
DHPG induces membrane depolarization by inhibiting a background K+ channel in interneurons
Whereas the result that DHPG increases input resistance did not support the idea that DHPG depolarizes GABAergic interneurons via activation of a cationic conductance, we still tested the possibility that activation of mGluR5 receptors opens cationic channels to generate membrane depolarization. We first tested the potential involvement of H-channels. DHPG-induced increases in inward HCs were not significantly altered by bath application of two H-channel blockers, Cs+ (3 mM, -10.4±3.3 pA, n=6, p=0.69, Fig. 5A) and ZD7288 (100 μM, -13.9±4.5 pA, n=6, p=0.61, Fig. 5A) suggesting that DHPG-mediated depolarization of entorhinal interneurons is not mediated by interaction with H-channels. We then examined the roles of other cationic channels. DHPG-induced increases in inward HCs were not significantly changed by bath application of two non-specific cationic channel blockers, Gd3+ (-16.9±3.2 pA, n=5, p=0.21, Fig. 5B) and La3+ (-16.4±3.8 pA, n=5, p=0.25, Fig. 5B). Together, these results suggest that DHPG-induced depolarization is not mediated via activation of a cationic conductance.
Fig. 5.
DHPG inhibits K+ channels of the interneurons in layer III to modulate GABA release. A, Bath application of H-channel blockers, Cs+ and ZD7288, did not significantly change DHPG-induced increases in inward HCs (unpaired t-test). B, Bath application of Gd3+ and La3+, two nonselective cationic channel blockers did not change DHPG-induced increases in inward HCs (unpaired t-test). C, Voltage-current relationship recorded by a ramp protocol (from -140 mV to -60 mV, at a speed of 0.1 mV/ms) before and during the application of DHPG (10 μM) when the extracellular K+ concentration was 3.5 mM. Traces in the figure were averaged traces from 10 cells. DHPG-induced net current has a reversal potential at ∼-90.5 mV close to the calculated K+ reversal potential (∼-92.2 mV). D, DHPG-induced increases in HCs were insensitive to TEA (n=5, unpaired t-test vs. DHPG alone). E, Bath application of 4-AP did not significantly change DHPG-induced increases in inward HCs (n=6, unpaired t-test vs. DHPG alone). F, Bath application of Ba2+ (3 mM) induced an inward HC by itself (n=7, p=0.01, paired t-test vs. baseline) but did not significantly change DHPG-induced increases in HCs (n=5, p=0.12, unpaired t-test vs. DHPG alone).
Because application of DHPG significantly increased the input resistance in interneurons (Fig. 4C and 4D), we next tested the hypothesis that DHPG-induced depolarization is mediated by inhibition of a background K+ channel. If so, DHPG-induced increase in HCs should have a reversal potential close to the K+ reversal potential. We used a ramp protocol (from -140 mV to -60 mV, at a speed of 0.1 mV/ms) to construct the voltage-current curve before and during the application of DHPG (10 μM). The extracellular solution was supplemented with (in μM) 1 TTX, 100 Cd2+, 100 Ni2+, 10 DNQX, 50 dl-APV and 10 bicuculline to block synaptic current and other voltage-gated ion channels. In this condition, DHPG induced a current which had a reversal potential (-90.5±2.9 mV, n=10) close to the calculated K+ reversal potential (-92.2 mV) in our recording conditions (Fig. 5C) suggesting that DHPG induces membrane depolarization by inhibiting background K+ channels.
We next characterized the properties of the involved K+ channels. DHPG-induced increases in inward HCs recorded from interneurons in the EC were not significantly altered (vs. DHPG alone) by application of TEA (10 mM, -9.7±3.1 pA, n=5, p=0.14, Fig. 5D) and 4-aminopyridine (4-AP, 2 mM, -12.7±3.0 pA, n=6, p=0.78, Fig. 5E). As described above, DHPG-induced depolarization was not sensitive to extracellular application of Cs+ (Fig. 5A) suggesting that DHPG-mediated inhibition of K+ channels is insensitive to the classic K+ channel blockers. Furthermore, bath application of Ba2+ (2 mM) alone induced an inward HC (-45±14 pA, n=7, p=0.01, Fig. 5F) by itself but did not significantly change DHPG-induced increases in inward HCs (-10.3±0.8 pA, n=7, p=0.12, Fig. 5F). We therefore conclude that DHPG-induced depolarization of GABAergic interneurons in the EC is mediated by a background K+ channel that is insensitive to Ba2+ and other classic K+ channel blockers.
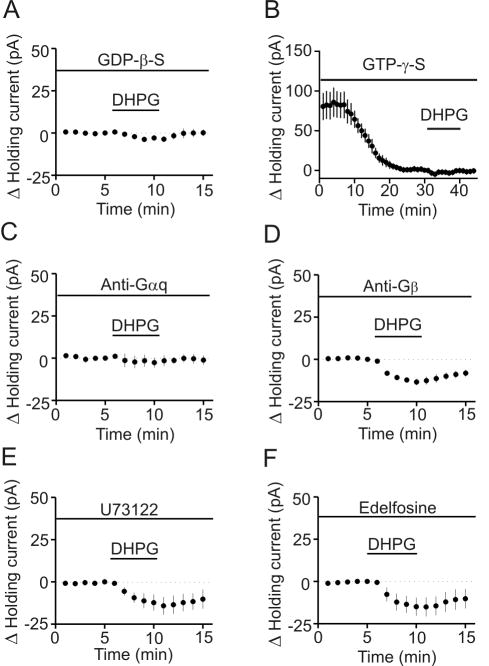
Signal transduction mechanism
Our results demonstrate that DHPG modulates GABA release by activating mGluR5 receptors on GABAergic interneurons. Activation of mGluR5 receptors is coupled to Gαq/11 resulting in activation of phospholipase C (PLC). We initially tested whether G-proteins were involved in DHPG-induced modulations of GABAergic transmission. We replaced GTP in the intracellular solution with GDP-β-S (4 mM), a G-protein inactivator, and recorded the HCs at -55 mV from interneurons in layer III. Intracellular application of GDP-β-S significantly reduced DHPG-induced increases in inward HCs (-3.8±1.2 pA, n=5, p=0.01, Fig. 6A). Moreover, intracellular application of GTP-γ-S (1 mM) by itself induced an inward HC (-80.3±16.6 pA, n=6, p=0.005, Fig. 6B) and significantly reduced DHPG-induced increases in inward HCs (-5.0±2.1 pA, n=6, p=0.03 vs. DHPG alone, Fig.6B). These data together indicate that G-proteins are required for DHPG-induced depolarization in entorhinal interneurons.
Fig. 6.
DHPG-induced increases in inward HCs in interneurons require the functions of Gαq but are independent of PLC activity. A, Intracellular dialysis of GDP-β-S blocked DHPG-induced increases in inward HCs (p=0.01, unpaired t-test vs. DHPG alone). B, Intracellular dialysis of GTP-γ-S induced an inward HC by itself (p=0.005, paired t-test vs. baseline) and significantly reduced DHPG-induced increases in inward HCs (p=0.03, unpaired t-test vs. DHPG alone). C, Intracellular dialysis of antibody to Gαq blocked DHPG-induced increases in inward HCs (p=0.41, paired t-test vs. baseline). D, Intracellular dialysis of antibody to Gβ failed to change significantly DHPG-induced increases in inward HCs (p=0.58, unpaired t-test vs. DHPG alone). E, Pretreatment of slices with and continuous bath application of U73122 did not significantly change DHPG-induced increases in inward HCs (p=0.59, unpaired t-test vs. DHPG alone). F, Pretreatment of slices with and continuous bath application of edelfosine did not significantly change DHPG-induced increases in inward HCs (p=0.49, unpaired t-test vs. DHPG alone).
We then tested the role of Gαq and Gβγ by intracellular dialysis of antibodies to Gαq and Gβ. Intracellular dialysis of ant-Gαq (20 μg/ml) blocked DHPG-induced increases in inward HCs (-2.6±2.9 pA, n=6, p=0.41, Fig. 6C) whereas intracellular application of ant-Gβ (20 μg/ml) failed to change significantly DHPG-induced increases in inward HCs (-13.4±1.9 pA, n=6, p=0.58, Fig. 6D). These data demonstrate that DHPG-induced depolarization is mediated by Gαq.
Because activation of mGluR5 receptors increases PLCβ activity, we next tested whether PLCβ was required for DHPG-induced depolarization. Slices were pretreated with U73122 (20 μM) for >2 h and the bath was continuously perfused with the same concentration of U73122. Under these conditions, DHPG-induced increases in inward HCs recorded from layer III interneurons were not significantly changed (-14.2±5.3 pA, n=5, p=0.59, Fig. 6E). Similarly, pretreatment of slices with and continuous bath application of edelfosine (20 μM), another PLC inhibitor, failed to change significantly DHPG-induced increases in inward HCs (-15.1±5.6 pA, n=6, p=0.49, Fig. 6F). These results suggest that PLC activity is not required for DHPG-induced depolarization of entorhinal interneurons.
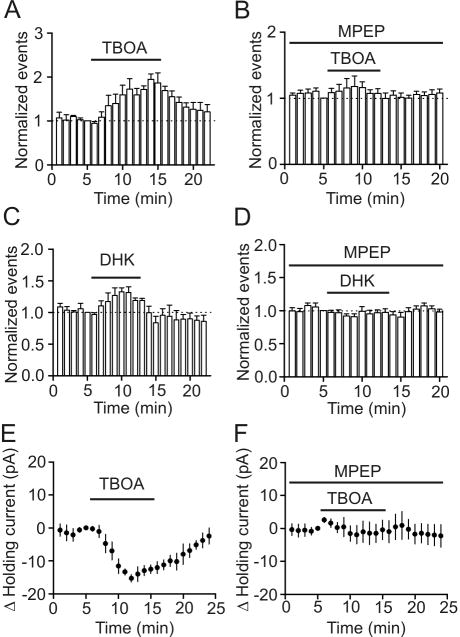
Glutamate spillover from excitatory synapses increases sIPSC frequency and amplitude via mGluR5-mediated depolarization of GABAergic interneurons
We next tested whether mGluR5-mediated modulation of GABAergic transmission was physiologically relevant. Glutamate can reach GABAergic synapses via spillover from the excitatory glutamatergic synapses in physiological condition (Bergles et al., 1999; Kullmann, 2000). We next tested whether glutamate spillover can modulate GABAergic transmission onto the principal neurons in the superficial layer of the EC. Because glutamate released from the excitatory synapses is removed mainly by glutamate transportor, we used TBOA, a nontransportable neuronal and glial glutamate transport blocker to increase glutamate concentration at GABAergic synapses. Bath application of TBOA (100 μM) significantly increased sIPSC frequency (172±24% of control, n=6, p<0.001, Fig. 7A) and amplitude (119±5% of control, n=6, p=0.01). To test the involvement of mGluR5, we applied TBOA in the presence of MPEP (10 μM). Under these conditions, application of TBOA (100 μM) failed to increase sIPSC frequency (118±16% of control, n=5, p=0.81, Fig. 7B) and amplitude (103±5% of control, n=5, p=0.65) significantly suggesting that the effect was mediated by activation of mGluR5. We also applied DHK, a more selective glial glutamate transport blocker. Bath application of DHK (200 μM) also significantly increased sIPSC frequency (135±5% of control, n=4, p=0.002, Fig. 7C) and amplitude (115±3% of control, n=4, p=0.01). Co-application of MPEP (10 μM) also blocked DHK-induced increases in sIPSC frequency (99±7% of control, n=6, p=0.91, Fig. 7D) and amplitude (105±6% of control, n=6, p=0.46) suggesting the involvement of mGluR5. To test the involvement of GABAergic interneurons, we also examined the effects of TBOA on HCs recorded from identified interneurons. The extracellular solution was supplemented with dl-APV (50 μM) and DNQX (10 μM) to block glutamate receptors and bicuculline (10 μM) to block GABAA receptors. Under these conditions, application of TBOA (100 μM) induced an inward HC (-15.3±1.1 pA, n=5, p<0.001, Fig. 7E) by activation of mGluR5 because TBOA-induced depolarization was blocked by pretreatment of slices with and continuous bath application of MPEP (10 μM, n=5, p=0.99, Fig. 7F). Together, these results demonstrate that glutamate spillover from the excitatory synapses activates mGluR5 on GABAergic neurons to modulate GABAergic transmission.
Fig. 7.
Elevation of synaptic glutamate concentration by application of glutamate transporter inhibitors increases sIPSC frequency recorded from principal neurons and induced an inward HC in layer III interneurons of the EC. A, Bath application of TBOA (100 μM), a neuronal and glial glutamate transporter inhibitor, significantly enhanced sIPSC frequency (p<0.001, two-way ANOVA). B, Pretreatment of slice with and continuous bath application of MPEP (10 μM), a mGluR5 inhibitor, blocked TBOA-induced increases in sIPSC frequency (p=0.81, two-way ANOVA). C, Bath application of DHK (200 μM), a glial glutamate transporter inhibitor, significantly increased sIPSC frequency (p=0.002, two-way ANOVA). D, Pretreatment of slices with and continuous bath application of MPEP (10 μM) blocked DHK-induced increases in sIPSC frequency (p=0.91, two-way ANOVA). E, Bath application of TBOA (100 μM) induced an inward HC in layer III interneurons of the EC (p<0.001, two-way ANOVA). F, Pretreatment of slices with and continuous bath application of MPEP (10 μM) blocked the effect of TBOA on HCs recorded from entorhinal interneurons (p=0.99, two-way ANOVA).
Discussion
Our results demonstrate two distinct modes of modulation of GABAergic transmission by mGluR5 receptors in the superficial layers of the EC; activation of mGluR5 receptors facilitates sIPSC frequency and amplitude but depresses the amplitude of eIPSCs. mGluR5 activation modulates GABAergic transmission by inhibiting a type of background K+ channels that are insensitive to Ba2+, resulting in membrane depolarization and increases in AP firing frequency of GABAergic interneurons in the EC. mGluR5-induced modulation of GABAergic transmission requires the function of G-proteins but is independent of PLC activity. Elevation of synaptic glutamate concentration by application of glutamate transporter inhibitors mimics the effects of mGluR5 agonists on GABAergic transmission suggesting that mGluR5-mediated modulation of GABAergic transmission is physiologically relevant.
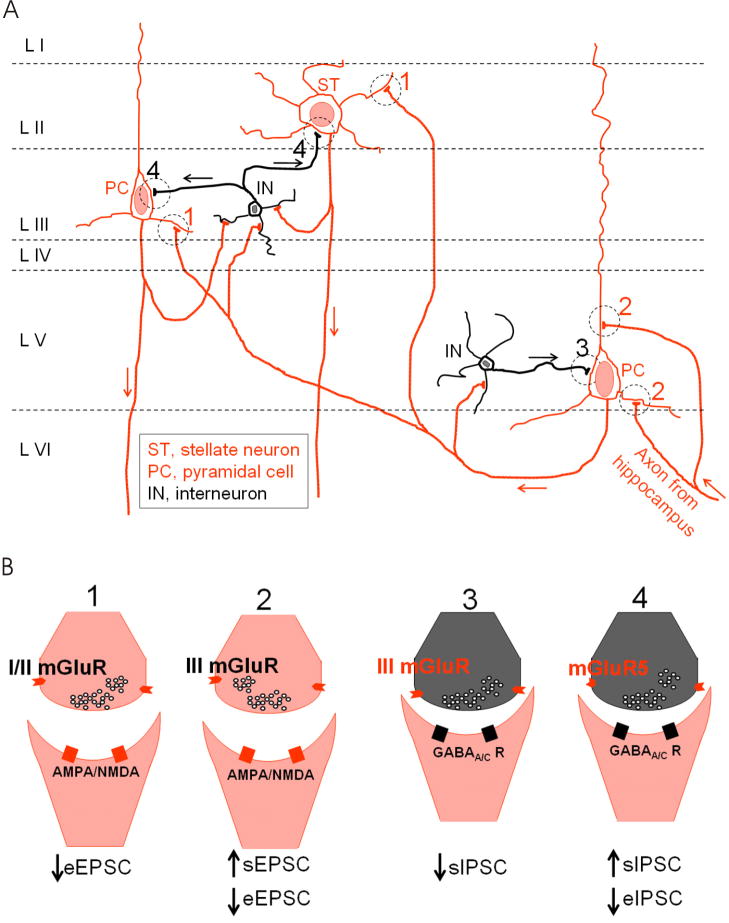
To date, the EC has been shown to express mGluR1 (Shigemoto et al., 1992), mGluR2 (Fotuhi et al., 1994; Ohishi et al., 1998; Ohishi et al., 1993), mGluR3 (Petralia et al., 1996), mGluR4 (Ohishi et al., 1995; Thomsen et al., 1992), mGluR5 (Fotuhi et al., 1994) and mGluR7 (Kinoshita et al., 1998; Ohishi et al., 1995). As shown in Fig. 8, activation of these receptors modulates synaptic functions at several entorhinal synapses. Our results that DHPG has opposite effects on sIPSCs and eIPSCs but has no effects on mIPSCs suggest that DHPG-mediated modulation of GABA release is AP- and Ca2+-dependent. Activation of mGluR5 depolarizes GABAergic interneurons resulting in increases in AP firing frequency and reduction of AP amplitudes. DHPG-induced increases in AP firing frequency of GABAergic interneurons could explain its effect on sIPSC frequency. DHPG-mediated increases in sIPSC amplitude could be explained by two possible mechanisms. First, DHPG-induced increases in sIPSC events could be synchronized. More than one event could be temporally summated and the amplitudes of the integrated sIPSCs are likely larger. Second, DHPG-induced enhancement of AP firing in GABAergic interneurons could potentially increase GABA concentration in the synaptic cleft. Elevation of GABA concentration in the synaptic cleft likely results in increased sIPSC amplitudes. However, the precise mechanisms whereby DHPG reduces eIPSC amplitude are unknown. Although we have observed a small but significant reduction in AP amplitude recorded from GABAergic interneurons due to DHPG-induced membrane depolarization, DHPG-induced reduction of AP amplitude could not explain the attenuated eIPSC amplitude because injection of a negative current to compensate DHPG-induced depolarization returned the AP amplitude to the initial level but failed to rectify the attenuated eIPSC amplitude. An alternative mechanism whereby DHPG depresses eIPSCs is that DHPG-induced increases in sIPSC frequency could deplete or at least reduce the readily releasable pool of GABA at the GABAergic synapses if sIPSCs and eIPSCs share the same pool of GABA, which leads to insufficient GABA release when the terminals or the soma of interenurons are stimulated exogenously.
Fig. 8.
Schematic diagram illustrating distinct effects of mGluRs on synaptic transmission in the EC. A, Scheme of neural circuit in the EC. The EC is usually divided into superficial (layer II-III) and deep layers (layer V-VI). Hippocampal output arrives at the deep layers (layer V/VI). The axons of the neurons in layer V/VI synapse onto the superficial layer neurons including layer II stellate neurons (denoted as ST) and layer III pyramidal cells (denoted as PC). Interneurons (denoted as IN) are distributed in each layer and they form GABAergic synapses with principal cells or other interneurons in the EC. Interneurons also receive glutamatergic excitation from the principal cells in the EC. B, Summarized effects of mGluRs at 4 different synapses labeled in A as 1, 2, 3 and 4. At synapse 1, activation of presynaptic group I/II mGluRs reduces eEPSC recorded from layer II/III principal neurons (Iserhot et al., 2004). At synapse 2, activation of presynaptic group III mGluRs increases the frequency of sEPSC and depresses the amplitude of eEPSC recorded from layer V pyramidal cells (Woodhall et al., 2007). At synapse 3, group III mGluRs in layer V interneurons inhibits sIPSCs recorded from layer V pyramidal cells (Woodhall et al., 2001). At synapse 4, we have shown in the present study that activation of mGluR5 in layer III interneurons increases sIPSC frequency and amplitude, but inhibits the amplitude of eIPSCs recorded from the principal neurons in the superficial layers.
Our results demonstrate that DHPG-induced depolarization of interneuron is not mediated by activation of cationic channels but by inhibition of a background K+ channels based on the following lines of evidence. First, application of DHPG increases the input resistance of interneurons suggesting that DHPG reduces but not increases ionic conductance of interneurons. Second, application of the inhibitors to cationic channels failed to block DHPG-induced depolarization. Third, DHPG-induced net current has a reversal potential close to K+ reversal potential. Whereas our results support the involvement of K+ channels, the identity of the K+ channels has not been identified. DHPG-induced depolarization was not sensitive to the classical K+ channel blockers including TEA, Cs+ and 4-AP. Neither did bath application of Ba2+ significantly alter DHPG-induced depolarization in interneuron. Although the inwardly rectifier K+ channels are involved in controlling resting membrane potentials, our results do not support a role for this type of K+ channels in DHPG-induced depolarization because the inward rectifier K+ channels are sensitive to TEA whereas DHPG-induced depolarization was insensitive to TEA. Two-pore domain K+ (K2P) channels are the principal K+ channels involved in controlling resting membrane potentials and these channels are insensitive to the classic K+ channel blockers such as TEA, Cs+ and 4-AP. Because we have shown that application of these K+ channel blockers failed to change DHPG-induced depolarization, it is highly likely that K2P channels are involved in DHPG-induced depolarization in interneuron. Seventeen members of K2P channels have been cloned and they can be grouped according to sequence and functional similarities into 6 subfamilies: TWIK, THIK, TREK, TASK, TALK and TRESK (Bayliss et al., 2003). Among the K2P channels, TASK-1 (Han et al., 2002), TASK-3 (Han et al., 2002; Kim et al., 2000), TREK-1 (Fink et al., 1996), TREK-2 (Han et al., 2002), TWIK-1 (Lesage et al., 1996) and TRESK (Kang et al., 2004; Sano et al., 2003) are sensitive to Ba2+. Our result that DHPG-induced depolarization was insensitive to Ba2+ suggests that K2P channels other than TASK-1, TASK-3, TREK-1, TREK-2, TWIK-1 and TRESK are involved.
The result that elevation of synaptic glutamate concentration by application of glutamate transporter inhibitors increases sIPSC frequency and amplitude suggests that the modulation of GABAergic transmission mediated by mGluR5 occurs when the ambient glutamate concentration is higher. The functional involvement of mGluR5 in the modulation synaptic transmission in the EC is also consistent with the histological expression of mGluR5 in this brain region (Fotuhi et al., 1994). Because sIPSCs and eIPSCs represent two different modes of GABAergic transmission, our results that activation of mGluR5 enhances the frequency and amplitude of sIPSCs but reduces the amplitude of eIPSCs suggest that glutamate may exert feedback (via sIPSCs) or feedforward (via eIPSCs) modulation of GABAergic transmission. Consistent with our results, activation of mGluR5 inhibits eIPSCs in the midbrain periaqueductal gray (Drew et al., 2008) but facilitates sIPSCs in the ventral lateral geniculate nucleus (Govindaiah and Cox, 2009) and the main olfactory bulb granule cells and periglomerular cells (Dong et al., 2007).
Our results demonstrate that the DHPG-induced membrane depolarization in interneurons is dependent on G-proteins but independent of PLC. Our results support an action mode in which activation of mGluR5 receptors depolarizes entorhinal interneurons via intracellular signals other than PLC or a direct interaction of G-proteins with K+ channels possibly the K2P channels. Consistent with our results, K2P channels are inhibited by Gq-coupled receptors. Gq-mediated inhibition of K2P channels is mediated via either intracellular signaling molecules (Chemin et al., 2003; Kang et al., 2006; Mathie, 2007; Veale et al., 2007) or direct G protein-coupling (Chen et al., 2006; Deng et al., 2006) depending on the involved types of K2P channels. Consistent with our results, many physiological functions of mGluRs are independent of PLC activities (Balazs et al., 1998; Huang and Hsu, 2006; Tozzi et al., 2001).
Group I mGluRs are involved in modulation of learning and memory (Riedel et al., 2003) and epilepsy (Alexander and Godwin, 2006; Ure et al., 2006), two major functions of the EC. Application of DHPG has been shown to enhance persistent firing induced by current injection in layer III pyramidal neurons of the EC (Yoshida et al., 2008) and persistent firing in the EC is believed to be an important mechanism for working memory (Fransen et al., 2002; Fransen et al., 2006; Hasselmo and Eichenbaum, 2005; Hasselmo and Stern, 2006). These results suggest that group I mGluRs could modulate learning and memory by facilitating neuronal excitability in the EC. Consistent with this scenario, our results have shown that activation of group I mGluRs inhibits eIPSCs, which could contribute to the facilitation of the excitability of entorhianl neurons and potentially learning and memory. Furthermore, synchronized synaptic potentials observed in epileptic human mesial temporal lobe tissue were also found to be dependent on fast GABAergic transmission (Schwartzkroin and Haglund, 1986) suggesting that the epileptogenic synaptic events are probably the compound action of both glutamatergic-mediated EPSPs and GABAergic-mediated IPSPs (Schwartzkroin and Knowles, 1984). As demonstrated in Fig. 8, different types of mGluRs exert distinct effects on synaptic transmission, which may underlies the diverse effects of mGluRs on epilepsy. Usually, group I mGluRs facilitate whereas group II and III mGluRs inhibits epilepsy (Alexander and Godwin, 2006; Doherty and Dingledine, 2002; Moldrich et al., 2003; Ure et al., 2006). Our results that activation of mGluR5 exerts opposite effects on sIPSCs and eIPSCs suggest that mGluR5-mediated modulation of GABAergic transmission may play a fine tune on epilepsy. In conclusion, our results may serve as a cellular mechanism to explain the roles of mGluRs in epilepsy as well.
Acknowledgments
This work was supported by NIH grants R01MH082881 (S. L.), 5P20RR017699 (S. L.).
References
- Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006;71(1):1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48(7):625–32. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68(3):167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Balazs R, Miller S, Chun Y, O'Toole J, Cotman CW. Metabotropic glutamate receptor agonists potentiate cyclic AMP formation induced by forskolin or beta-adrenergic receptor activation in cerebral cortical astrocytes in culture. J Neurochem. 1998;70(6):2446–58. doi: 10.1046/j.1471-4159.1998.70062446.x. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3(4):205–19. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9(3):293–8. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22(20):5403–11. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci U S A. 2006;103(9):3422–7. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J. 1997;73(1):220–9. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol. 2006;572(Pt 2):425–42. doi: 10.1113/jphysiol.2005.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol. 2007;97(1):727–37. doi: 10.1152/jn.01089.2006. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci. 2008;39(2):273–84. doi: 10.1016/j.mcn.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577(Pt 2):497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72(1):208–18. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. The roles of metabotropic glutamate receptors in seizures and epilepsy. Curr Drug Targets CNS Neurol Disord. 2002;1(3):251–60. doi: 10.2174/1568007023339355. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998a;398(1):49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998b;398(1):25–48. [PubMed] [Google Scholar]
- Dong HW, Hayar A, Ennis M. Activation of group I metabotropic glutamate receptors on main olfactory bulb granule cells and periglomerular cells enhances synaptic inhibition of mitral cells. J Neurosci. 2007;27(21):5654–63. doi: 10.1523/JNEUROSCI.5495-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew GM, Mitchell VA, Vaughan CW. Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. J Neurosci. 2008;28(4):808–15. doi: 10.1523/JNEUROSCI.4876-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region--a morphometric study. Biol Psychiatry. 1988;24(5):515–21. doi: 10.1016/0006-3223(88)90162-x. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15(24):6854–62. [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Standaert DG, Testa CM, Penney JB, Jr, Young AB. Differential expression of metabotropic glutamate receptors in the hippocampus and entorhinal cortex of the rat. Brain Res Mol Brain Res. 1994;21(3-4):283–92. doi: 10.1016/0169-328x(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Fransen E, Alonso AA, Hasselmo ME. Simulations of the role of the muscarinic-activated calcium-sensitive nonspecific cation current INCM in entorhinal neuronal activity during delayed matching tasks. J Neurosci. 2002;22(3):1081–97. doi: 10.1523/JNEUROSCI.22-03-01081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen E, Tahvildari B, Egorov AV, Hasselmo ME, Alonso AA. Mechanism of graded persistent cellular activity of entorhinal cortex layer v neurons. Neuron. 2006;49(5):735–46. doi: 10.1016/j.neuron.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci. 1998;1(6):479–86. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Distinct roles of metabotropic glutamate receptor activation on inhibitory signaling in the ventral lateral geniculate nucleus. J Neurophysiol. 2009;101(4):1761–73. doi: 10.1152/jn.91107.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4(11):1139–45. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542(Pt 2):431–44. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18(9):1172–90. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10(11):487–93. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Sustained activation of metabotropic glutamate receptor 5 and protein tyrosine phosphatases mediate the expression of (S)-3,5-dihydroxyphenylglycine-induced long-term depression in the hippocampal CA1 region. J Neurochem. 2006;96(1):179–94. doi: 10.1111/j.1471-4159.2005.03527.x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Iserhot C, Gebhardt C, Schmitz D, Heinemann U. Glutamate transporters and metabotropic receptors regulate excitatory neurotransmission in the medial entorhinal cortex of the rat. Brain Res. 2004;1027(1-2):151–60. doi: 10.1016/j.brainres.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Laakso MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, Alakare B, Rakkolainen V, Salokangas RK, Hietala J. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51(12):1005–7. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol. 2006;291(4):C649–56. doi: 10.1152/ajpcell.00047.2006. [DOI] [PubMed] [Google Scholar]
- Kang D, Mariash E, Kim D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J Biol Chem. 2004;279(27):28063–70. doi: 10.1074/jbc.M402940200. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K(+) channel family. J Biol Chem. 2000;275(13):9340–7. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J Comp Neurol. 1998;393(3):332–52. [PubMed] [Google Scholar]
- Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986;246(2):149–69. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer's disease. J Mol Neurosci. 2001;17(2):225–32. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. Prog Brain Res. 2000;125:339–51. doi: 10.1016/S0079-6123(00)25023-1. [DOI] [PubMed] [Google Scholar]
- Lei S, Deng PY, Porter JE, Shin HS. Adrenergic facilitation of GABAergic transmission in rat entorhinal cortex. J Neurophysiol. 2007;98(5):2868–77. doi: 10.1152/jn.00679.2007. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15(5):1004–11. [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442(1):131–8. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578(Pt 2):377–85. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich RX, Chapman AG, De Sarro G, Meldrum BS. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur J Pharmacol. 2003;476(1-2):3–16. doi: 10.1016/s0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360(4):555–70. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30(1):65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53(4):1009–18. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71(4):949–76. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161(9):1612–9. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140(1-2):1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Ritzen A, Mathiesen JM, Thomsen C. Molecular pharmacology and therapeutic prospects of metabotropic glutamate receptor allosteric modulators. Basic Clin Pharmacol Toxicol. 2005;97(4):202–13. doi: 10.1111/j.1742-7843.2005.pto_156.x. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278(30):27406–12. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Haglund MM. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia. 1986;27(5):523–33. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Knowles WD. Intracellular study of human epileptic cortex: in vitro maintenance of epileptiform activity? Science. 1984;223(4637):709–12. doi: 10.1126/science.6695179. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322(1):121–35. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35(4):721–7. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169(3):347–70. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Kristensen P, Mulvihill E, Haldeman B, Suzdak PD. L-2-amino-4-phosphonobutyrate (L-AP4) is an agonist at the type IV metabotropic glutamate receptor which is negatively coupled to adenylate cyclase. Eur J Pharmacol. 1992;227(3):361–2. doi: 10.1016/0922-4106(92)90018-q. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Guatteo E, Caputi L, Bernardi G, Mercuri NB. Group I mGluRs coupled to G proteins are regulated by tyrosine kinase in dopamine neurons of the rat midbrain. J Neurophysiol. 2001;85(6):2490–7. doi: 10.1152/jn.2001.85.6.2490. [DOI] [PubMed] [Google Scholar]
- Ure J, Baudry M, Perassolo M. Metabotropic glutamate receptors and epilepsy. J Neurol Sci. 2006;247(1):1–9. doi: 10.1016/j.jns.2006.03.018. [DOI] [PubMed] [Google Scholar]
- van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13(8):943–52. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. G(alpha)q-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007;71(6):1666–75. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33(3):161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000a;10(4):398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000b;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Evans DI, Jones RS. Activation of presynaptic group III metabotropic glutamate receptors depresses spontaneous inhibition in layer V of the rat entorhinal cortex. Neuroscience. 2001;105(1):71–8. doi: 10.1016/s0306-4522(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Woodhall GL, Ayman G, Jones RS. Differential control of two forms of glutamate release by group III metabotropic glutamate receptors at rat entorhinal synapses. Neuroscience. 2007;148(1):7–21. doi: 10.1016/j.neuroscience.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Deng PY, Rojanathammanee L, Yang C, Grisanti L, Permpoonputtana K, Weinshenker D, Doze VA, Porter JE, Lei S. Noradrenergic Depression of Neuronal Excitability in the Entorhinal Cortex via Activation of TREK-2 K+ Channels. J Biol Chem. 2009;284(16):10980–91. doi: 10.1074/jbc.M806760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Fransen E, Hasselmo ME. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. Eur J Neurosci. 2008;28(6):1116–26. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]