Abstract
The effects of ionizing irradiation on the brain are associated with oxidative stress. While oxidative stress following irradiation is generally viewed as detrimental for hippocampal function, it might have beneficial effects as part of an adaptive or preconditioning response to a subsequent challenge. Here we show that in contrast to what is seen in wild-type mice, irradiation enhances hippocampus-dependent cognitive measures in mice lacking extracellular superoxide dismutase. These outcomes were associated with genotype-dependent effects on measures of oxidative stress. When cortices and hippocampi were analyzed for nitrotyrosine formation as an index of oxidative stress, the levels were chronically elevated in mice lacking extracellular superoxide dismutase. However, irradiation caused a greater increase in nitrotyrosine levels in wild-type mice than mice lacking extracellular superoxide dismutase. These paradoxical genotype-dependent effects of irradiation on measures of oxidative stress and cognitive function underscore potential beneficial effects associated with chronic oxidative stress if it exists prior to a secondary insult such as irradiation.
Introduction
Radiation-induced brain injury is a dose limiting factor during therapeutic irradiation of the brain (Tofilon and Fike, 2000). While overt tissue injury generally occurs only after relatively high doses, there is a strong likelihood of developing adverse reactions in terms of cognitive decline after relatively lower doses (Meyers and Brown, 2006). Such impairment has a diverse character, and in humans and animals often includes hippocampus-dependent functions involving learning, memory, and spatial information processing (Abayomi, 1996; Fan et al., 2007; Raber et al., 2004; Rola et al., 2004). The underlying mechanisms responsible for radiation-induced cognitive impairment have remained elusive, but important possibilities include alterations in the neurogenic cell populations in the dentate gyrus (Mizumatsu et al., 2003; Raber et al., 2004; Rola et al., 2004), loss of mature neurons in the dentate gyrus (Fan et al., 2007), alterations in NMDA subunits (Shi et al., 2006), genetic risk factors (Villasana et al., 2006), and reductions in the immediate early gene Arc (Rosi et al 2008). Additionally, radiation-induced reductions in cognitive performance may be associated with changes in the microenvironment (Fike et al., 2007; Rola et al., 2004), including oxidative stress, which can regulate the fate of neurogenic cells associated with cognitive function (Fike et al., 2007; Limoli et al., 2004; Smith et al., 2000).
The redox environment is of particular importance in the central nervous system (CNS), where there is a relatively high rate of oxygen consumption and metabolic turnover (Lewen et al., 2000) and a relatively low level of endogenous antioxidants (Peuchen et al., 1997). There are several pathways that mitigate the physiological and pathological effects of reactive oxygen species (ROS) like superoxide, in mammalian cells (Riley, 1994). One of these pathways involves the superoxide dismutase (SOD) enzymes, which are critical elements of the cellular antioxidant defense mechanism (Muscoli et al., 2003). The SODs are oxidoreductases that remove superoxide by catalyzing the dismutation of the superoxide radical to hydrogen peroxide. Hydrogen peroxide is then metabolized to molecular oxygen and water by catalase or glutathione peroxidase. There are three different SOD isoforms that catalyze the same chemical reaction, but have different enzymatic properties and distinct subcellular localizations: CuZnSOD (SOD1) is localized in the cytoplasm, MnSOD (SOD2) is localized in the mitochondria, and extracellular SOD (EC-SOD, SOD3) is extracellular. Although the physiological roles of the SOD isoforms in mammalian cells are not completely understood, the extracellular isoform was shown to be associated with certain cognitive functions (Levin et al., 1998; Thiels et al., 2000), and alterations in EC-SOD expression using transgenic or mutant mice impaired learning (Levin et al., 1998). The mechanisms associated with this effect are not yet understood, but likely involve superoxide, which has been shown to have both positive and negative effects (Hu et al., 2007; Kamsler et al., 2007; Kishida and Klann, 2007; Serrano and Klann, 2004). In rodents, superoxide is a necessary signaling component of long-term potentiation (LTP), which is a widely studied form of synaptic plasticity (Hu et al., 2007; Hu et al., 2006). The source of superoxide is yet to be determined, but may include mitochondrial metabolism, monoamine oxidase and cyclo-oxygenase, nitric oxide synthase, and NADPH oxidase (Kishida and Klann, 2007). Regardless of the source and relevant targets of superoxide signaling (e.g. kinases, phosphatases), clearly ROS are important molecules involved in the regulation of learning and memory (Kishida and Klann, 2007). One way to characterize the extent to which ROS affects cognition, and if it is modulated by ionizing irradiation, is to use mutant mice that lack a specific antioxidant molecule, like an SOD isoform.
A persistent level of oxidative stress in EC-SOD deficient mice (i.e. knock out, KO mice) is associated with a lower baseline level of neurogenesis relative to wild type (WT) mice (Rola et al., 2007). However, a modest dose of x-rays has no effect on neurogenesis in KO mice, but causes a highly significant reduction in WT animals (Rola et al., 2007). These data indicate that while oxidative stress can be generally viewed as detrimental for neurogenesis, it might also have a beneficial effect, at least in the context of an adaptive or preconditioning response to a subsequent challenge such as irradiation. The present study was performed to determine if such a beneficial effect would be seen in the context of cognitive performance and whether such an effect would be associated with genotype-dependent effects on measures of oxidative stress.
Materials and Methods
Mice
Congenic EC-SOD KO mice (Carlsson et al., 1995) on the C57BL/6J (B6) background were initially obtained from Dr. James Crapo at the National Jewish Medical and Research Center, Denver, Colorado. The colony was maintained by backcrossing to B6 mice purchased from the Jackson Laboratory (Bar Harbor, ME). Two-month-old male homozygous KO mice (n = 14 for the behavioral studies and n = 4–5 for the western blot analysis) and their wild type (WT) littermate controls (n = 16 for the behavioral study and n = 3–4 for the western blot analysis), generated from the intercross of heterozygous KO mice, were used in the current study. All animal handling procedures were done in accordance with Federal guidelines and approved by the OHSU IACUC and VA Palo Alto IACUC. Mice were maintained in a temperature- and light-controlled environment with a 12 h light/dark cycle and were provided food and water ad libitum.
Irradiation
Mice were anesthetized (i.p., 80 mg/kg ketamine (Sigma, St. Louis, MO) and 20 mg/kg xylazine (Sigma)) and sham-irradiated (controls) or irradiated at 2 months of age with a dose of 10 Gy using a Mark 1 Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). The cerebellum, eyes, and body were shielded with lead (see (Acevedo et al., 2008b) for details on the irradiation set up). The 10 Gy dose used here was selected because it was shown to induce hippocampus-dependent cognitive impairments (Raber et al., 2004; Villasana et al., 2006). After irradiation, mice for the behavioral study were group housed until 3 days prior to the behavioral testing. Mice for the western blot analyses were killed by cervical dislocation at 3 months of age.
Behavioral Testing
Cognitive assessments began three months following irradiation or sham-irradiation, and consisted of four weeks of testing both hippocampus-dependent and hippocampus-independent cognitive performance. Three different hippocampus-dependent tests were included to increase the ability to detect potential genotype-dependent effects of irradiation on hippocampus-dependent cognitive performance. Because irradiation effects on the brain might not be limited to hippocampal function, non-hippocampus-dependent cognitive tests were also included. Mice were tested for hippocampus-dependent novel location and hippocampus-independent novel object recognition during the first week, for hippocampus-dependent spatial learning and memory in the water maze in the second week, for sensorimotor function on the rotorod in the third week, and for hippocampus-dependent contextual and hippocampus-independent cued fear conditioning in the last week. The individual researcher testing the mice was blinded to genotype and radiation treatment.
a) Novel Location and Novel Object Recognition Tasks
To assess object recognition, mice were individually habituated for three consecutive days to a 16 × 16 inch open-field with clear plexiglass walls (Kinder Scientific, Poway, CA) for 5 minutes. On the fourth day, the mice were first given three 10-minute trials with three plastic objects in different corners of the open field. In subsequent trials, the familiar objects were exchanged with replicas. For the fourth 10-minute trial, one of the familiar objects was moved from one corner of the field to another to evaluate hippocampus-dependent novel location recognition. For the fifth 10-minute trail, a familiar object was replaced by a novel object to assess hippocampus-independent novel object recognition. There was a 3-minute interval between each trial. During this time, the mice were placed back in their home cage and the open field and the objects were cleaned with 5% acetic acid to remove potential odors. The total time spent exploring all objects was compared between trials to assess the familiarization of each mouse with the objects (Acevedo et al., 2008b). The difference between the percent time spent exploring the object in the novel location (trial 4) and the percent time spent exploring the same object in its original location (trial 3) was calculated to assess novel location recognition. The percent time spent exploring the novel object during trial 5 was calculated to assess novel object recognition. The percent time spent exploring the objects were then compared based on genotype and treatment.
b) Water maze
The water maze test was used to assess hippocampus-dependent spatial learning and memory. A circular pool (diameter 140 cm) was filled with opaque water (24°C) and mice were trained to locate a submerged platform (luminescence: 200 lux). To determine if irradiation affected the ability to swim or learn the water maze task, mice were first trained to locate a clearly marked platform (visible platform, days 1 and 2). Mice were subsequently trained to locate the platform when it was hidden beneath the surface of opaque water (days 3–5). Training during the hidden platform sessions (acquisition) required the mice to learn the location of the hidden platform based on extra-maze cues. For both visible and hidden sessions, there were 2 daily sessions, morning and afternoon, which were 2 hours apart. Each session consisted of 3 trials (with 10-min inter-trial intervals). A trial ended when the mice located the platform. Mice that failed to locate the platform within 60 seconds were led to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they were physically on the platform for a minimum of 3 seconds. During visible platform training, the platform was moved to a different quadrant of the pool for each session. For the hidden platform training, the platform location was kept constant. Mice were placed into the water facing the edge of the pool in one of nine randomized locations. The start location was changed for each trial. The swimming patterns of the mice were recorded with the Noldus Ethovision video tracking system (Ethovision XT, Noldus Information Technology, Wageningen, Netherlands) set at 6 samples/sec. The time to locate the platform (latency) was used as a measure of performance for the visible and hidden sessions. Because swim speeds can influence the time it takes to reach the platform, they were also analyzed to assess if there were genotype or treatment differences in this measure.
To measure spatial memory retention, probe trials (platform removed) were conducted 1 hr after the last hidden trial of each mouse on each day of hidden platform training (i.e., a total of 3 probe trials). The time spent in the target quadrant, the quadrant where the platform was previously located during hidden platform training, was compared to the time spent in the 3 non-target quadrants. For the probe trials, mice were placed into the water in the quadrant opposite from the target quadrant.
c) Rotorod
To exclude the possibility that radiation-induced alterations in motor function could negatively impact performance in the cognitive tests, all mice were tested on the rotorod (Rotamex-5, Columbus Instruments, Columbus, OH). Mice were placed on an elevated rod (3 cm × 9.5 cm spindle 44.5 cm elevated) initially rotating at 5 rpm. The speed of the rotating rod was increased by 1 rpm every 3 sec to a maximum of 24 rpm. Each trial ended when a fall was recorded by photo beams aligned with each individual mouse or if a mouse did not fall from the rod within 300 sec. Mice received 3 trials each day, 30 minutes apart, for 3 consecutive days.
d) Conditioned Fear
Hippocampal function was also assessed using the contextual fear conditioning task. In this task, mice learned to associate the environmental context (fear conditioning chamber) with a mild foot shock (unconditioned stimulus, US). Because contextual fear conditioning is hippocampus- and amygdala-dependent, the mild foot shock was also paired with a tone (conditioned stimulus, CS) to allow assessment of cued fear conditioning, which is amygdala-but not hippocampus-dependent. When mice were re-exposed to the context or the tone, conditioned fear resulted in freezing behavior. Mice displayed this conditioned fear by ceasing all movement except for respiration (i.e. freezing). On day 1, each mouse was placed in a fear conditioning chamber (Kinder Scientific, Poway, CA) and allowed to explore for 2 minutes before delivery of a 30 second tone (80 db) which was immediately followed by a 2 second foot shock (0.6 mA). Two minutes later, a second CS-US pair was delivered. On day two each mouse was first placed in the fear conditioning chamber containing the exact same context, but there was no administration of a tone or foot shock. Freezing was analyzed for 3 minutes. One hour later, the mice were placed in a new context (containing a different odor, cleaning solution, floor texture, walls and shape) where they were allowed to explore for 3 minutes before being re-exposed to the fear conditioning tone and freezing was assessed for an additional 3 minutes. Freezing was measured using a Noldus Ethovision video tracking system.
Nitrotyrosine analysis
To determine the level of oxidative stress in KO and WT mice at baseline and after irradiation, cortices and hippocampi were dissected on ice and processed as described {Zou, 2008 #15828}. A mouse monoclonal antibody (clone 1A6, Upstate/Millipore, Billerica, MA) was used as the primary antibody at a 1:2,000 dilution and HRP-tagged goat anti-mouse IgG (Bio-Rad, Hercules, CA) was used as the secondary antibody at a 1:10,000 dilution. Chemiluminescence signals from western blots were captured either by x-ray film or by the Typhoon system (GE Healthcare, Piscataway, NJ). Actin was used as an internal control for slight variations in protein loading and transferring. As tissues may contain low levels of nitrotyrosine, a nitrotyrosine immunoblotting control (Upstate/Millipore), which contains proteins that were nitrated with peroxynitrite, was used as a positive control for the primary antibody. Gel images were analyzed by Image J using the gel analysis function. Protein bands that belonged to the same group (the upper and lower nitrotyrosine bands or β-actin) were analyzed together using the Gels/Plot Lanes function. The area under each peak was subsequently calculated as a measure for the band intensity. Because the actual band intensities were different for different proteins and thresholds for the two groups of protein bands (the upper and lower nitrotyrosine bands or β-actin) were automatically adjusted by Image J, the generated measures reflect the relative differences in protein levels within the same group of samples. The band intensity obtained for each upper and lower nitrotyrosine band was then normalized to that of its corresponding β-actin to obtain relative expression levels.
Statistical Analyses
Data were assessed for normality and homogeneity of variance to determine whether to use parametric or non-parametric statistical tests. All statistical analyses were performed using SPSS software (SPSS Inc, Chicago, Il, USA) or GraphPad Prism software (San Diego, CA, USA). A two-way (2 X 2) AVOVA was used with genotype and treatment as between-subject factors to measure the effects of genotype and irradiation on novel location recognition, novel object recognition, and contextual and cued fear conditioning. To determine whether each group showed novel location recognition, a paired t-test was used to compare the percent time exploring the object in the new location (trial 4) versus the old location (trial 3). To determine whether each group showed novel object recognition, a one-way ANOVA (within each group) was used to compare the percent time exploring the novel and familiar objects and, when appropriate, a Neuman-Keuls post hoc test was used. For the fear conditioning tasks, an unpaired t-test was used to compare between group performances. For the water maze learning curves, visible and hidden platform sessions were analyzed separately. To compare water maze and rotorod learning curves in the different groups, a three-way repeated measures ANOVA was used with genotype and irradiation treatment as between-subject factors and session number as a within-subject factor. To compare mean water maze latency during the visible and hidden sessions in the different groups, a two-way ANOVA with genotype and irradiation treatment as between subject factors was also used, and this analysis was followed using a Bonferroni post hoc test when appropriate. In the water maze probe trials, one-way ANOVAs were used for each group to assess spatial memory retention by comparing the percent time spent in each quadrant and when appropriate, a Neuman-Keuls post hoc test was used to compare the percent time spent in the target quadrant and the three non-target quadrants. For an overall analysis of the western blot data, a three-way repeated measures ANOVA was used with treatment, genotype, and brain region as between-subjects factors and the upper and lower band as within-subject factor. T-tests were used to assess potential genotype differences in the upper band, lower band, or combined upper and low band for each treatment condition and brain region. Data were expressed as means ± SEM unless otherwise noted. P < 0.05 was considered significant for all tests.
Results
There was no effect of irradiation on novel location recognition. Both sham-irradiated (t =2.3, P <0.05) and irradiated WT (t = 2.7, P < 0.05) mice showed novel location recognition (Fig. 1A). In contrast, while irradiated KO mice showed novel location recognition (t = 2.1, P < 0.05), sham irradiated KO mice did not (t = 0.7, P > 0.05) (Fig. 1A).
Fig. 1.
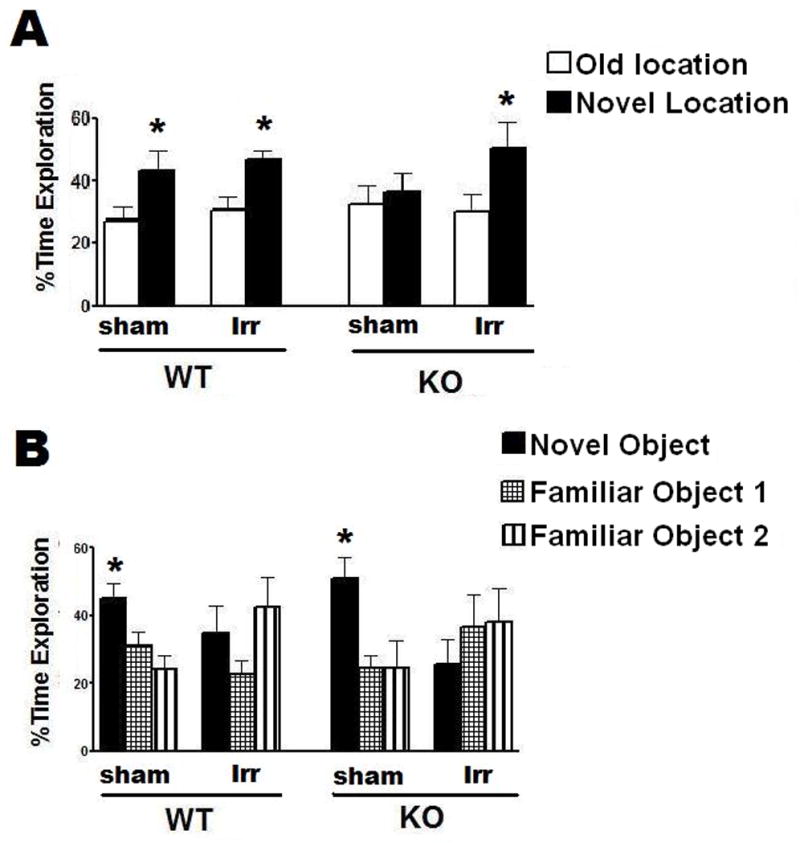
Effects of 137Cs irradiation on object recognition of WT and KO mice. A. While sham-irradiated and irradiated WT mice and irradiated KO mice showed novel location recognition, sham-irradiated KO mice did not. *p < 0.05, paired t-test. B. Irradiation impaired novel object recognition in both WT and KO mice. *p < 0.05 novel object versus familiar objects 1 and 2, one-way ANOVA, Dunnett’s posthoc test. WT, n = 8 each and KO, n = 7 each.
In the hippocampus-independent novel object recognition task, sham-irradiated WT and sham-irradiated KO mice showed novel object recognition (sham-irradiated WT: ANOVA object preference, F(2,18) = 6.9, P < 0.01; sham-irradiated KO: F(2,12) = 6.3, P < 0.01). They showed greater preference for the novel object than the two familiar objects. In contrast, irradiated WT and KO mice showed no novel object recognition (Fig. 1B).
In the water maze task, there were no significant group differences in swim speeds during the visible platform sessions (WT sham: 15.04 ± 0.44 cm/sec; WT irradiated: 15.81 ± 0.46 cm/sec; KO sham: 15.77 ± 0.46 cm/sec; KO irradiated: 16.03 ± 0.35 cm/sec). Therefore, time to reach the platform (latency) was used as performance measure. All groups showed improvement in the visible (F(3,78) = 81.04, P < 0.001) and hidden platform sessions (F(5, 130) = 7.87, P < 0.001) and there were no session by genotype or session by treatment interactions. During the visible, but not during the hidden, platform sessions, there was a genotype by treatment interaction for the average latency across the sessions (ANOVA genotype × treatment interaction, F(1, 26) = 9.53, P < 0.01). Therefore, we assessed the effects of treatment in the two genotypes separately. There was an effect of irradiation in the WT mice (ANOVA effect of treatment, F(1,14) = 9.6, P < 0.01) not seen in KO mice (sham-irradiated: 24.8 ± 3.1 sec; irradiated: 21.4 ± 2.3 sec). The average time to reach the platform across the visible platform sessions was lower in sham-irradiated (17.3 ± 2.5 sec) than irradiated (21.0 ± 2.5 sec) WT mice (Bonferroni test, P < 0.01), indicating that irradiation impaired task learning in WT mice. However, irradiation did not affect spatial learning to locate the hidden platform in either genotype (data not shown). In the first probe trial of the water maze test, sham-irradiated and irradiated WT mice and sham-irradiated KO mice did not show spatial memory retention (Fig. 2), as they did not spend more time searching in the target quadrant than in any other quadrant (ANOVA, quadrant within group analysis). In contrast, irradiated KO mice showed spatial memory retention (ANOVA quadrant preference, F(3,20) = 13.65, P < 0.001) and spent most of their time searching in the target quadrant (Neuman-Keuls, P < 0.01 target versus any other quadrant; Fig. 2). In the probe trial following an additional day of hidden platform training (Probe 2), sham-irradiated and irradiated WT mice and irradiated KO mice showed spatial memory retention but sham-irradiated KO mice still did not (ANOVA quadrant preference, F(3, 16) = 1.1; P = 0.38); Fig. 2). Following a third day of hidden platform training (Probe 3), all groups showed spatial memory retention and spent more time searching in the target quadrant than in any other quadrant (Fig. 2). When sensorimotor function was assessed on the rotorod, all groups of mice improved their performance with training (ANOVA, effect of session: F(2,52) = 24.56, P < 0.001) and there were no effects of genotype, treatment, or genotype by treatment interactions.
Fig. 2.
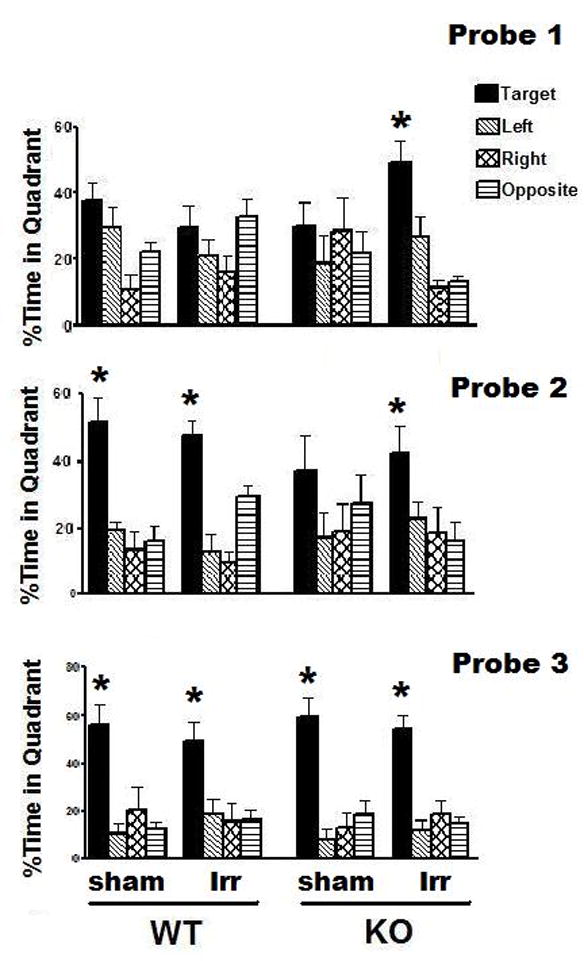
Effects of 137Cs irradiation on water maze performance of WT and KO mice. C. Spatial memory retention of sham-irradiated and irradiation WT and KO mice in the first 30 seconds of the water maze probe trials. In the probe trial following the first day of hidden platform training (Probe 1), only irradiated KO mice spent more time searching in the target quadrant than any other quadrant. In the probe trial following the second day of hidden platform training (Probe 2), sham-irradiated and irradiated WT mice and irradiated KO mice spent more time searching in the target quadrant than any other quadrant. In the probe trial following the third day of hidden platform training (Probe 3), all groups spent more time searching in the target quadrant than any other quadrant. *p < 0.05 target versus any other quadrant, ANOVA and Newman-Keuls post hoc test. WT, n = 8 each and KO, n = 7 each.
Finally, hippocampal function was assessed using the contextual fear conditioning task. There were no significant effects of genotype or treatment on % baseline freezing (first 2 minutes prior to delivery of tone on day 1) (WT sham: 10.6 ± 0.9; WT irradiated: 11.6 ± 1.6; KO sham: 9.7 ± 1.7; KO irradiated: 10.6 ± 1.3). However, there were opposing genotype-dependent effects of irradiation on hippocampus-dependent contextual freezing (ANOVA, genotype × treatment interaction, F(1,22) = 13.6, P < 0.01, Fig. 3A). Compared to sham-irradiated WT mice, irradiated WT mice showed impairments in contextual fear conditioning (t = 2.6, P < 0.05). In contrast, compared to sham-irradiated KO mice, irradiation enhanced contextual fear conditioning in the KO mice (t = 2.6, P < 0.05). As a result, irradiated KO mice showed significantly more contextual freezing than irradiated WT mice (t = 3.9, P < 0.01). All groups of mice showed cued fear conditioning (paired t-test, Pre-CS versus Post-CS within each group, P < 0.05) and there was no effect of genotype or treatment and no significant interaction (Fig. 3B).
Fig. 3.
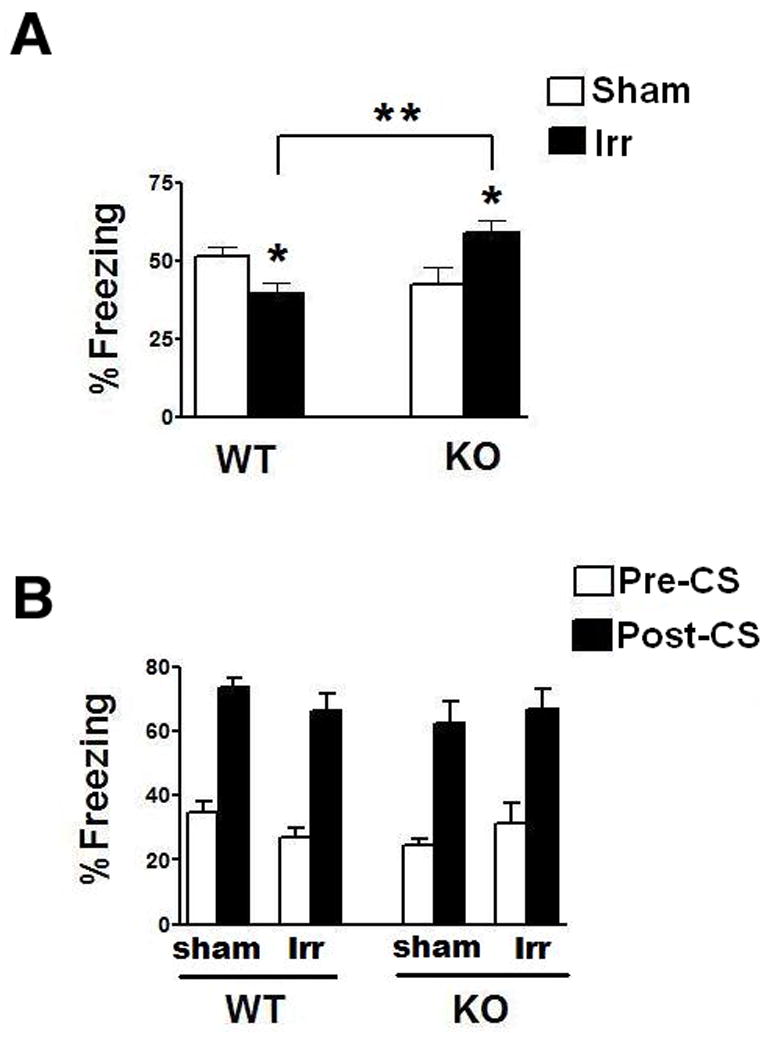
Effects of 137Cs irradiation on fear conditioning of WT and KO mice. A. While irradiation impaired contextual fear conditioning of WT mice, it enhanced contextual fear conditioning of KO mice. *p < 0.05 versus sham-irradiated genotype matched mice. **p < 0.01between irradiated WT and irradiated KO mice. B. Sham-irradiated and irradiated WT and KO showed robust and comparable hippocampus-independent cued fear conditioning. WT, n = 8 each and KO, n = 7 each.
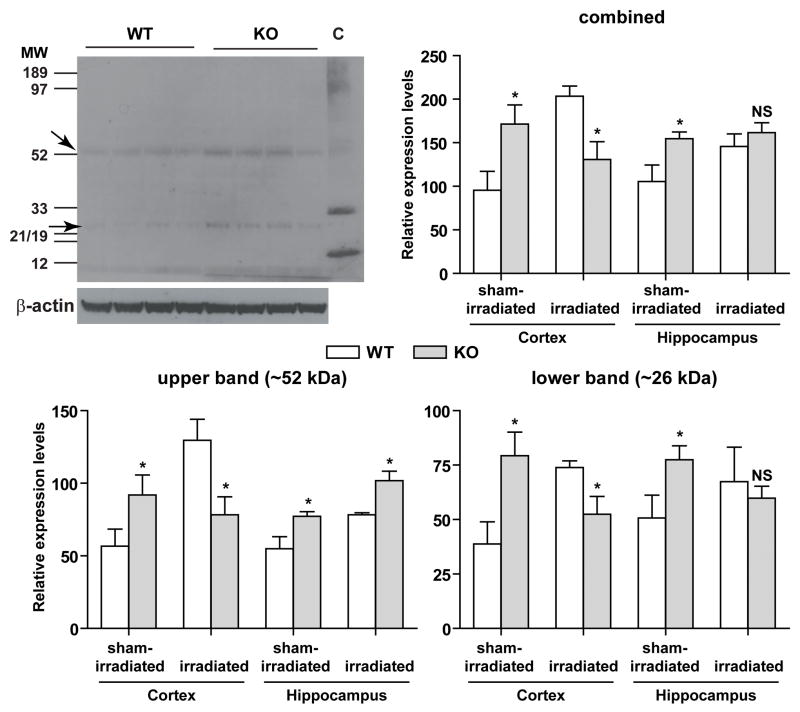
To determine whether these cognitive changes were associated with genotype-dependent effects on measures of oxidative stress, nitrotyrosine western blot analyses were used (Fig. 4). There were two main nitrotyrosine bands of approximately 26 and 52 kDa (Fig. 4, top left panel). In cortex and hippocampus, the levels of both bands were higher in sham-irradiated KO than WT mice (Fig. 4, lower panels). There was an effect of treatment (F = 6.822, P = 0.015), a treatment × genotype interaction (F = 17.214, P < 0.0001), and a treatment × genotype × brain region interaction (F(2,52) = 6.909, P = 0.014). In the cortex, irradiation increased the levels of the lower and the upper band and the combined levels in WT mice. In contrast, no increase in these levels was seen in KO mice. In the hippocampus, irradiation increased the combined levels in WT but not in KO mice. When the individual bands were analyzed, irradiation increased the levels of the upper band in both genotypes and the intensity of the lower band only in WT, but not in KO mice.
Fig. 4.
Nitrotyrosine western blot analyses of hippocampal and cortical extracts of sham-irradiated and irradiated WT and KO mice. Upper left panel, a representative western blot image showing the upper and lower nitrotyrosine-positive bands at approximately 52 and 26 kDa, respectively. Chemiluminescence signals from western blots were captured either by x-ray film or by the Typhoon system and β-actin was used as an internal control. A nitrotyrosine immunoblotting control (lane C) containing proteins nitrated with peroxynitrite was used as a positive control. The band intensity obtained for each upper (bottom left panel) and lower (bottom right panel) nitrotyrosine band was normalized to that of its corresponding β-actin to obtain relative expression levels. Combined relative expression levels of the upper and lower band (upper right panel) were also calculated. *P < 0.05 versus treatment matched WT. NS, not significant. n = 3–4 WT and n = 4–5 KO mice.
Discussion
The primary finding of the current study is that while EC-SOD deficiency is associated with some hippocampus-dependent impairments prior to irradiation, a finding also shown by others (Levin et al., 1998), after irradiation, hippocampus-dependent cognitive measures are enhanced in mice lacking EC-SOD. This paradoxical effect supports the idea that ROS can have both positive and negative effects, depending upon the circumstances (Kamsler and Segal, 2003; Levin et al., 1998; Valko et al., 2007). While the precise mechanism(s) responsible for the ‘protective’ type of response seen here is (are) not yet known, in a general sense this effect resembles a preconditioning (Gori and Forconi, 2005; Pespeni et al., 2005), adaptive (reviewed in (Yu and Chung, 2006)), or inducible-like radioprotective response (Qutob et al., 2006), where a sublethal or potentially injurious stimulus (e.g. oxidative stress) induces tolerance to a subsequent and potentially more damaging insult (e.g. irradiation). For instance, ischemic/hypoxic preconditioning has been shown to protect the brain under some conditions (Murry et al., 1986; Kitagawa et al., 1991; Chen et al., 1996; for review Liu at al., 2009) but those effects can change with age (for review Schaller, 2007). Additionally, the same EC-SOD KO mice were shown to be more sensitive to focal cerebral ischemia injury (Sheng et al., 1999). These and other data highlight the complexity of preconditioning or adaptive responses and highlight the fact that such responses may be context-dependent.
The relationship between EC-SOD and processes involved in learning and memory is not simple, with both over and under expression reported to impair learning, (Hu et al., 2006; Levin et al., 1998). Whether this represents differences in superoxide regulation, differing levels of hydrogen peroxide, components of the nitric oxide (NO) pathway and bio-availability of NO, or alterations in stress signaling pathways, needs to be determined. In the present study we used EC-SOD KO mice, which show indications of persistent oxidative stress but which do not display any compensatory changes in levels or activities of the other SOD isoforms, catalase or glutathione peroxidase (Rola et al., 2007). Prior to irradiation these mice showed some cognitive impairments, i.e. hippocampus-dependent novel location recognition, but for other cognitive measures there were no differences observed between sham-irradiated WT and sham-irradiated KO mice. This indicated that compared to WT mice, the EC-SOD phenotype was relatively subtle, which might relate to the fact that ROS might have both positive and negative effects on brain function.
The nitrotyrosine analyses revealed two predominant nitrated proteins, of about 26 and 52 kDa, respectively. As increased levels of nitrated proteins have been shown in various neurodegenerative disorders (Castegna et al., 2003; Sacksteder et al., 2006), future efforts are warranted to identify these two proteins.
In this study we used a battery of cognitive tests to address hippocampus dependent function. Inclusion of multiple hippocampus-dependent cognitive tests is particularly important in comparative studies of wild-type and mutant mice, because the tests differ in the amount of training, complexity, and motivation, and might differ in their sensitivity for detecting potential detrimental or beneficial effects of irradiation. Additionally, the ability to detect potential effects of irradiation may depend upon the specific level of functioning under baseline conditions. That is, detrimental effects of irradiation may be more likely revealed when the cognitive function at baseline is intact while beneficial effects of irradiation may be more likely revealed when the cognitive function at baseline is impaired.
In the present study, WT mice showed novel location recognition both before and after irradiation. This suggested that the novel location recognition test was not very sensitive in detecting detrimental effects of gamma irradiation in male mice. The situation is different for female mice, however, where it was shown that 10 Gy of 137Cs irradiation impaired novel location recognition in WT mice and those lacking apolipoprotein E (Acevedo et al., 2008a). On the other hand, the novel location recognition paradigm used here was sensitive enough to detect the beneficial effects of irradiation in mutant male mice not showing novel location recognition under baseline conditions (Fig. 1A). That is, sham-irradiated KO mice showed impairments in this test, which is consistent with impairments in hippocampus-dependent learning and memory of EC-SOD KO mice reported by others (Levin et al., 1998), but KO mice did show novel location recognition after irradiation. Thus, this measure of hippocampal function was fully recovered following radiation exposure. These data indicate that the sensitivity of the novel location recognition test to detect effects of cranial irradiation is critically influenced by genetic and environmental factors.
We also included a hippocampus-independent version of the object recognition test. In the present investigation, both sham-irradiated WT and sham-irradiated KO mice showed hippocampus-independent novel object recognition (Fig. 1B), that is, they spent more time exploring the novel object. In both genotypes, this behavior was impaired by irradiation, indicating that the effects of radiation on cognitive function were not limited to the hippocampus but also involved the cortex, and that pre-existing and persistent oxidative stress did not impact this response. To the best of our knowledge, this is the first time that effects of gamma irradiation on hippocampus-independent novel object recognition have been reported. These data, together with the water maze data discussed below, also highlight the importance of including both hippocampus-dependent and non-hippocampus-dependent versions of cognitive tests in the evaluation of radiation effects on brain function.
This study involved the assessment of spatial learning and memory using the Morris water maze, a test we have used previously after irradiation of adult WT (Raber et al., 2004) and mutant mice (Villasana et al., 2006). In our earlier study in WT male mice irradiated at 2 months of age, this test involved a single probe trial performed after 3 days of testing, and under that testing paradigm, radiation did not have any discernible effects (Raber et al., 2004). The current results confirmed this finding in that there were no differences between sham-irradiated and irradiated WT mice when the probe trial was done after the third day of hidden platform training (Fig. 2). Similar results were seen in EC-SOD KO mice. However, when probe trial performance was analyzed after the first and second days of testing, there were significant differences between sham-irradiated and irradiated KO mice, and in the probe trial following the first day of hidden platform training the irradiated KO mice clearly outperformed sham-irradiated WT mice (Fig. 2). These data, together with our studies in mutant and WT female mice (Villasana et al., 2006; Acevedo et al., 2008b), highlight the strength of including multiple probe trials in the design of the water maze to detect effects of irradiation, particularly in mutant mice. Thus, a water maze paradigm including only one probe trial at the end of 3 days of hidden platform training would not have revealed the genotype-dependent effects of irradiation in the current study.
Given the complexities associated with cognitive function and the differential sensitivities of cognitive tests to detect effects of irradiation, we also used contextual fear conditioning to assess hippocampus-dependent emotional learning and memory. Sham-irradiated and irradiated mice showed genotype-dependent contextual fear conditioning. While contextual fear conditioning was impaired in WT mice following irradiation, it was enhanced in KO mice (Fig. 3A). These data show that the contextual fear conditioning test is particularly sensitive to detect effects of cranial irradiation in WT and mutant male mice. Sham-irradiated and irradiated WT and KO both showed robust and comparable hippocampus-independent cued fear conditioning, suggesting that the effects of irradiation on contextual fear conditioning are hippocampus-dependent and not due to general impairments in fear conditioning, which is more amygdala based.
With regard to the overall radiation response, it was particularly striking that in contrast to what is seen in WT mice, performance in hippocampus-dependent novel location recognition, spatial memory retention in the first and second water maze probe trials, and contextual fear conditioning was enhanced in animals deficient in EC-SOD. This improvement was associated with genotype-dependent effects on measures of oxidative stress. In cortex and hippocampus, nitrotyrosine levels were chronically elevated in KO mice but irradiation caused a greater increase in oxidative stress in wild-type mice than in mice lacking extracellular superoxide dismutase. In addition to measures of oxidative stress, genotype-dependent effects of hippocampal neurogenesis following irradiation might have contributed to the cognitive performance. In our earlier neurogenesis study that did not involve behavioral assessments, baseline neurogenesis was lower in KO than WT mice but following irradiation, neurogenesis was strongly reduced in WT, but not in KO mice (Rola et al., 2007). Thus the net results are that measures of oxidative stress are lower and hippocampal neurogenesis is higher in irradiated KO than WT mice. Whether or not measures of oxidative stress and/or neurogenesis play a causal or contributory role in the “protection” against radiation-induced cognitive impairments or the enhanced cognitive performance of irradiated mice when compared to sham-irradiated mice needs to be established. A new animal model that involves the conditional expression of EC-SOD now exists that may provide a novel way to address this idea (Zou et al., 2009). Given that radiation affected the hippocampus-independent novel object recognition cognitive measure in WT as well as KO mice, it seems unlikely that neurogenesis alone is causally responsible for the improved cognitive performance seen here. Therefore, to understand the functional effects seen here, other avenues also need to be explored, including, perhaps, how specific molecular markers associated with learning and memory (Rosi et al., 2008) are affected in animals deficient in EC-SOD. While the molecular mechanisms underlying the opposing genotype-dependent effects of irradiation reported here are not yet known, increased efforts are warranted to study potential beneficial effects associated with chronic oxidative stress in the context of a secondary insult such as irradiation. Ultimately understanding how such an effect develops may provide new insight into the evolution of cognitive injury after irradiation and provide information useful for the development of new approaches for the management of radiation-induced brain injury.
Acknowledgments
This work was supported by NIH grants R01 NS46051 and R01 AG24400, Alzheimer’s Association Grant IIRG-05-14021 and NASA Grant NNJ05HE63G.
References
- Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- Acevedo SE, McGinnis G, Raber J. Effects of 137Cs gamma irradiation on cognitive performance and measures of anxiety in Apoe−/− and wild-type female mice. Radiat Res. 2008a;170:422–8. doi: 10.1667/rr1494.1. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Tittle S, Raber J. Transgenic expression of androgen receptors improves spatial memory retention in both sham-irradiated and 137Cs gamma-irradiated female mice. Radiat Res. 2008b;170:572–8. doi: 10.1667/RR1435.1. [DOI] [PubMed] [Google Scholar]
- Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A. 1995;92:6264–8. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesberry WR, Butterfield A. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85:1394–1401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Graham SH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;16:566–577. doi: 10.1097/00004647-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18:115–27. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Gori T, Forconi S. The role of reactive free radicals in ischemic preconditioning--clinical and evolutionary implications. Clin Hemorheol Microcirc. 2005;33:19–28. [PubMed] [Google Scholar]
- Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–84. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–41. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler A, Avital A, Greenberger V, Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal. 2007;9:181–9. doi: 10.1089/ars.2007.9.181. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci. 2003;23:10359–67. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–44. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuli T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. Ischemic tolerance phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Levin ED, Brady TC, Hochrein EC, Oury TD, Jonsson LM, Marklund SL, Crapo JD. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav Genet. 1998;28:381–90. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–90. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat Res. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- Liu X-Q, Sheng R, Qin Z-H. The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol Sin advanced online publication. 2009 July 20; doi: 10.1038/aps.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Can Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–60. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pespeni M, Hodnett M, Pittet JF. In vivo stress preconditioning. Methods. 2005;35(2):158–64. doi: 10.1016/j.ymeth.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–81. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Qutob SS, Multani AS, Pathak S, McNamee JP, Bellier PV, Liu QY, Ng CE. Fractionated X-radiation treatment can elicit an inducible-like radioprotective response that is not dependent on the intrinsic cellular X-radiation resistance/sensitivity. Radiat Res. 2006;166:590–9. doi: 10.1667/RR0514.1. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt DR, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-Induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rola R, Zou Z, Huang T-T, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. Lack of EC-SOD in the microenvironment impacts radiation-induced changes in neurogenesis. Free Rad Biol & Med. 2007;42:1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68:9763–70. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacksteder CA, Qian W-J, Knyushko TV, Wang H, Chin MH, Lacan G, Melega WP, Camp DG, II, Smith RD, Smith DJ, Squier TC, Bigelow DJ. Endogenously Nitrated Proteins in Mouse Brain: Links to Neurodegenerative Disease. Biochemistry. 2006;45:8009–8022. doi: 10.1021/bi060474w. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–56. [PubMed] [Google Scholar]
- Schaller BJ. Influence of age on stroke and preconditioning-induced ischemic tolerance in the brain. Exp Neurol. 2007;205:9–19. doi: 10.1016/j.expneurol.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–43. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Shao G, Zhang R, Wang Z-L, Gao C-Y, Huo X, Lu G-W. Hypoxic Preconditioning improves spatial cognitive ability in mice. Neurosignals. 2008;15:314–321. doi: 10.1159/000121368. [DOI] [PubMed] [Google Scholar]
- Sheng H, Brady TC, Pearlstein RD, Crapo JD, Warner DS. Extracellular superoxide dismutase deficiency worsens outcome from focal cerebral ischemia in the mouse. Neurosci Lett. 1999;267:13–16. doi: 10.1016/s0304-3940(99)00316-x. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D’Agostino R, Brunso-Bechtold JK. Spatial Learning and Memory Deficits after Whole-Brain Irradiation are Associated with Changes in NMDA Receptor Subunits in the Hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20(20):7631–9. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofilon PJ, Fike JR. The radioresponse of the central nervous system: A dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006;127:436–43. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Zou Y, Chen CH, Fike JR, Huang TT. A new mouse model for temporal- and tissue-specific control of extracellular superoxide dismutase. Genesis. 2009 doi: 10.1002/dvg.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]