STRUCTURED ABSTRACT
Objective
To determine if genetic variation in chemotherapy metabolism are associated with risk of ovarian failure in breast cancer patients after adjuvant chemotherapy.
Design
Prospective cohort study.
Setting
Comprehensive cancer center.
Patients
Early stage breast cancer patients who were premenopausal at cancer diagnosis and treatment.
Interventions
None.
Main outcomes measures
Chemotherapy related ovarian failure (CROF)
Results
127 breast cancer subjects who were premenopausal at cancer diagnosis and underwent cyclophosphamide-based chemotherapy were genotyped for 9 single nucleotide polymorphisms (SNPs) in enzymes involved in cyclophosphamide activation (CYP3A4, CYP2B6, CYP3A5) and detoxification (GSTA1, GSTM1, GSTP1, GSTT1). Median age at chemotherapy was 43.2 years. Median years of follow up since chemotherapy were 5.2 years. For the entire cohort, there was no significant association between CROF and SNPs. However, the association between CROF and SNPs was modified by age at chemotherapy. In subjects younger than 45 at chemotherapy, CYP3A4*1B variants had significantly longer time to CROF than CYP3A4*1A homozygotes in an adjusted multivariable Cox model (HR 0.25 [95% CI 0.07–0.9]). Age and tamoxifen use were also independently associated with CROF.
Conclusions
A common SNP in a cyclophosphamide drug metabolizing enzyme appears to be related to ovarian failure after cyclophosphamide-based chemotherapy in young women with breast cancer. Larger prospective studies to validate these results should be directed toward women less than 45 years of age at chemotherapy.
Keywords: Ovarian failure, chemotherapy metabolism, genetic polymorphisms, breast cancer
Introduction
More than two million American women are breast cancer survivors (1). One-third of these women are less than 54 years old, and 10 percent of the total are between ages 35 and 45 (2). While chemotherapy has decreased recurrences and improved survival, these medications also induce ovarian damage and aging, resulting in premature ovarian failure and early menopause in a large proportion of women (3–5).
Predicting ovarian failure after chemotherapy is important to this large population of reproductive-aged breast cancer survivors. The degree of ovarian function affects breast cancer prognosis, choice of cancer adjuvant therapy, need for contraception, fertility potential and menopausal concerns (6–8). Thus, the ability to predict ovarian function after cancer therapy would impact patient counseling, medical and surgical treatment decisions, and consideration of fertility preservation options.
Currently, only age and chemotherapy regimen have been found to be consistent clinical risk factors for ovarian failure after adjuvant chemotherapy (3, 9). However, these two risk factors (singly or in combination) are unable to predict ovarian function or ovarian failure reliably. Therefore, there is a significant need for both increased understanding of the biological factors related to chemotherapy-related ovarian failure and identification of biomarkers or combinations of biomarkers to predict subsequent ovarian function after adjuvant chemotherapy.
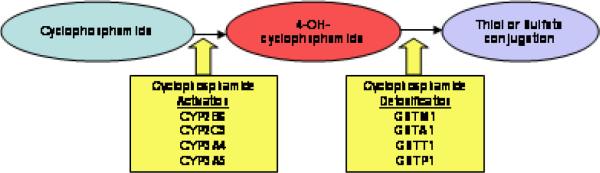
Little is known about the genetics of chemotherapy related ovarian failure (CROF). Alkylating agents like cyclophosphamide have been associated with ovarian damage; ovarian histology after cyclophosphamide exposure shows decreased follicles, absent follicles and ovarian fibrosis (10–14). By intercalating DNA, cyclophosphamide behaves in a non-cell cycle specific manner; this mechanism affects not only growing ovarian follicles but also quiescent primordial ones (15). Cyclophosphamide is inactive until metabolized by cytochrome P450 (CYP) enzymes to its cytotoxic form 4-hydroxycyclophosphamide (Figure 1) (16). Inactivation of cytotoxic 4-hydroxycyclophosphamide occurs through conjugation with glutathione by several GST enzymes (17). Several studies have demonstrated that functional single nucleotide polymorphisms (SNPs) in drug metabolizing enzymes (DME) are associated with cancer-related outcomes such as recurrence and survival (18–22).
Figure 1.
Schematic of cyclophosphamide activation and detoxification by durg metabolizing enzymes
Because genetic variation in chemotherapy metabolism, specifically cyclophosphamide metabolism, can contribute to inter-individual differences in drug response, we hypothesized that cyclophosphamide-DME SNPs may also be associated with CROF. We further hypothesized that there would be an interaction between SNPs and CROF by age at chemotherapy. That is, SNPs would have greater effect in younger patients receiving chemotherapy than in older patients. The objective of the current study was to determine if cyclophosphamide DME polymorphisms are associated with CROF after cyclophosphamide-based adjuvant chemotherapy in a cohort of premenopausal breast cancer patients treated for early breast cancer.
Materials and Methods
Study population
We performed a prospective cohort study of 127 female breast cancer survivors from the Rena Rowan Breast Center of the Abramson Cancer Center at the University of Pennsylvania. Women eligible for the current study had been diagnosed with histologically confirmed, American Joint Committee on Cancer Stages I–III breast cancer. Additional eligibility criteria included premenopausal at time of breast cancer diagnosis (defined as menstruating during the 12 months preceding cancer diagnosis), treatment with cyclophosphamide-based adjuvant chemotherapy, and the presence of a uterus and at least one ovary. At enrollment, subjects were required to be within 4 years of initiating chemotherapy. Chemotherapy regimen decisions were made by the subjects' treating medical oncologists according to tumor characteristics and usual standard of care; doses of cyclophosphamide were weight-based. Hormonal therapy for breast cancer was not an exclusion criterion. Eligible patients were identified through the Abramson Cancer Center's Breast Cancer Database, which archives detailed demographic, diagnostic and treatment information on all patients evaluated in the medical oncology practice within the Rowan Breast Center. Eligible patients were approached after passive consent of the treating physician for enrollment, and written consent for participation was provided by all participants. This study was approved by the University of Pennsylvania Institutional Review Board and the Abramson Cancer Center Scientific Review and Monitoring Committee.
Data collection
Menstrual history was collected prior to chemotherapy, at enrollment between 2004 and 2005 (Assessment 1), and at a second follow up visit (Assessment 2) in 2007. At enrollment, subjects had blood collected, from which DNA was extracted and stored. Clinical data were abstracted from medical charts. CROF was determined by self-reported menstrual history and was defined as at least 12 months of amenorrhea occurring after start of chemotherapy.
SNP selection
SNPs were carefully selected based on evidence of association with breast cancer survival or on changes in coding that would affect enzyme expression or function (19, 23–35). All SNPs except for CYP3A4*1B were hypothesized to result in decreased drug metabolizing enzyme function. The CYP3A4*1B polymorphism has been associated with higher CYP3A4 gene expression in vitro (36, 37). Other SNPs in CYP genes were hypothesized to result in decreased cyclophosphamide activation and lower risk of ovarian failure. SNPs in GST genes were expected to result in decreased cyclophosphamide detoxification and higher risk of ovarian failure.
Sample preparation and genotyping
DNA was extracted from buffy coat specimens using the Qiagen QIAamp DNA Blood Kit (Qiagen, Inc., Valencia, California, United States) and quantitated using Invitrogen Pico Green dsDNA quantitation kit (Invitrogen, Ltd., Paisley, United Kingdom). Subjects were genotyped for 9 SNPs in 8 cyclophosphamide DME genes: CYP2B6, CYP2C9, CYP3A4, CYP3A5, GSTM1, GSTP1, GSTA1 and GSTT1. CYP2B6*5 and CYP2C9*2 genotypes were determined using Real-Time PCR assays on the MJ Research Chromo4 (Bio-Rad Laboratories, Hercules, California, United States) platform. CYP2C9*3, CYP3A4*1B, CYP3A5*3, GSTA1 and GSTP1*1B genotypes were determined by PyroSequencing (Biotage, Charlottesville, Virginia, United States). GSTM1 and GSTT1 null mutations were determined by gel electrophoresis assays (38, 39).
Data Analysis
STATA (Release 9, Stata Corporation, College Station, Texas, United States) software was used for data analyses. Categorical variables were summarized by frequencies and proportions, while continuous variables were summarized by the mean, median, standard deviation and range. For each polymorphism, genotypes frequencies were calculated, and results were grouped as “wildtype” versus “any variant”. Hardy-Weinberg equilibrium was assessed for each polymorphism using the Pearson chi-square test. Confounding by population stratification was examined by determining if both risks of CROF and genotype frequencies differed by self-reported ethnicity.
Time-to-event methods were used for analyses. Time to CROF was selected as the primary endpoint to detect the impact of DME polymorphisms on the rate of developing ovarian failure and account for different lengths of follow up time for each subject. For subjects who developed CROF, time to CROF was calculated by subtracting the start date of chemotherapy from the end date of twelve months of amenorrhea. Subjects who did not develop CROF included 1) subjects who did not develop 12 months of amenorrhea during study follow up and 2) subjects who had bilateral oophorectomies or medical ovarian suppression with GnRH agonists prior to 12 months of amenorrhea. None of the subjects underwent a hysterectomy without bilateral oophorectomies. Subjects who did not develop CROF were censored at the last follow up date, date of surgical menopause, or start date of GnRH agonists, as appropriate. They contributed time from start of chemotherapy to the date when they were censored.
We analyzed age as a dichotomous variable, grouping subjects as < 45 or ≥ 45 years at chemotherapy. Age was dichotomized because risk of menopause does not increase linearly with age. The cutoff was set at 45 because ovarian failure less than 45 years old is considered early menopause and pathologic (40). To address the hypothesis of potential effect modification by age at chemotherapy, we examined the interaction between age and genotype. A priori power calculations were not performed for this preliminary study on genetic variation in chemotherapy metabolism and risk of CROF. Post hoc power calculations had limited power. For example, for the GSTM1 SNP, power calculations for detecting an interaction used the following assumptions: 80% power, type I error 5%, 50% overall CROF risk, 30% risk of CROF in women younger than 45, 90% risk of ovarian failure in women ≥ 45, variant genotype prevalence of 50%, and variant genotype effect of 1.4. The study was powered to detect an interaction odds ratio of 8 (Power version 3.0, http://dceg.cancer.gov/power/power.html). The detectable interaction odds ratio would be higher for SNPs with low variant frequencies. Kaplan-Meier survival curves were generated and time to CROF was compared using log rank tests. To test for interaction between age and genotype, comparisons of survival curves by genotype were further stratified by age at chemotherapy (<45 versus ≥ 45) using an interaction term in Cox proportional hazard regression models. Finally, explanatory multivariable Cox models were developed to examine predictors of time to ovarian failure and control for confounding. All clinical and genetic variables with p<0.1 based on the Wald test from univariate Cox regression were included in the multivariable model. For this first study on the genetics of CROF, a p-value of 0.05 in the adjusted model was considered significant.
Results
One hundred twenty-seven subjects were enrolled in this cohort between 2004 and 2005 (Assessment 1, Table 1). At Assessment 1, these subjects were on average 2.3 years (range 1.0– 4.9) from chemotherapy. Median age at start of chemotherapy was 43.2 (range 26.7–57.8). Four subjects had undergone bilateral oophorectomies prior to Assessment 1, resulting in surgical menopause. At Assessment 2 in 2007, 88% (n=112) of the cohort were reached and provided additional menstrual data. Of the 15 women who were lost to follow up at Assessment 2, 2 were deceased, 1 declined follow up and 12 could not be reached. Subjects lost to follow up were similar in all baseline characteristics except that they were less likely to be Caucasian, and, by definition, had shorter total follow up time and fewer CROF events (Table 1). Chemotherapy related ovarian failure occurred in 65 of 127 (51%) subjects. Overall, participants were followed for a median of 4.2 years since chemotherapy (range 1.0–7.6). The total person-time of follow up was 374 years. While all subjects underwent cyclophosphamide-based chemotherapy regimens, 94% received adriamycin/cyclophosphamide with or without a taxane. No subject was on oral contraceptives or hormonal replacement therapy throughout the study duration. Four subjects were placed on Lupron, and 77% of subjects received tamoxifen therapy.
Table 1.
Cohort characteristics (n = 127)
| Overall cohort (n=127) | Completed Assessment 2 (n=112) | Lost to follow up (n=15) | p-value | |
|---|---|---|---|---|
| Median age at start of chemotherapy (range) | 43.2 (26.7 – 57.8) | 43.4 (28.1–56) | 42.0 (26.7–57.8) | 0.12a |
| Median age at enrollment (range) | 45.3 (28.9–60.6) | 45.8 (29.2–59.3) | 43.5 (28.9–60.6) | 0.10a |
| Frequency (%) | ||||
| Race | ||||
| Caucasian | 113 (89%) | 102 (91%) | 11 (73%) | 0.04b |
| African American | 5 (4%) | 5 (4.5%) | 0 (0%) | |
| Other/Not reported | 9 (7%) | 5 (4.5%) | 4 (27%) | |
| Breast cancer stage | ||||
| I | 27 (21%) | 27 (24%) | 0 (0%) | 0.08b |
| II | 80 (63%) | 68 (61%) | 12 (80%) | |
| III | 20 (16%) | 17 (15%) | 3 (13%) | |
| Estrogen receptor + | 95 (75%) | 84 (75%) | 12 (75%) | 1.0b |
| Progesterone receptor + | 87 (68%) | 78 (70%) | 10 (63%) | 0.81b |
| Her-2/neu + | 27 (21%) | 23 (21%) | 4 (25%) | 0.58b |
| Median tumor size (cm) (range) | 2 (0–8.5) | 2 (0–8.5) | 2.5 (0.6–4.2) | 0.31c |
| Median lymph node + (range) | 1 (0–20) | 1 (0–20) | 1.5 (0–17) | 0.14c |
| Chemotherapy regimend | ||||
| Cyclophosphamide based chemotherapy | 127 (100%) | |||
| AC | 48 (38%) | 42 (37%) | 5 (33%) | 0.60b |
| AC/T | 69 (54%) | 61 (55%) | 8 (53%) | |
| FAC | 4 (3%) | 3 (3%) | 1 (7%) | |
| Othere | 4 (3%) | 6 (5%) | 1 (0%) | |
| Tamoxifen therapy | ||||
| Yes | 94 (75%) | 85 (77%) | 9 (60%) | 0.17b |
| No | 32 (25%) | 26 (23%) | 6 (40%) | |
| Median years of follow-up since chemotherapy (range) | 5.2 (1.0–7.6) | 5.4 (2.7–7.6) | 2.1 (1.0–3.9) | <0.001a |
| Chemotherapy related ovarian failure | 65 (51%) | 62 (55%) | 3 (20%) | <0.01b |
Student's t-test
Chi-square, Fisher's exact test as appropriate
Wilcoxon ranksum test
A = Doxorubicin, C = Cyclophosphamide, T = Taxane, F = 5-Flurouracil, N = Vinorelbine, M = Methotrexate
AC/N, AC/T/N, CMF, CMF/T
Genotype frequencies for the nine SNPs are depicted in Table 2. The genotype distributions were in Hardy-Weinberg equilibrium and were consistent with reported reference SNP frequencies. Overall, the number of samples that failed genotyping was small and did not differ by CROF status. GSTA1 genotype were highly correlated with CYP3A4*1B (p=0.03) and CYP3A5*3 (p=0.01) genotypes. GSTM1 and GSTT1 genotypes were also significantly associated (p<0.001).
Table 2.
Genotype frequencies and unadjusted hazard ratios of CROF by drug metabolizing enzyme genotypes (Wildtype to Variants) and by age at chemotherapy
| SNP | Genotype frequencies n (%) | All subjects (n = 127) | Age at chemotherapy < 45 years (n = 78) | Age at chemotherapy ≥ 45 years (n = 49) | Interaction p-valuee | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) WT to variant genotypese | p-value | HR (95% CI) WT to variant genotypese | p-value | HR (95% CI) WT to variant genotypese | p-value | ||||
| CYP2B6*5 (rs3211371)a | WT | 96 (75.6) | 1.0 (0.6–1.8) | 1.0 | 2.2 (0.5–9.4) | 0.3 | 1.1 (0.6–2.2) | 0.69 | 0.38 |
| Variant | 29 (22.6) | ||||||||
| Missing | 2 (1.6) | ||||||||
| CYP2C9*2 (rs1799853)a | WT | 103 (81.1) | 1.2 (0.6–2.4) | 0.52 | 1.9 (0.4–8.2) | 0.4 | 1.3 (0.6–2.7) | 0.55 | 0.77 |
| Variant | 22 (17.3) | ||||||||
| Missing | 2 (1.6) | ||||||||
| CYP2C9*3 (rs1057910)b | WT | 94 (74.0) | 1.0 (0.5–2.0) | 1.0 | 1.5 (0.3–6.5) | 0.6 | 0.9 (0.4–1.9) | 0.76 | 0.67 |
| Variant | 18 (14.2) | ||||||||
| Missing | 15 (11.8) | ||||||||
| CYP3A4*1B (rs2740574)c | WT | 113 (89.0) | 0.5 (0.2–1.1) | 0.10 | 0.3 (0.07–0.9) | 0.03 | 0.6 (0.2–2.1) | 0.45 | 0.26 |
| Variant | 8 (6.3) | ||||||||
| Missing | 6 (4.7) | ||||||||
| CYP3A5*3 (rs776746)b | WT | 3 (2.4) | 0.7 (0.1–4.7) | 0.67 | 0 | - | 2.1 (0.3–15.7) | 0.46 | - |
| Variant | 108 (85.0) | ||||||||
| Missing | 16 (12.6) | ||||||||
| GSTA1 (rs4715332)b | WT | 27 (21.3) | 1.2 (0.7–2.1) | 0.57 | 0.5 (0.1–2.2) | 0.37 | 1.3 (0.7–2.5) | 0.49 | 0.38 |
| Variant | 94 (66.2) | ||||||||
| Missing | 16 (12.6) | ||||||||
| GSTM1 (no rs # available)d | Non-null | 42 (33.1) | 0.7 (0.4–1.2) | 0.27 | 0.9 (0.3–2.3) | 0.79 | 1.1 (0.6–2.1) | 0.78 | 0.66 |
| Null | 82 (64.6) | ||||||||
| Missing | 3 (2.3) | ||||||||
| GSTT1 (no rs # available)d | Non-null | 108 (85) | 2.0 (0.8–5.0) | 0.14 | 0 | - | 1.2 (0.5–3.1) | 0.66 | - |
| Null | 8 (6.7) | ||||||||
| Missing | 3 (2.3) | ||||||||
| GSTP1*B (rs1695)c | WT | 52 (40.9) | 1.1 (0.7–1.9) | 0.66 | 1.3 (0.5–3.5) | 0.60 | 0.8 (0.4–1.5) | 0.49 | 0.38 |
| Variant | 59 (46.5) | ||||||||
| Missing | 16 (12.6) | ||||||||
Genotyped by real-time PCR
Genotyped by Pyrosequencing Simplex Assay
Genotyped by Pyrosequencing Multiplex Assay
Genotyped by gel electropheresis
Cox regression
Unadjusted associations between genotype, clinical factors and time to CROF
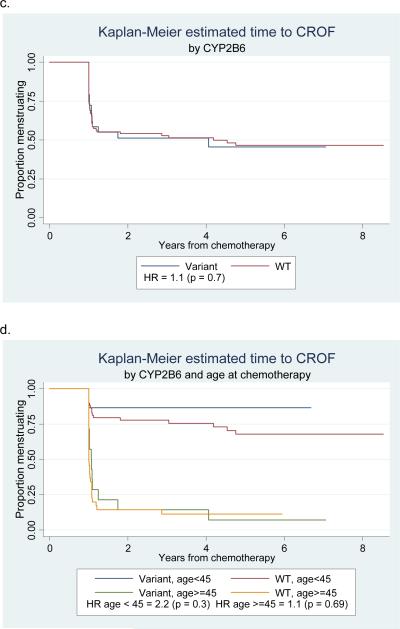
For the entire cohort, no association between genotype and time to CROF was significant (Table 2). Age at chemotherapy, addition of taxane, treatment schedule, and tamoxifen exposure also did not differ by genotype (all p>0.3). Stratified by age at chemotherapy as pre-specified, subjects younger than 45 at chemotherapy who had CYP3A4*1B wildtype genotypes had significantly longer time to ovarian failure (median 4.3 years, range 1–8.5 years) compared to younger subjects who had variant genotypes (median 1.0 year, range 1–6.8 years) (p=0.03). Hazard ratios for CROF were consistently near 1 for older women (45 years or older at chemotherapy). Kaplan-Meier curves for these analyses show that time to CROF in younger women begins to diverge by genotype for multiple SNPs, though these were not statistically significant (Figure 2).
Figure 2.
Kaplan-Meier survival curves of time to CROF by genotype alone (a, c) and by genotype and age at chemotherapy (b, d)
Of clinical and chemotherapy regimen-related factors, younger age at chemotherapy and taxane exposure had significantly decreased hazards of ovarian failure (Table 3). Progesterone receptor (PR) status, treatment schedule and tamoxifen exposure had associations of borderline significance to ovarian failure. Age at chemotherapy was significantly correlated with taxane therapy (p=0.02), but not with dose dense therapy (p=0.14), or tamoxifen exposure (p=0.91). Tamoxifen exposure and progesterone receptor status were highly correlated (p<0.001). For model building, age at chemotherapy, treatment schedule, taxane therapy and tamoxifen exposure were included as covariates of CYP3A4*1B genotype. PR status was not included in the model because it was co-linear with tamoxifen use.
Table 3.
Hazard ratios of CROF by individual risk factors
| Risk factor | Comparison | HR (95% CI)a | p-value |
|---|---|---|---|
| Age at chemotherapy | ≥ 45 years to < 45 years | 7.5 (4.3–13.0) | <0.001 |
| Race | Caucasian to Others | 0.76 (0.3–1.7) | 0.50 |
| Dose Dense therapy | Dose dense to non-Dose Dense | 1.5 (0.9–2.5) | 0.10 |
| Taxane therapy | Taxane exposure to no taxane exposure | 0.59 (0.4–0.9) | 0.03 |
| Tamoxifen therapy | Tamoxifen exposure to no tamoxifen exposure | 1.7 (0.9–3.0) | 0.10 |
| Smoking | Ever smoked to no smoke | 1.0 (0.6–1.6) | 0.92 |
Cox regression
Adjusted associations with time to CROF
Table 4 shows adjusted Cox modeling of the associations between genotype, CROF and other factors, including age at chemotherapy, the interaction between genotype and age at chemotherapy, dose dense therapy, taxane exposure and tamoxifen exposure. In this model, survivors who had the wildtype CYP3A4*1B genotype and younger than 45 at chemotherapy continued to be significantly less likely to develop CROF than survivors in this age group who had variant genotypes (HR 0.25 [0.07–0.9], p=0.03). Independent of genotype, subjects who were treated with tamoxifen were twice as likely to experience CROF (HR 2.0[1.0–3.7], p=0.04).
Table 4.
Final multivariable Cox Model for CROF
| Risk factor | Comparison | HR (95% CI) | p-value |
|---|---|---|---|
| CYP3A4*1B (Age at chemotherapy < 45) | WT to Variant | 0.25 (0.07–0.9) | 0.03 |
| CYP3A4*1B (Age at chemotherapy ≥ 45) | WT to Variant | 0.69 (0.2–2.3) | 0.54 |
| Age at chemotherapy | ≥ 45 to < 45 | 4.1 (0.8–22.0) | 0.10 |
| Treatment schedule | Dose dense to non-Dose Dense | 1.2 (0.6–2.3) | 0.54 |
| Addition of taxane | Taxane exposure to no taxane exposure | 1.1 (0.6–2.2) | 0.76 |
| Receipt of Tamoxifen therapy | Tamoxifen exposure to no tamoxifen exposure | 2.0 (1.0–3.7) | 0.04 |
Population stratification
CYP3A4*1B genotype frequencies differed by ethnicity (p<0.001), while CROF risks did not (p=0.56), Therefore, by definition, population stratification did not confound the association between genotype and CROF. Furthermore, all directions of the hazard ratios for each genotype remain unchanged if analyses are restricted to self-reported Caucasians (data not shown). Specifically, for the CYP3A4 genotype, wildtype genotype remained protective against CROF in unadjusted (HR 0.06, p<0.001) and adjusted COX models (HR 0.07, p<0.001) in subjects who were younger than 45 at chemotherapy.
Discussion
After cyclophosphamide exposure, the risk of ovarian failure in young women with breast cancer is variable and difficult to predict. To our knowledge, this is the first study to explore the contribution of genetic variation in chemotherapy metabolism to these inter-individual differences in cancer survivors. We found that a common SNP in cyclophosphamide metabolism, age at chemotherapy and tamoxifen use are all independently associated with CROF in our cohort. Moreover, this study suggests that the relationship between genotype and CROF is modified by age at chemotherapy.
Our data demonstrated a higher risk of ovarian failure after cyclophosphamide-based chemotherapy in young breast cancer survivors with the CYP3A4*1B variant genotypes compared to the wildtype genotype. CYP3A4 accounts for 30–60% of liver cytochrome P450 protein and metabolizes alkylating agents such as cyclophosphamide (36). The CYP3A4*1B SNP consists of an A to G nucleotide change at position −290 in the gene promoter. The transcription regulation of this gene and functional significance of this polymorphism are controversial (41, 42). Several in vitro cell-based assays suggest that CYP3A4*1B variant is associated with higher CYP3A4 expression than the wildtype (36, 37). This observation would result in higher cyclophosphamide activation with variant genotypes and is consistent with the higher risk of ovarian failure found in this study. However, some clinical studies have linked CYP3A4*1B variants with worse cancer survival compared to wildtype (21). These studies would suggest decreased CYP3A4 function in carriers of the CYP3A4*1B variant genotypes (43, 44). One plausible explanation for the disparate findings is that the mechanisms for both cancer survival and ovarian failure are more complex than chemotherapy metabolism, requiring further investigation. Further, our finding may be a false positive association or Type I error. Therefore, we are in the process of conducting validation studies in new cohorts.
To our knowledge, there are only two other studies on the impact of DME polymorphisms on ovarian function. These studies were in young women with systemic lupus who received cyclophosphamide. Testing different SNPs than the present study, both studies found a protective effect of CYP2C19*2 variants on ovarian function (45, 46). The CYP2C19*2 variant is known to result in truncated and nonfunctional enzyme (47). While this finding also requires further validation, it strengthens the biologic plausibility of our hypothesis that genetic polymorphisms in drug metabolism of gonadotoxic drugs are associated with ovarian function.
Our data also suggested CROF risk conferred by cyclophosphamide drug metabolizing enzyme SNPs differs by age at chemotherapy. We found that risk of ovarian failure is virtually 100% in our subjects who were over age 45 at chemotherapy, limiting our ability to assess the impact of additional risk factors such as DME polymorphisms on ovarian failure on these older women. The study had limited power to detect a statistically significant interaction between genotype and age, particularly for genotypes with low variant frequencies. Despite this limitation, the biology of ovarian aging and observed trend across genotypes in this dataset both support the hypothesis of an interaction between age and genotype. Therefore, we believe this is an important issue to highlight in this dataset. Our findings provide a basis for larger future studies to address such gene-environment interactions more definitively.
We do not believe population stratification confounded the observed association between genotype and CROF risk, as the risk of CROF did not differ by self-reported ethnicity. Furthermore, both adjusting for race and restricting analyses to Caucasians did not change the observed association.
There are several limitations to this study. First, sample size limited the power of analyzing SNPs with low genotype frequencies, such as CYP3A5*3. SNPs selection in our future studies will be narrowed by cohort sample size and distribution of SNP genotypes observed by this current work. Second, we were unable to provide further age-specific or age-adjusted risks of CROF by genotype in subjects younger than 45, because of the low prevalence of variant genotype. The strength of our study was the long follow up and collection of menstrual history at three separate time points (prior to chemotherapy and two post-chemotherapy time points). Further, last menstrual period dates reported by our subjects with CROF are likely fairly accurate because a large proportion of them (73%) experienced their last period with start of chemotherapy, a date cancer survivors remember well and verified by chart review. We do not have reason to believe that inaccuracies in recalled LMP in CROF subjects are systematically biased, to be consistently more recent or distant than the true dates. Thus, we do not believe that recalled LMP biased our results away from the null. Next, there are limited data correlating genotype to cyclophosphamide pharmacokinetics (48–52). A single, underpowered study did not detect an impact of CYP3A4*1B genotypes on cyclophosphamide and 4-hydroxycyclophosphamide clearance (48). Larger studies are required to assess adequately the effect on genotype on cyclophsphamide pharmacokinetics. Finally, loss to follow up occurred for 12% of our cohort. We postulate that if no loss to follow up occurred, these 15 subjects would have contributed more CROF events. However, complete follow up would not likely impact the association between genotype and CROF because of similar genotype frequencies between those who stayed in the cohort and those who were lost to follow up (p=0.6)
Ovarian failure significantly impacts quality of life in breast cancer survivors. Therefore, it is important to have tools to predict the outcome prior to adjuvant chemotherapy. We have demonstrated that DME polymorphisms may be an additional risk factor for chemotherapy related ovarian failure. Further, this novel risk factor is likely to have the highest impact on risk of ovarian failure in women who are younger than 45 at chemotherapy. In an era where individualized therapy is a goal and survival from breast cancer continues to improve, studies like this one work toward defining risk of adverse outcomes after chemotherapy for each patient. In addition, results from this study will aid in design of future studies.
Acknowledgments
FUNDING: NIH 5-T32-HD007440-12 Reproductive Epidemiology Grant (IS), Commonwealth of Pennsylvania Health Research Formula Funds (AD), Doris Duke Clinical Research Fellowship (JG), FOCUS Medical Student Fellowship in Women's Health/Bertha Dagan Berman Award (CS)
Footnotes
CONFLICTS OF INTEREST: None
CAPSULE: A common single nucleotide polymorphism in a cyclophosphamide drug metabolizing enzyme is associated with ovarian failure after cyclophosphamide-based chemotherapy in young breast cancer patients. Genetic variation in chemotherapy metabolism may contribute to inter-individual differences in risk of chemotherapy related ovarian failure.
References
- 1.Ries LAG, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2005. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site 2008. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–29. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 4.Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. 1992;166(3):788–93. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17(8):2365–70. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 6.(EBCTCG) EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184–93. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 8.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 9.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24(36):5769–79. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 10.Brincker H, Rose C, Rank F, Mouridsen HT, Jakobsen A, Dombernowsky P, et al. Evidence of a castration-mediated effect of adjuvant cytotoxic chemotherapy in premenopausal breast cancer. J Clin Oncol. 1987;5(11):1771–8. doi: 10.1200/JCO.1987.5.11.1771. [DOI] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. The International Breast Cancer Study Group. Ann Oncol. 1990;1(3):183–8. doi: 10.1093/oxfordjournals.annonc.a057718. [DOI] [PubMed] [Google Scholar]
- 12.Miller JJ, 3rd, Williams GF, Leissring JC. Multiple late complications of therapy with cyclophosphamide, including ovarian destruction. Am J Med. 1971;50(4):530–5. doi: 10.1016/0002-9343(71)90341-x. [DOI] [PubMed] [Google Scholar]
- 13.Nicosia SV, Matus-Ridley M, Meadows AT. Gonadal effects of cancer therapy in girls. Cancer. 1985;55(10):2364–72. doi: 10.1002/1097-0142(19850515)55:10<2364::aid-cncr2820551011>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Pagani O, O'Neill A, Castiglione M, Gelber RD, Goldhirsch A, Rudenstam CM, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34(5):632–40. doi: 10.1016/s0959-8049(97)10036-3. [DOI] [PubMed] [Google Scholar]
- 15.Warne GL, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289(22):1159–62. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- 16.Scripture CD, Sparreboom A, Figg WD. Modulation of cytochrome P450 activity: implications for cancer therapy. Lancet Oncol. 2005;6(10):780–9. doi: 10.1016/S1470-2045(05)70388-0. [DOI] [PubMed] [Google Scholar]
- 17.Choi JY, Nowell SA, Blanco JG, Ambrosone CB. The role of genetic variability in drug metabolism pathways in breast cancer prognosis. Pharmacogenomics. 2006;7(4):613–24. doi: 10.2217/14622416.7.4.613. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosone CB, Sweeney C, Coles BF, Thompson PA, McClure GY, Korourian S, et al. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 2001;61(19):7130–5. [PubMed] [Google Scholar]
- 19.Sweeney C, Ambrosone CB, Joseph L, Stone A, Hutchins LF, Kadlubar FF, et al. Association between a glutathione S-transferase A1 promoter polymorphism and survival after breast cancer treatment. Int J Cancer. 2003;103(6):810–4. doi: 10.1002/ijc.10896. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney C, McClure GY, Fares MY, Stone A, Coles BF, Thompson PA, et al. Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res. 2000;60(20):5621–4. [PubMed] [Google Scholar]
- 21.De Michele AG, P, Aplenc R, Colligon T, Foulkes AS, Rebbeck TR. Drug Metabolizing Enzyme Polymorphisms in Breast Cancer Patients Receiving Cyclophosphamide-Based Adjuvant Therapy: Corrected Results of a Pilot Study. J Clin Oncol. 2007 in press. [Google Scholar]
- 22.Gor PG, RJ, Gimotty PA, Horn M, Aplenc R, Tallman MS, Rebbeck TR, DeMichele A. Association of Drug Metabolizing Enzyme Polymorphisms with Survival Outcomes in Node Positive Breast Cancer Patients Treated on the Eastern Cooperative Oncology Group/Intergroup 0121. manuscript in submission. [Google Scholar]
- 23.Aithal GP, Day CP, Leathart JB, Daly AK. Relationship of polymorphism in CYP2C9 to genetic susceptibility to diclofenac-induced hepatitis. Pharmacogenetics. 2000;10(6):511–8. doi: 10.1097/00008571-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Chang TK, Yu L, Goldstein JA, Waxman DJ. Identification of the polymorphically expressed CYP2C19 and the wild-type CYP2C9-ILE359 allele as low-Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics. 1997;7(3):211–21. doi: 10.1097/00008571-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61(3):154–66. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 26.Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287(13):1690–8. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 27.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 28.Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11(5):399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Petros WP, Hopkins PJ, Spruill S, Broadwater G, Vredenburgh JJ, Colvin OM, et al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol. 2005;23(25):6117–25. doi: 10.1200/JCO.2005.06.075. [DOI] [PubMed] [Google Scholar]
- 30.Rettie AE, Haining RL, Bajpai M, Levy RH. A common genetic basis for idiosyncratic toxicity of warfarin and phenytoin. Epilepsy Res. 1999;35(3):253–5. doi: 10.1016/s0920-1211(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 31.Seidegard J, Pero RW. The genetic variation and the expression of human glutathione transferase mu. Klin Wochenschr. 1988;66(Suppl 11):125–6. [PubMed] [Google Scholar]
- 32.Seidegard J, Vorachek WR, Pero RW, Pearson WR. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988;85(19):7293–7. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6(4):341–9. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19(2):275–80. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki H, Inoue K, Chiba K, Ozawa N, Kawai T, Suzuki Y, et al. Comparative studies on the catalytic roles of cytochrome P450 2C9 and its Cys- and Leu-variants in the oxidation of warfarin, flurbiprofen, and diclofenac by human liver microsomes. Biochem Pharmacol. 1998;56(2):243–51. doi: 10.1016/s0006-2952(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 36.Amirimani B, Ning B, Deitz AC, Weber BL, Kadlubar FF, Rebbeck TR. Increased transcriptional activity of the CYP3A4*1B promoter variant. Environ Mol Mutagen. 2003;42(4):299–305. doi: 10.1002/em.10199. [DOI] [PubMed] [Google Scholar]
- 37.Amirimani B, Walker AH, Weber BL, Rebbeck TR. RESPONSE: re: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 1999;91(18):1588–90. doi: 10.1093/jnci/91.18.1588. [DOI] [PubMed] [Google Scholar]
- 38.Aplenc R, Glatfelter W, Han P, Rappaport E, La M, Cnaan A, et al. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. British Journal of Haematology. 2003;122(2):240–4. doi: 10.1046/j.1365-2141.2003.04430.x. [DOI] [PubMed] [Google Scholar]
- 39.Davies SM, Robison LL, Buckley JD, Radloff GA, Ross JA, Perentesis JP. Glutathione S-transferase polymorphisms in children with myeloid leukemia: a Children's Cancer Group study. Cancer Epidemiol Biomarkers Prev. 2000;9(6):563–6. [PubMed] [Google Scholar]
- 40.Fritz LSMA. Clinical Gynecologic Endocrinology & Infertility Lippincott. Williams & Wilkins; 2006. [Google Scholar]
- 41.Lamba JK, Lin YS, Thummel K, Daly A, Watkins PB, Strom S, et al. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12(2):121–32. doi: 10.1097/00008571-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Spurdle AB, Goodwin B, Hodgson E, Hopper JL, Chen X, Purdie DM, et al. The CYP3A4*1B polymorphism has no functional significance and is not associated with risk of breast or ovarian cancer. Pharmacogenetics. 2002;12(5):355–66. doi: 10.1097/00008571-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Rebbeck TR, Jaffe JM, Walker AH, Wein AJ, Malkowicz SB. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 1998;90(16):1225–9. doi: 10.1093/jnci/90.16.1225. [DOI] [PubMed] [Google Scholar]
- 44.Paris PL, Kupelian PA, Hall JM, Williams TL, Levin H, Klein EA, et al. Association between a CYP3A4 genetic variant and clinical presentation in African-American prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1999;8(10):901–5. [PubMed] [Google Scholar]
- 45.Singh G, Saxena N, Aggarwal A, Misra R. Cytochrome P450 polymorphism as a predictor of ovarian toxicity to pulse cyclophosphamide in systemic lupus erythematosus. J Rheumatol. 2007;34(4):731–3. [PubMed] [Google Scholar]
- 46.Takada K, Arefayene M, Desta Z, Yarboro CH, Boumpas DT, Balow JE, et al. Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum. 2004;50(7):2202–10. doi: 10.1002/art.20338. [DOI] [PubMed] [Google Scholar]
- 47.Nakamoto K, Kidd JR, Jenison RD, Klaassen CD, Wan YJ, Kidd KK, et al. Genotyping and haplotyping of CYP2C19 functional alleles on thin-film biosensor chips. Pharmacogenet Genomics. 2007;17(2):103–14. doi: 10.1097/FPC.0b013e32801152c2. [DOI] [PubMed] [Google Scholar]
- 48.Ekhart C, Doodeman VD, Rodenhuis S, Smits PH, Beijnen JH, Huitema AD. Influence of polymorphisms of drug metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide. Pharmacogenet Genomics. 2008;18(6):515–23. doi: 10.1097/FPC.0b013e3282fc9766. [DOI] [PubMed] [Google Scholar]
- 49.Timm R, Kaiser R, Lotsch J, Heider U, Sezer O, Weisz K, et al. Association of cyclophosphamide pharmacokinetics to polymorphic cytochrome P450 2C19. Pharmacogenomics J. 2005;5(6):365–73. doi: 10.1038/sj.tpj.6500330. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima M, Komagata S, Fujiki Y, Kanada Y, Ebi H, Itoh K, et al. Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genomics. 2007;17(6):431–45. doi: 10.1097/FPC.0b013e328045c4fb. [DOI] [PubMed] [Google Scholar]
- 51.Xie H, Griskevicius L, Stahle L, Hassan Z, Yasar U, Rane A, et al. Pharmacogenetics of cyclophosphamide in patients with hematological malignancies. Eur J Pharm Sci. 2006;27(1):54–61. doi: 10.1016/j.ejps.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Xie HJ, Yasar U, Lundgren S, Griskevicius L, Terelius Y, Hassan M, et al. Role of polymorphic human CYP2B6 in cyclophosphamide bioactivation. Pharmacogenomics J. 2003;3(1):53–61. doi: 10.1038/sj.tpj.6500157. [DOI] [PubMed] [Google Scholar]