Abstract
Many anti-viral vaccines elicit neutralizing antibodies as a correlate of protection. For HIV, given the huge variability of the virus, it is widely believed that the induction of a broadly neutralizing antibody (bNAb) response will be crucial in a successful vaccine against the virus. Unfortunately, despite many efforts, the development of an immunogen that elicits bNAbs remains elusive. However, recent structural studies of HIV-1 Env proteins, generation of novel bNAbs, maturation of technologies for the isolation of further antibodies, insights into the requirements for antibody-mediated protection, and novel vaccination approaches are providing grounds for renewed optimism.
Introduction
The hallmark of many successful anti-viral vaccines is the ability to induce neutralizing antibodies [1]. One method of showing that antibodies can provide protection is by passive administration followed by virus challenge in animal models. For many viruses, this approach shows good correlation between protection in vivo and neutralizing activity in vitro [2]. For HIV-1, passively administered NAbs provide protection after intravenous, vaginal, rectal, and oral virus challenge in non-human primate models [3•,4•,5,6,7]. Importantly, several studies demonstrate that vaccine-induced NAb responses can confer complete protection against homologous SHIV challenge in macaques [8,9••,10], indicating that a vaccine capable of eliciting sufficient levels of NAb against HIV-1 could prevent the establishment of infection. For many viruses, extra-neutralizing mechanisms, such as those dependent on interaction of antibody with Fc receptors, e.g. antibody-dependent cellular cytotoxicity (ADCC), or on interaction with complement, also contribute to protection [2,11,12]. For HIV-1, experiments in the macaque model suggest the importance of the interaction of antibody with Fc receptors [11]. Although non-neutralizing antibodies can mediate extra-neutralizing activities, these types of antibodies provide little or no protection against SHIV challenge in non-human primates [5,13], suggesting that a vaccine should focus on the induction of NAbs. Overall, given the observations in animal models, it seems highly likely that neutralizing antibodies to HIV-1 induced by a vaccine would provide benefit on exposure to the virus.
There are, however, major challenges in the development of immunogens that induce bNAbs. These challenges include the extraordinary genetic diversity of the virus, the relative inaccessibility of conserved epitopes that are targeted by bNAbs, the instability of the envelope glycoprotein (Env, the only known target for neutralizing antibodies), and difficulties sustaining NAb titers following vaccination. Optimism in the field has risen following recent studies in humans and non-human primate models. First, a series of serum mapping studies show that 10–30% of HIV-1 infected individuals develop moderate to broadly neutralizing sera over time, demonstrating that the human immune system is capable of generating bNAb responses against HIV-1 [14]. Studies underway on how these bNAb responses develop may prove valuable in vaccine design. Second, broadly neutralizing monoclonal antibodies with outstanding potency have recently been isolated from infected donors [15••, GJ Nabel et al., personal communication]. Third, recent passive immunization studies in non-human primate models indicate that bNAbs can provide benefit against SHIV challenge at much lower serum neutralizing titers than originally considered obligatory for protection [3•,16••]. Fourth, several studies show that productive HIV-1 infection may often be mediated by one or a few virions, providing a vulnerability of the virus to antibody neutralization early in infection [17].
Initial attempts to generate a protective vaccine against HIV-1 focused on the elicitation of Env-specific humoral immune responses using gp120 subunit immunogens. Unfortunately, the results of Phase 1 clinical trials indicated that the antibodies elicited by monomeric gp120 failed to neutralize HIV-1 primary isolates (representative of circulating viruses as opposed to laboratory adapted strains) [18,19]. The results of two Phase 3 efficacy trials showed that the vaccine failed to prevent HIV-1 infection, reduce viral loads, or delay disease progression [20,21]. Over the ensuing years, HIV-1 researchers have pursued many different approaches to the generation of an antibody-based vaccine, but none of the immunogens generated to date have induced NAb responses of the required breadth and potency. Much effort has focused on rational vaccine design. The Env proteins, more specifically the functional Env heterotrimer (gp120)3(gp41)3, and bNAbs are the two molecular species that are at the heart of such rational HIV-1 vaccine design. Insight into the molecular structures of Env proteins, both alone and in complex with bNAbs, is crucial for attempts to, in effect, reverse engineer vaccine candidates [22] (Fig 1). This review will discuss recent advances in HIV vaccine design with a focus on rational approaches.
Figure 1. An approach to vaccines for highly mutable pathogens.
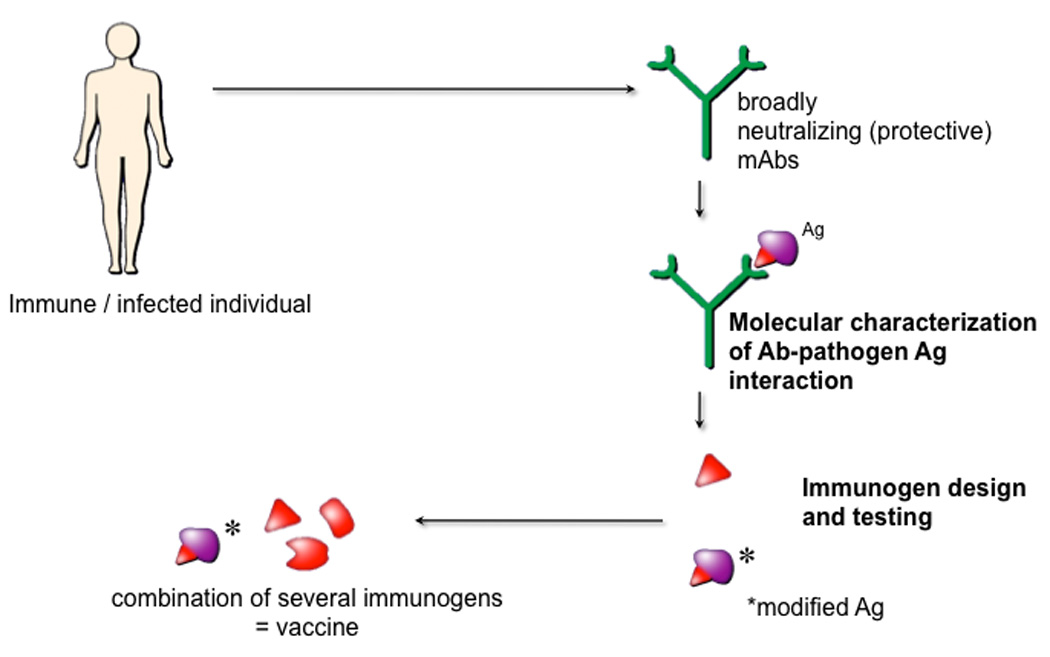
For these pathogens, e.g. HIV, classical vaccine approaches are problematic but a subset of individuals do produce the types of antibody response that, if they could be elicited by vaccination, would likely provide benefit on exposure to the pathogen. Isolation of monoclonal antibodies (mAbs) constituting these responses together with a molecular characterization of the interaction of the mAbs with their pathogen target antigens (Ag) is proposed as a route to the design of immunogens that can elicit protective responses. For HIV, Ag is the envelope spike.
Soluble trimers
The native functional HIV-1 trimer (either prior to or during the fusion process) is the sole target for neutralizing antibodies, and it seems that antibody binding to the trimer is necessary and sufficient for neutralization [2]. Therefore, in principle, a recombinant native trimer represents an ideal immunogen for the elicitation of NAb responses. However, the inherent instability of the functional HIV-1 spike has presented challenges to the development of native recombinant trimers [23]. Various strategies, including the introduction of disulfide bonds to covalently link gp120 and gp41, deletion of the furin cleavage site in gp160, and the incorporation of a number of trimerization motifs into the gp41 ectodomain, have been employed to stabilize recombinant trimers [23]. Although none of these recombinant trimers display antigenic profiles that accurately mimic the native HIV-1 spike, some elicit antibodies that neutralize heterologous isolates with very modest potency [24,25,26,27]. For example, both YU2 gp140 GCN4 and KNH1144 gp140 SOSIP trimers induce NAbs with increased breadth and potency relative to those elicited by monomeric gp120 [25,26], although the improvements are small. On a positive note, recent cryoelectron tomographic structures of native HIV-1 trimers [28••,29,30], as well as the isolation of new broadly neutralizing trimer-specific antibodies [15••], will likely aid the design of recombinant trimers that better mimic the native HIV-1 spike.
Immunogens based on the epitopes recognize by bNAbs
HIV-1 tends to elicit a high abundance of NAbs against variable regions of the virus, whereas NAbs that target conserved regions are rare and only develop in a subset of individuals [14]. Nonetheless, a small number of broadly neutralizing human monoclonal antibodies have been isolated, and the epitopes targeted by these antibodies have served as templates for immunogen design (Figure 1 and Figure 2) [23]. Recent crystallographic, cryoelectron tomographic, and molecular modeling studies have provided valuable insights into the molecular surfaces recognized by the antibodies and assisted the rational design of immunogens [31].
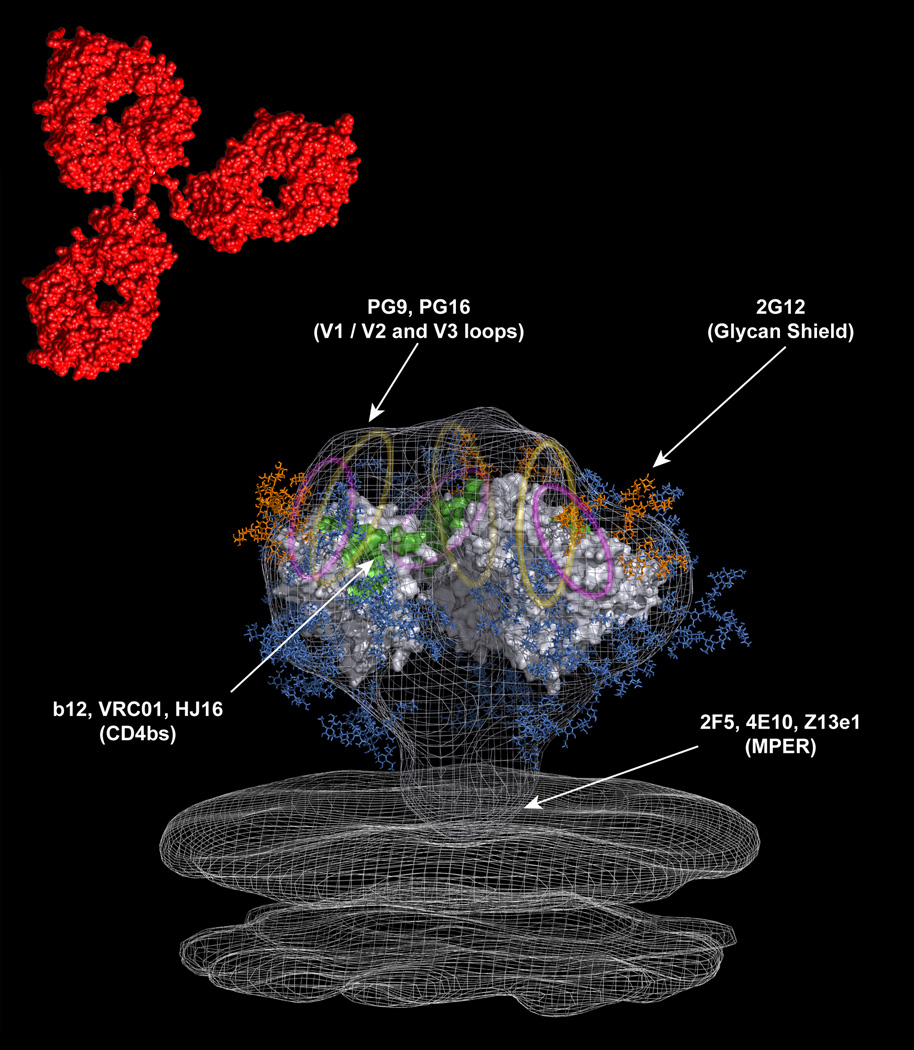
Figure 2. Modeling the epitopes recognized by bNAbs onto the HIV-1 trimer.
The above model is adapted from a recent cryo-electron tomographic structure of the HIV-1 trimer [28••,86]. The crystal structure of the b12-bound monomeric gp120 core has been fitted into the density map [39]. The V1/V2 and V3 loops, which are not resolved in the structure, are represented as yellow and magenta ovals, respectively. The red structure located above the trimer is representative of a human IgG1 molecule. The approximate locations of the epitopes targeted by the existing bNAbs are indicated with arrows. Carbohydrate chains are shown in blue, and the oligomannose cluster targeted by mAb 2G12 is shown in orange.
The CD4 binding site (CD4bs) is a prime target for the elicitation of bNAbs due to its high degree of conservation and the requirement for accessibility, at least to CD4. Indeed, the well-characterized bNAb b12, as well as several broadly neutralizing sera, have been shown to target this site [14,32]. Furthermore, two new CD4bs-directed bNAbs have recently been reported [33, GJ Nabel et al., personal communication]. One of these, HJ16, exhibits similar breadth and potency as b12 but shows a different pattern of neutralization sensitivity [33]. The other bNAb, VRC01, displays greater breadth and potency compared to b12 [GJ Nabel et al., personal communication]. Until the recent findings, b12 was the most broad and potent CD4bs-directed mAb, and therefore a variety of strategies were employed to focus the immune response on its epitope. In a series of studies, gp120 and gp140 were engineered using alanine substitutions and hyperglycosylation to maintain b12 binding while reducing the binding of most non-neutralizing mAbs [34,35,36]. However, several designs failed to induce b12-like antibodies in rabbits [37]. This may be due to the inherent conformational flexibility of recombinant gp120 and gp140 that militates against uniquely fixing the b12 epitope. Interestingly, a recent crystal structure of b13, a non-neutralizing CD4bs-directed antibody, bound to gp120 revealed a remarkably similar footprint to that of b12 [38•]. This result suggests that either extremely precise targeting induced by the immunogen or presentation of the b12 epitope in the context of the functional trimer may be required for the elicitation of b12-like antibodies. Since b12 and 2G12 primarily interact with the outer domain (OD) of gp120 [39], this surface also represents an attractive target for immunogen design. The OD is poorly immunogenic in the context of gp120, and therefore researchers have focused on the generation of isolated OD constructs that expose the b12 and 2G12 (see below) binding sites. However, when the OD of YU2 gp120 was expressed independently, it bound 2G12 and V3 loop-directed antibodies with high affinity but failed to bind b12 [40]. More recently, the crystal structure of the b12-gp120 complex was used to guide the design of a membrane-anchored OD construct that specifically bound to b12 but not most other non-neutralizing CD4bs-directed antibodies [41]. This construct is currently being tested as a vaccine immunogen.
The bNAb 2G12, which adopts an unusual domain-exchanged structure to recognize a conserved cluster of oligomannose residues on the OD of gp120, has provided a basis for the design of immunogens to target the HIV-1 glycan shield. Attempts to generate immunogens based on the 2G12 epitope initially focused on the multivalent display of chemically synthesized oligomannose-containing glycoconjugates [42,43]. 2G12 recognized the glycoconjugates weakly, and they were poorly immunogenic in rabbits and predominately elicited linker-specific antibody responses. The next generation of synthetic glycoconjugates better mimicked the oligomannose cluster that comprises the 2G12 epitope on the HIV-1 trimer and bound 2G12 in the nM affinity range. Some of these approaches, including the construction of oligomannose dendrons [44], Man4 containing neoglycoconjugates [45], and cyclic glycopeptides [46], successfully induced anti-carbohydrate antibody responses, but to date these responses have failed to cross-react with gp120 or neutralize HIV-1. A second approach to the design of immunogens for the elicitation of 2G12-like antibodies has been to identify heterologous glycoproteins that express carbohydrates structures that mimic the clustered high mannose glycans on the HIV-1 trimer [47,48]. Recently, Luallen et al. engineered a S. cerevisiae triple mutant that exclusively produced homogenous high mannose glycans [49•]. Since 2G12 efficiently bound to the triple mutant, but not wild-type S. cerevisiae, whole yeast cells were used in preliminary immunization studies. Although the triple mutant-immunized rabbit sera cross-reacted with a diverse range of HIV-1 Env proteins in a glycan-specific manner, the sera failed to neutralize the corresponding HIV-1 isolates. These results suggest that the glycan epitopes recognized by these antibodies differ from that of 2G12, and/or that the titer of 2G12-like antibodies was too low to observe potent neutralization activity.
The bNAbs 2F5, 4E10, and Z13e1 bind to a conserved tryptophan rich region on gp41 referred to as the membrane-proximal external region (MPER), and this region has attracted considerable interest as a vaccine target. This interest is enhanced by the recent demonstration that both 2F5 and 4E10 can protect against mucosal SHIV challenge [4•]. Of note, some reports suggest that 4E10, and controversially 2F5, cross-react with lipids, and it has been proposed that these types of antibodies may be difficult to elicit by vaccination due to B cell tolerance mechanisms [50,51]. The crystal structures of 2F5, 4E10, and Z13e1 bound to their cognate peptides reveal that 2F5 recognizes an extended loop structure, 4E10 recognizes a helical conformation, and Z13e1 binds to an elbow in the MPER [52,53,54•]. These structural studies, as well as complementary biochemical studies [55,56••,57], also suggest that the viral membrane may play a role in formation of the 2F5 and 4E10 epitopes. Notably, recent studies illustrate the importance of hydrophobic residues at the tip of the 4E10 CDRH3 loop for interaction with the viral membrane and potent neutralization activity [57,58,59]. The crystal structure data has been used to rationally design constrained peptides that mimic the conformations recognized by 2F5 and 4E10 [60,61] and/or to present the 2F5 and 4E10 peptides in the context of a lipid membrane [62,63,64]. However, none of these immunogens have to date elicited 4E10 or 2F5-like antibodies.
Recently, two new broad and potent NAbs, PG9 and PG16, were isolated from a clade A infected donor using a high-throughput functional screening approach [15••,65]. These somatically related antibodies bind to conserved residues in the V1/V2 and V3 loops of gp120 and their epitopes are preferentially expressed on trimeric HIV-1 Env. Both antibodies neutralize a diverse range of HIV-1 isolates at concentrations (sub-µg/ml range) about 10- to 100-fold lower than the previously identified bNAbs. Such concentrations might readily be achieved through vaccination. Vaccination strategies are currently being explored to focus the immune response on conserved regions of the variable loops in the context of the trimeric spike. The identification of potent trimer-specific bNAbs again underscores the limitations of monomeric gp120 as an immunogen and emphasizes the importance of generating trimers that closely resemble the functional spike.
Virus-like particle based immunogens
Although most successful anti-viral vaccines have relied on the use of live-attenuated viruses [66], they are not currently considered safe for HIV-1 vaccine development due to the risk of mutation and reversion to a pathogenic form [67,68]. As an alternative, researchers have turned their attention to the use of virus-like particles (VLPs). VLPs resemble infectious virions but are non-pathogenic because they lack a viral genome. Since viruses display multivalent structures, VLPs are usually highly immunogenic and induce antibody responses in the absence of adjuvant. Another attraction of VLP-based immunogens is the presence of native trimers on the VLP surface. However, due to the instability of the HIV-1 spike, non-functional forms of Env are also expressed on the surface of VLPs [69]. Indeed, in a comparative immunogenicity study of VLPs bearing various forms of HIV-1 Env, the binding activity of the VLP immunized sera was primarily focused on non-functional forms of Env [70]. Furthermore, there are relatively few native spikes on the surface of HIV-1, which is likely to reduce the elicitation of antibodies against native structures. Other complicating factors, such as the induction of antibodies against cellular proteins and the elicitation of strain-specific NAbs, also contribute to the limited success of VLPs as immunogens. Although a number of strategies to overcome these hurdles have been employed, including pseudotyping HIV-1 with heterologous envelopes [71,72], truncating the cytoplasmic tail of gp41 to increase Env expression [73], and generating VLPs with cleavage-defective or disulfide-shackled Env to prevent gp120-gp41 dissociation [70], so far none of these approaches have induced potent heterologous antibody responses in non-human primate models.
Prime-boost strategies
DNA vaccines are typically poorly immunogenic in non-human primates and humans; multiple immunizations are required to elicit even moderate titers of NAbs and these titers rise and fall with successive immunizations. On the other hand, results from numerous studies suggest that DNA priming followed by Env protein boosting induces NAbs with increased titer and persistence relative to either approach alone. In proof-of-concept studies, DNA priming and Env protein boosting induced high titers of NAbs that correlated with protection against homologous SHIV challenge in rhesus macaques [8,74,75]. In a recent Phase I clinical trial, polyvalent Env antigens were delivered to healthy human volunteers in a DNA prime-boost approach [76]. Despite the weak neutralizing activity in the sera of the vaccinated individuals, a single Env protein boost elicited higher titers of anti-Env antibodies than has been previously achieved with multiple immunizations of recombinant Env proteins alone [76,77]. Thus, although further modifications of the vaccine formulation may be required to elicit NAbs with increased breadth and potency, the results of this study demonstrate the potential of the DNA prime-protein boost strategy for vaccine development. Indeed, the DNA prime-boost approach is currently being employed for many ongoing and planned Phase I and II clinical trials.
DNA priming followed by viral vector boosting has also been shown to improve the magnitude of the NAb response. For example, results from a non-human primate study demonstrate that a DNA prime-recombinant serotype 5 adenovirus boost strategy elicits higher levels of NAbs than either approach alone [78]. Viral vectors have also been used to prime B cells for higher titer NAb responses after boosting with recombinant Env proteins or heterologous viral vectors. For instance, several studies in human volunteers show that ALVAC priming and Env protein boosting elicits higher NAb titers than vaccination with Env protein alone [79,80,81]. Recently, a Phase III HIV-1 vaccine clinical trial in Thailand (RV 144) tested a heterologous prime-boost regimen using a canary pox-HIV vector (ALVAC-HIV) prime and a recombinant gp120 boost (AIDSVAX B/E) [82••]. To the surprise of many HIV researchers, the vaccine showed modest efficacy in preventing HIV-1 infections. Interestingly, the modest protective effect appeared limited to low-risk individuals, and there was suggestion that the effect was confined to the first year following the commencement of vaccination. Also, in contrast to the results of many protection studies in non-human primate models, vaccinated individuals who became infected did not have lower viral loads or decreased loss of CD4+ T cells compared to the placebos. Efforts are currently focused on evaluating the immune responses induced by the vaccine to establish potential correlates of protection.
Genetic approaches
The difficulties faced in eliciting broad and potent NAbs using the approaches described above have led researchers to develop innovative genetic strategies that essentially bypass immunization. In a proof-of-concept study, Lewis et al. used an adeno-associated virus (AAV) vector to deliver the IgG1 b12 gene into mouse muscle and discovered that the antibody molecules imparted neutralization activity in the sera for over 6 months [83]. Encouraged by this result, this approach was then tested in a non-human primate model by delivering neutralizing immunoadhesins (antigen-binding variable fragment domains of Fabs fused to the Fc fragment of a rhesus IgG2 molecule) into macaques [84••]. The immunoadhesins were expressed in the macaque muscle myofibers, and serum neutralization activity was sustained for over 1 year. More importantly, sterilizing protection against SIV challenge was achieved in six out of nine immunized monkeys, and all nine monkeys were protected from AIDS. In another genetic approach, lentiviral vectors were used to engineer human hematopoietic stem cells to produce IgG1 b12 after in vitro maturation into B cells [85••]. This study substantiates that human B cells can be “programmed” to secrete antibodies of pre-defined specificity in a tissue culture system.
Conclusion
Traditional vaccination approaches have thus far failed to elicit NAb responses against HIV-1 of sufficient breadth and potency, and therefore the field has turned to alternative, particularly rational structure-based, vaccine design strategies. Although these approaches have provided insight into the link between Env antigenicity and immunogenicity, immunogens that focus the antibody responses to conserved epitopes still remain elusive. However, recent technological and scientific advances have reignited the field, and hopes for an antibody-based vaccine are notably higher than in previous years.
Acknowledgments
We thank William Schief for providing us with the model used in Figure 2. We also thank Christina Corbaci for artwork in Figure 2 and Samantha Arnett, Katie Doores, Rich Wyatt, and Wayne Koff for helpful comments on the manuscript. Our laboratory is supported by funds from NIAID, the International AIDS Vaccine Initiative, and the Ragon Institute of MGH, MIT, and Harvard.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Hum Vaccin. 2008;4:316–319. doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. This study shows that the bNAb 2G12 is unusually efficient in protection relative to its neutralizing ability, suggesting that the glycan shield may have advantages as vaccine target.
- 4. Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, et al. Broadly Neutralizing Monoclonal Antibodies 2F5 and 4E10, Directed Against the Human Immunodeficiency Virus Type 1 (HIV-1) gp41 Membrane Proximal External Region (MPER), Protect Against SHIVBa-L Mucosal Challenge. J Virol. 2009 doi: 10.1128/JVI.01272-09. This study definitively shows that the bNAbs 2F5 and 4E10 can provide complete protection against mucosal SHIV challenge, supporting immunogen design based on the MPER.
- 5.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 7.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogers WM, Davis D, Baak I, Kan E, Hofman S, Sun Y, Mortier D, Lian Y, Oostermeijer H, Fagrouch Z, et al. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology. 2008;382:217–225. doi: 10.1016/j.virol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, Ferrai MG, Weiss DE, Letvin NL, Montefiori D, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS. 2008;22:339–348. doi: 10.1097/QAD.0b013e3282f3ca57. The results of this study demonstate that vaccine-induced NAb responses can provide protection against homologous SHIV challenge.
- 10.Pal R, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, Hudacik L, Rose N, Mboudjeka I, Shen S, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348:341–353. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 12.Baldridge JR, Buchmeier MJ. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J Virol. 1992;66:4252–4257. doi: 10.1128/jvi.66.7.4252-4257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 14.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 15. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. In this study, high throughput direct screening for neutralization of about 30,000 B cell clones was used to isolate two new bNAbs with outstanding potency. The epitopes recognized by these antibodies were mapped to conserved regions of variable loops in the context of the trimeric spike.
- 16. Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. This study demonstrate that lower titers of antibody than originally considered obligatory for protection are effective in a low dose repeated macaque challenge model that may better mimic a sizable proportion of human heterosexual exposures to HIV-1.
- 17.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore JP, Cao Y, Qing L, Sattentau QJ, Pyati J, Koduri R, Robinson J, Barbas CF, 3rd, Burton DR, Ho DD. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, Clements ML, Dolin R, Graham BS, Gorse GJ, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 20.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 21.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 22.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 23.Phogat S, Wyatt R. Rational modifications of HIV-1 envelope glycoproteins for immunogen design. Curr Pharm Des. 2007;13:213–227. doi: 10.2174/138161207779313632. [DOI] [PubMed] [Google Scholar]
- 24.Kothe DL, Decker JM, Li Y, Weng Z, Bibollet-Ruche F, Zammit KP, Salazar MG, Chen Y, Salazar-Gonzalez JF, Moldoveanu Z, et al. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology. 2007;360:218–234. doi: 10.1016/j.virol.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang YK, Andjelic S, Binley JM, Crooks ET, Franti M, Iyer SP, Donovan GP, Dey AK, Zhu P, Roux KH, et al. Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine. 2009;27:5120–5132. doi: 10.1016/j.vaccine.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundner C, Li Y, Louder M, Mascola J, Yang X, Sodroski J, Wyatt R. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology. 2005;331:33–46. doi: 10.1016/j.virol.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Beddows S, Schulke N, Kirschner M, Barnes K, Franti M, Michael E, Ketas T, Sanders RW, Maddon PJ, Olson WC, et al. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2005;79:8812–8827. doi: 10.1128/JVI.79.14.8812-8827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455:109–113. doi: 10.1038/nature07159. Using cryo-electron tomography, the authors report the three-dimensional structures of native HIV-1 trimers in the unliganded state, in complex with the bNAb b12, and in a ternary complex with CD4 and the CD4i antibody 17b.
- 29.Zhu P, Liu J, Bess J, Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 31.Zanetti G, Briggs JA, Grunewald K, Sattentau QJ, Fuller SD. Cryo-electron tomographic structure of an immunodeficiency virus envelope complex in situ. PLoS Pathog. 2006;2:e83. doi: 10.1371/journal.ppat.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin G, Nara PL. Designing immunogens to elicit broadly neutralizing antibodies to the HIV-1 envelope glycoprotein. Curr HIV Res. 2007;5:514–541. doi: 10.2174/157016207782418489. [DOI] [PubMed] [Google Scholar]
- 33.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, et al. Analysis of Memory B Cell Responses and Isolation of Novel Monoclonal Antibodies with Neutralizing Breadth from HIV-1-Infected Individuals. PLoS ONE. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantophlet R, Wilson IA, Burton DR. Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Protein Eng Des Sel. 2004;17:749–758. doi: 10.1093/protein/gzh085. [DOI] [PubMed] [Google Scholar]
- 35.Pantophlet R, Wilson IA, Burton DR. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J Virol. 2003;77:5889–5901. doi: 10.1128/JVI.77.10.5889-5901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantophlet R, Ollmann Saphire E, Poignard P, Parren PW, Wilson IA, Burton DR. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol. 2003;77:642–658. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvarajah S, Puffer B, Pantophlet R, Law M, Doms RW, Burton DR. Comparing antigenicity and immunogenicity of engineered gp120. J Virol. 2005;79:12148–12163. doi: 10.1128/JVI.79.19.12148-12163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen L, Do Kwon Y, Zhou T, Wu X, O'Dell S, Cavacini L, Hessell AJ, Pancera M, Tang M, Xu L, et al. Structural Basis of Immune Evasion at the Site of CD4 Attachment on HIV-1 gp120. Science. 2009;326:1123–1127. doi: 10.1126/science.1175868. This study demonstrates that non-neutralizing CD4bs-directed antibodies exhibit only slight differences in gp120 recognition relative to the broadly neutralizing CD4bs-directed antibody b12
- 39.Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Tomov V, Kurteva S, Wang L, Ren X, Gorny MK, Zolla-Pazner S, Sodroski J. Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1. J Virol. 2004;78:12975–12986. doi: 10.1128/JVI.78.23.12975-12986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L, Zhou T, Yang ZY, Svehla K, O'Dell S, Louder MK, Xu L, Mascola JR, Burton DR, Hoxie JA, et al. Enhanced exposure of the CD4-binding site to neutralizing antibodies by structural design of a membrane-anchored human immunodeficiency virus type 1 gp120 domain. J Virol. 2009;83:5077–5086. doi: 10.1128/JVI.02600-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LX. Toward oligosaccharide- and glycopeptide-based HIV vaccines. Curr Opin Drug Discov Devel. 2006;9:194–206. [PubMed] [Google Scholar]
- 43.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 44.Wang SK, Liang PH, Astronomo RD, Hsu TL, Hsieh SL, Burton DR, Wong CH. Targeting the carbohydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc Natl Acad Sci U S A. 2008;105:3690–3695. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astronomo RD, Lee HK, Scanlan CN, Pantophlet R, Huang CY, Wilson IA, Blixt O, Dwek RA, Wong CH, Burton DR. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol. 2008;82:6359–6368. doi: 10.1128/JVI.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyce JG, Krauss IJ, Song HC, Opalka DW, Grimm KM, Nahas DD, Esser MT, Hrin R, Feng M, Dudkin VY, et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc Natl Acad Sci U S A. 2008;105:15684–15689. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luallen RJ, Fu H, Agrawal-Gamse C, Mboudjeka I, Huang W, Lee FH, Wang LX, Doms RW, Geng Y. A yeast glycoprotein shows high-affinity binding to the broadly neutralizing human immunodeficiency virus antibody 2G12 and inhibits gp120 interactions with 2G12 and DC-SIGN. J Virol. 2009;83:4861–4870. doi: 10.1128/JVI.02537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunlop DC, Ulrich A, Appelmelk BJ, Burton DR, Dwek RA, Zitzmann N, Scanlan CN. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS. 2008;22:2214–2217. doi: 10.1097/QAD.0b013e328314b5df. [DOI] [PubMed] [Google Scholar]
- 49. Luallen RJ, Lin J, Fu H, Cai KK, Agrawal C, Mboudjeka I, Lee FH, Montefiori D, Smith DF, Doms RW, et al. An engineered Saccharomyces cerevisiae strain binds the broadly neutralizing human immunodeficiency virus type 1 antibody 2G12 and elicits mannose-specific gp120-binding antibodies. J Virol. 2008;82:6447–6457. doi: 10.1128/JVI.00412-08. This study demonstrates that modified yeast proteins may serve as molecular scaffolds that recapitulate important features of the glycan shield of HIV-1 Env.
- 50.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum Antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 51.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pejchal R, Gach JS, Brunel FM, Cardoso RM, Stanfield RL, Dawson PE, Burton DR, Zwick MB, Wilson IA. A conformational switch in human immunodeficiency virus gp41 revealed by the structures of overlapping epitopes recognized by neutralizing antibodies. J Virol. 2009;83:8451–8462. doi: 10.1128/JVI.00685-09. This study reports the crystal structure of the affinity enhanced bNAb Z13e1 in complex with a peptide corresponding to the core epitope. The results demonstrate that neutralization can occur by the recognition of different conformations and faces of the MPER.
- 55.Lorizate M, Cruz A, Huarte N, Kunert R, Perez-Gil J, Nieva JL. Recognition and blocking of HIV-1 gp41 pre-transmembrane sequence by monoclonal 4E10 antibody in a Raft-like membrane environment. J Biol Chem. 2006;281:39598–39606. doi: 10.1074/jbc.M605998200. [DOI] [PubMed] [Google Scholar]
- 56. Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, Qiao Z, Wang JH, Wagner G, Reinherz EL. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28:52–63. doi: 10.1016/j.immuni.2007.11.018. Using a combination nuclear magnetic resonance, electron paramagnetic resonance, and surface plasmon resonance techniques, the authors demonstrate how a membrane-embedded epitope can be recognized by an antibody.
- 57.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, et al. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Song L, Kim M, Holmes MA, Kraft Z, Sellhorn G, Reinherz EL, Stamatatos L, Strong RK. Interactions Between Lipids And Human Anti-HIV Antibody 4E10 Can Be Reduced Without Ablating Neutralizing Activity. J Virol. 2009 doi: 10.1128/JVI.02113-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scherer EM, Leaman DP, Zwick MB, McMichael AJ, Burton DR. Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0909680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGaughey GB, Citron M, Danzeisen RC, Freidinger RM, Garsky VM, Hurni WM, Joyce JG, Liang X, Miller M, Shiver J, et al. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry. 2003;42:3214–3223. doi: 10.1021/bi026952u. [DOI] [PubMed] [Google Scholar]
- 61.Joyce JG, Hurni WM, Bogusky MJ, Garsky VM, Liang X, Citron MP, Danzeisen RC, Miller MD, Shiver JW, Keller PM. Enhancement of alpha -helicity in the HIV-1 inhibitory peptide DP178 leads to an increased affinity for human monoclonal antibody 2F5 but does not elicit neutralizing responses in vitro. Implications for vaccine design. J Biol Chem. 2002;277:45811–45820. doi: 10.1074/jbc.M205862200. [DOI] [PubMed] [Google Scholar]
- 62.Phogat S, Svehla K, Tang M, Spadaccini A, Muller J, Mascola J, Berkower I, Wyatt R. Analysis of the human immunodeficiency virus type 1 gp41 membrane proximal external region arrayed on hepatitis B surface antigen particles. Virology. 2008;373:72–84. doi: 10.1016/j.virol.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson DS, Szoka FC., Jr Role of lipid structure in the humoral immune response in mice to covalent lipid-peptides from the membrane proximal region of HIV-1 gp41. Vaccine. 2009;27:4672–4683. doi: 10.1016/j.vaccine.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim M, Qiao Z, Yu J, Montefiori D, Reinherz EL. Immunogenicity of recombinant human immunodeficiency virus type 1-like particles expressing gp41 derivatives in a pre-fusion state. Vaccine. 2007;25:5102–5114. doi: 10.1016/j.vaccine.2006.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwong PD, Mascola JR, Nabel GJ. Mining the B cell repertoire for broadly neutralizing monoclonal antibodies to HIV-1. Cell Host Microbe. 2009;6:292–294. doi: 10.1016/j.chom.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11:S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds MR, Weiler AM, Weisgrau KL, Piaskowski SM, Furlott JR, Weinfurter JT, Kaizu M, Soma T, Leon EJ, MacNair C, et al. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med. 2008;205:2537–2550. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Learmont JC, Geczy AF, Mills J, Ashton LJ, Raynes-Greenow CH, Garsia RJ, Dyer WB, McIntyre L, Oelrichs RB, Rhodes DI, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340:1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 69.Poignard P, Moulard M, Golez E, Vivona V, Franti M, Venturini S, Wang M, Parren PW, Burton DR. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J Virol. 2003;77:353–365. doi: 10.1128/JVI.77.1.353-365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crooks ET, Moore PL, Franti M, Cayanan CS, Zhu P, Jiang P, de Vries RP, Wiley C, Zharkikh I, Schulke N, et al. A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120. Virology. 2007;366:245–262. doi: 10.1016/j.virol.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuate S, Stahl-Hennig C, Stoiber H, Nchinda G, Floto A, Franz M, Sauermann U, Bredl S, Deml L, Ignatius R, et al. Immunogenicity and efficacy of immunodeficiency virus-like particles pseudotyped with the G protein of vesicular stomatitis virus. Virology. 2006;351:133–144. doi: 10.1016/j.virol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Marsac D, Loirat D, Petit C, Schwartz O, Michel ML. Enhanced presentation of major histocompatibility complex class I-restricted human immunodeficiency virus type 1 (HIV-1) Gag-specific epitopes after DNA immunization with vectors coding for vesicular stomatitis virus glycoprotein-pseudotyped HIV-1 Gag particles. J Virol. 2002;76:7544–7553. doi: 10.1128/JVI.76.15.7544-7553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poon B, Hsu JF, Gudeman V, Chen IS, Grovit-Ferbas K. Formaldehyde-treated, heat-inactivated virions with increased human immunodeficiency virus type 1 env can be used to induce high-titer neutralizing antibody responses. J Virol. 2005;79:10210–10217. doi: 10.1128/JVI.79.16.10210-10217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu SL, Abrams K, Barber GN, Moran P, Zarling JM, Langlois AJ, Kuller L, Morton WR, Benveniste RE. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 75.Pal R, Wang S, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, Hudacik L, Rose N, Cristillo A, et al. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J Med Primatol. 2005;34:226–236. doi: 10.1111/j.1600-0684.2005.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JH, Pitisuttithum P, Kamboonruang C, Chuenchitra T, Mascola J, Frankel SS, DeSouza MS, Polonis V, McLinden R, Sambor A, et al. Specific antibody responses to vaccination with bivalent CM235/SF2 gp120: detection of homologous and heterologous neutralizing antibody to subtype E (CRF01.AE) HIV type 1. AIDS Res Hum Retroviruses. 2003;19:807–816. doi: 10.1089/088922203769232601. [DOI] [PubMed] [Google Scholar]
- 78.Mascola JR, Sambor A, Beaudry K, Santra S, Welcher B, Louder MK, Vancott TC, Huang Y, Chakrabarti BK, Kong WP, et al. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J Virol. 2005;79:771–779. doi: 10.1128/JVI.79.2.771-779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta K, Hudgens M, Corey L, McElrath MJ, Weinhold K, Montefiori DC, Gorse GJ, Frey SE, Keefer MC, Evans TG, et al. Safety and immunogenicity of a high-titered canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS Vaccine Evaluation Group Protocol 022A. J Acquir Immune Defic Syndr. 2002;29:254–261. doi: 10.1097/00126334-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 80.Belshe RB, Stevens C, Gorse GJ, Buchbinder S, Weinhold K, Sheppard H, Stablein D, Self S, McNamara J, Frey S, et al. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus Type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J Infect Dis. 2001;183:1343–1352. doi: 10.1086/319863. [DOI] [PubMed] [Google Scholar]
- 81.Pialoux G, Excler JL, Riviere Y, Gonzalez-Canali G, Feuillie V, Coulaud P, Gluckman JC, Matthews TJ, Meignier B, Kieny MP, et al. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI). The AGIS Group, and l'Agence Nationale de Recherche sur le SIDA. AIDS Res Hum Retroviruses. 1995;11:373–381. doi: 10.1089/aid.1995.11.373. [DOI] [PubMed] [Google Scholar]
- 82. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. This study represents the first HIV-1 clinical trial to show even a modest protective effect against HIV-1 acquisition in humans.
- 83.Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 2002;76:8769–8775. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–906. doi: 10.1038/nm.1967. This study demonstrates that AAV gene transfer technology can be used to bypass immunization and provide continuous levels of NAbs able to protect against SIV challenge.
- 85. Luo XM, Maarschalk E, O'Connell RM, Wang P, Yang L, Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113:1422–1431. doi: 10.1182/blood-2008-09-177139. The results of this study suggest the possilibity that an HIV-1 vaccine might be delivered by in vitro "programming" of human B cells to produce a continuous supply of bNAbs.
- 86.Schief WR, Ban YE, Stamatatos L. Challenges for structure-based HIV vaccine design. Curr Opin HIV AIDS. 2009;4:431–440. doi: 10.1097/COH.0b013e32832e6184. [DOI] [PubMed] [Google Scholar]