Abstract
Aim
To create a Coronary Heart Disease (CHD) risk prediction model specific to type 1 diabetes.
Methods
Development of the model used data from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). EDC subjects had type 1 diabetes diagnosed between 1950 and 1980, received their first study exam between 1986 and 1988, and have been followed biennially since. The final cohort for model development consisted of 603 subjects and 46 incident events. Hard CHD was defined as CHD death, fatal/non-fatal MI or Q-waves. Baseline CHD risk factors were tested bivariately and introduced into a Weibull model. The prediction model was externally validated in the EURODIAB Prospective Complications Study.
Results
In males, predictors were higher white blood cell count, micro or macroalbuminuira, lower HDLc and longer diabetes duration. In females, larger waist/hip ratio, higher non-HDLc, higher systolic blood pressure, use of blood pressure medication, and longer diabetes duration were included. Models were robust to internal and external validation procedures.
Conclusions
CHD risk prediction models for hard CHD in those with type 1 diabetes should include risk factors not considered by existing models. Using models specifically developed for predicting CHD in type 1 diabetes may allow for more targeted prevention strategies.
Introduction
Cardiovascular disease is the leading cause of death in people with diabetes [1]. People with diabetes have a two to four fold increased risk of death due to coronary heart disease (CHD) compared to those without diabetes [1]. People with type 1 diabetes may experience as much as a 10 fold increased risk [2, 3]. Since people with type 1 diabetes are diagnosed at a younger age compared to those with type 2 diabetes, this group experiences significantly larger effects on overall life expectancy and quality of life.
Because of the magnitude of CHD in type 1 diabetes, the ability to predict the likelihood of experiencing a CHD event could prove beneficial for health education and risk factor treatment. Further, risk factor control is inadequate in type 1 diabetes [4], and increased awareness of the consequences of these risk factors in both patients and providers may be needed to improve control. One way to increase awareness is through the use of CHD prediction equations. While such equations exist, they were developed in general, nondiabetic populations [5] or in populations with only type 2 diabetes [6]. To the best of our knowledge, there are no CHD risk prediction models for type 1 diabetes. In previous work, we applied the Framingham [5] and UKPDS [6] risk equations in a type 1 diabetes cohort. These equations did not accurately predict risk, and showed a significant underestimation of events [7]. Risk factors that are important in predicting CHD in type 1 diabetes including renal disease, waist-hip ratio and inflammatory markers [8] are not taken into account in these existing models, likely accounting for their underestimation of events.
Given the shortcomings of existing prediction models, the accurate prediction of CHD outcomes in type 1 diabetes requires the development of a risk prediction tool that can account for the unique risk factors known to be important in those with type 1 diabetes. Our objective, therefore, was to develop a CHD prediction model for type 1 diabetes using data from an epidemiologically representative cohort, and to test the accuracy and generalizability of the developed model by applying it to another type 1 diabetes population.
Research Designs and Methods
Study Population
These analyses used data from the Epidemiology of Diabetes Complications Study (EDC), which includes subjects with childhood onset type 1 diabetes diagnosed between 1950 and 1980 before the age of 17. This is a prospective cohort study where all subjects were seen within one year of diagnosis at Children's Hospital of Pittsburgh. Although this population is clinic based, it has been shown to be epidemiologically representative of all type 1 diabetes cases in Allegheny County, Pennsylvania [9]. The 658 subjects participating in baseline exams have been followed biennially since 1986. Data included in these analyses included baseline risk factors (1986-1988) and outcome data (through 2001). Removed from these analyses were those that had prevalent CHD prior to study entry (n=52), and those whose CHD history was unknown (n=3). The final dataset consisted of 603 subjects and 46 incident hard CHD events (26 males and 20 females).
Baseline Measurements
This database includes numerous potential risk factors for complications in type 1 diabetes. The variables used to develop the models were collected at the baseline visit (1986-1988) and included, but were not limited to, the following: age at study entry, age at onset of diabetes, diabetes duration, current and past smoking status, Beck Depression Inventory (BDI) [10], waist hip ratio (WHR), non-HDLc (total cholesterol-HDLc), HDLc, LDLc (calculated from the Friedewald equation) [11], HbA1, white blood cell count, fibrinogen, estimated glucose disposal rate (eGDR) determined by a regression equation derived from hyperinsulinemic-euglycemic clamp studies [12], diastolic and systolic blood pressure measured after a five minute rest according to the Hypertension Detection and Follow-up Program [13], current use of antihypertensive or antihyperlipidemic medications, and presence of renal disease determined by the albumin excretion rate, >20 μg/min on 2 of 3 timed urines, renal failure or renal transplant.
CHD definitions
A hard CHD event was defined as an incident (first) non fatal MI that was confirmed by medical records or Q-waves on ECG's according to Minnesota codes 1.1 or 1.2 [14] and fatal CHD after review of death certificates, hospital records and autopsy reports as appropriate, and classified according to standard protocol of the Diabetes Epidemiology Research International [15].
Validation Dataset
The model was externally validated using the EURODIAB Prospective Complications Study (PCS) population. The EURODIAB PCS is a hospital based multi-center study designed to examine risk factors for micro and macrovascular complications in type 1 diabetes subjects in 31 centers from 16 countries across Europe [16]. Baseline exams took place in 1989-91 on 3250 subjects diagnosed with type 1 diabetes before age 36 who were on continuous insulin treatment within one year of diagnosis. Follow-up exams were conducted on 2328 participants 6-8 years later. Of these participants, 53 had incident hard CHD events at follow-up. Reasons for loss to follow-up were because four centers did not participate (n=437), participants and prevalent CHD at baseline (n=222), 8 participants did not meet inclusion criteria at baseline, and CHD was not measured in 14 participants. A more atherogenic risk factor profile was found in those who were lost to follow-up, after adjusting for age. Those who dropped out were older, had longer duration of diabetes, worse glycemic control, abnormal lipid levels, higher BP and more microvascular complications [17].
Statistical Analyses
Model development
First, descriptive statistics including means and frequencies were examined. Those variables exhibiting a non-normal distribution were appropriately transformed. Those variables having a p-value < 0.10 in the univariate models were considered for multivariate modeling. All models were adjusted for diabetes duration. Initial Cox models were found to violate the assumption of constant hazards using the Lagrange Multiplier Chi Square test. Therefore, the final multivariate models were estimated using a Weibull accelerated failure time model [18]. Since the subjects were not newly diagnosed with type 1 diabetes and diabetes duration is a known risk factor for CHD [19], duration of diabetes prior to entry into study was included in all models. When blood pressure was entered, the model was also adjusted for antihypertensive (Yes/No) use. After the most parsimonious multivariate model was selected, the survivor function was used to calculate yearly survival rates.
Internal Validation
Internal validity (reproducibility) was performed using Akaike's Information Criteria (AIC). This version of cross-validation was used at each step of the model building process. Additional reproducibility testing was performed with bootstrapping using STATA 8.0 [20]. There was concern about over fitting the model due to the small sample size. Bootstrapping with the extended information criterion can overcome the AIC's over fitting problems and was used in our modeling process. While both bootstrapping and shrinkage were carried out, we did not use the coefficients produced from the shrinkage calculations as it did not improve the validity of the model in the external independent validation sample (EURODIAB). The calibration and discrimination of the model was tested using Hosmer and Lemeshow's goodness of fit and c-statistics respectively.
External Validation
In the EURODIAB PCS (external validation dataset) there were no comparable inflammatory markers to EDC. Further, WBC was not measured in EURODIAB, therefore WBC was imputed using the following linear regression equation WBC= -.446 + .948*(presence of micro or macroalbumuira) + 7.319*(waist/hip ratio). This linear regression equation was derived from the variables significantly associated with WBC in the EDC dataset. The EURODIAB cohort was followed for eight years; therefore follow-up for the EDC cohort was limited to a maximum of eight years for the purposes of the external validation study.
All analyses were performed using SAS version 9 and STATA version 8. This study was approved by the University of Pittsburgh Institutional Review Board. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Six-hundred three subjects with type 1 diabetes were included in these analyses. Baseline characteristics of the EDC population are shown in table 1 and 2 for males and females. Initial steps in building the model involved examining associations between baseline characteristics and hard CHD outcomes (MI/CAD death, Q-waves). Age and duration are highly correlated in this cohort (>0.80), therefore only diabetes duration was forced into all models. All models were stratified by gender. In males standard cardiovascular risk factors (elevated LDLc, nonHDLc, AER, fibrinogen, SBP, WHR, and WBC) were associated with increased risk of CHD. Results were similar in females with the exception of BDI, where higher BDI was significantly associated univariately. The average HbA1 in males was 10.4% and in females 10.3%. HbA1 did not enter the model for either males or females.
Table 1.
Univariate Analysis of Baseline variables for Females (N=299) in the EDC study 1986-1988
| Variable Name | Mean [sd] or n [%] | Parameter Estimate | P-Value |
|---|---|---|---|
| LDLc (mg/dl) | 112.6 [30.9] | -2.11 | 0.008 |
| HDLc (mg/dl) | 58.5 [12.9] | 1.91 | 0.040 |
| Systolic Blood Pressure (mmHg)* | 109.4 [13.7] | -0.04 | < 0.001 |
| Total Chol/HDL | 3.4 [1.1] | -2.44 | < 0.001 |
| Beck Despression Index | 8.2 [6.4] | -0.05 | 0.040 |
| Currently smoking (%yes)* | 60 [20.6] | -0.44 | 0.230 |
| Renal Disease (%yes) | 121 [40.5] | -0.81 | 0.040 |
| Dur. Of disease prior entry (yrs) | 18.9 [7.5] | -0.91 | 0.002 |
| BMI (kg/m2) | 26.7 [56.6] | 0.00 | 0.890 |
| Antihypertensive (%yes) | 22 [7.6] | -1.30 | 0.004 |
| HbA1 (%) | 10.3 [1.8] | -0.14 | 0.100 |
| Ever smoke (%yes) | 116 [39.5] | -0.82 | 0.030 |
| Fibrinogen (mg/dl) | 300.5 [89.8] | -0.005 | 0.004 |
| Albumin Excretion Rate (μg/min) | 228.1 [572.4] | -0.22 | 0.009 |
| Wasit Hip Ratio | 0.78 [0.1] | -7.57 | 0.008 |
| Age at study entry (yrs) | 27.3 [7.9] | -0.09 | 0.002 |
| Non HDLc (mg/dl) | 133.2 [40.5] | -2.34 | 0.001 |
| WBC | 6.7 [2.0] | -2.14 | 0.001 |
Framingham variables
Table 2.
Univariate Analysis of Baseline variables for Males (N=304) in the EDC study 1986-1988
| Variable Name | Mean [sd] or n [%] | Parameter Estimate | P-Value |
|---|---|---|---|
| LDLc (mg/dl) | 117.1 [36.6] | -1.42 | 0.008 |
| HDLc (mg/dl) | 49.5 [9.8] | 1.97 | 0.008 |
| Systolic Blood Pressure (mmHg)* | 116.3 [15.1] | -0.03 | <0.001 |
| Total Chol/HDL | 3.9 [1.2] | -1.97 | <0.001 |
| Beck Despression Index | 5.7 [5.6] | 0.00 | 0.870 |
| Currently smoking (%yes)* | 68 [23.7] | -0.51 | 0.070 |
| Renal Disease (%yes) | 148 [48.7] | -1.59 | 0.001 |
| Dur. Of disease prior entry (yrs) | 19.1 [7.3] | -0.59 | 0.002 |
| BMI (kg/m2) | 30.0 [79.1] | 0.00 | 0.080 |
| Antihypertensive (%yes) | 30 [10.5] | -0.46 | 0.001 |
| HbA1 (%) | 10.4 [1.8] | -0.09 | 0.160 |
| Ever smoke (%yes) | 137 [46.1] | -0.61 | 0.030 |
| Fibrinogen (mg/dl) | 274.3 [85.9] | 0.00 | 0.001 |
| Albumin Excretion Rate (μg/min) | 385.2 [1023.9] | -0.33 | <0.001 |
| Wasit Hip Ratio | 0.87 [0.1] | -5.20 | 0.020 |
| Age at study entry (yrs) | 27.1 [7.6] | -0.06 | 0.002 |
| Non HDLc (mg/dl) | 138.3 [42.9] | -1.60 | 0.001 |
| WBC | 6.4 [1.8] | -1.65 | 0.002 |
Framingham variables
Using the methods described above, the following prediction models were derived.
Males: X =1.8738 - 0.9452 (log white blood cell count) - 1.0625 (presence of microalbuminuria or greater) + 1.4808 (log HDLc) - 0.2286 (sqrt diabetes duration at baseline)
Females: X =21.62 - 4.4331 (waist/hip ratio) - 1.594(log nonHDLc) - 0.023 (systolic blood pressure) - 0.498 (use of blood pressure medication) - 0.705 (sqrt diabetes duration at baseline).
Following this calculation, the probability of not having an event was calculated using St(t)= exp{ - [tie-βxi]1/σ } where σ=0.6417 for males and σ=0.6215 for females. 1-Si(10)=probability of an event in 10 years.
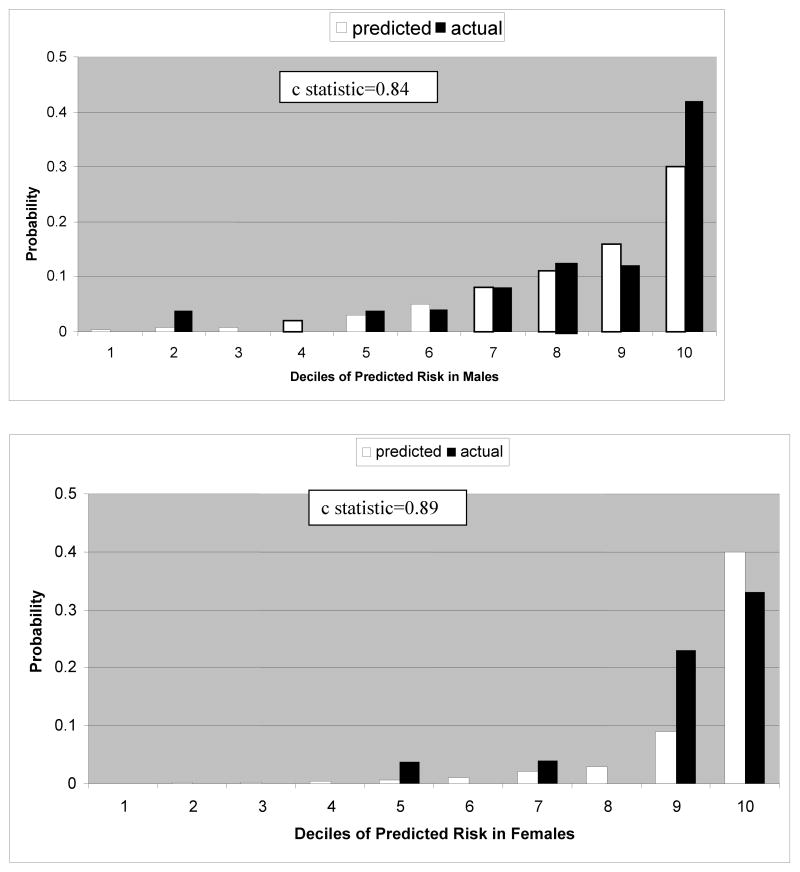
Observed and expected events using the Pittsburgh CHD in Type 1 Diabetes Risk Model are shown for males and females in Figure 1. In males, the observed and expected events are similar until the tenth (highest risk decile) where the model underpredicted events. In females, the model predicted well until the ninth and tenth deciles where the model underpredicted in the ninth decile and overpredicted in the tenth. The c statistics were 0.84 and 0.89, females and males respectively.
Figure 1.
10 year prediction of hard CHD events using the Pittsburgh CHD in Type 1 Diabetes Risk Model
There were differences noted when the model was externally validated in the EURODIAB PCS cohort. Age at onset was significantly younger in EDC 8.0 years vs 16.9 years in females; 8.0 vs 17.9 in males, respectively. Age at the baseline visit was also significantly younger in the EDC population 27.1 vs 31.4 years in females; 27.0 vs 31.7 years in males. Systolic blood pressures were lower in EDC 109.6 mmHg vs 116.6 mmHg in females; and 116.1 mmHg vs 121.7 mmHg in males with a similar proportion of subjects treated for high blood pressure. WHR, HDLc and non HDLc were similar in both cohorts.
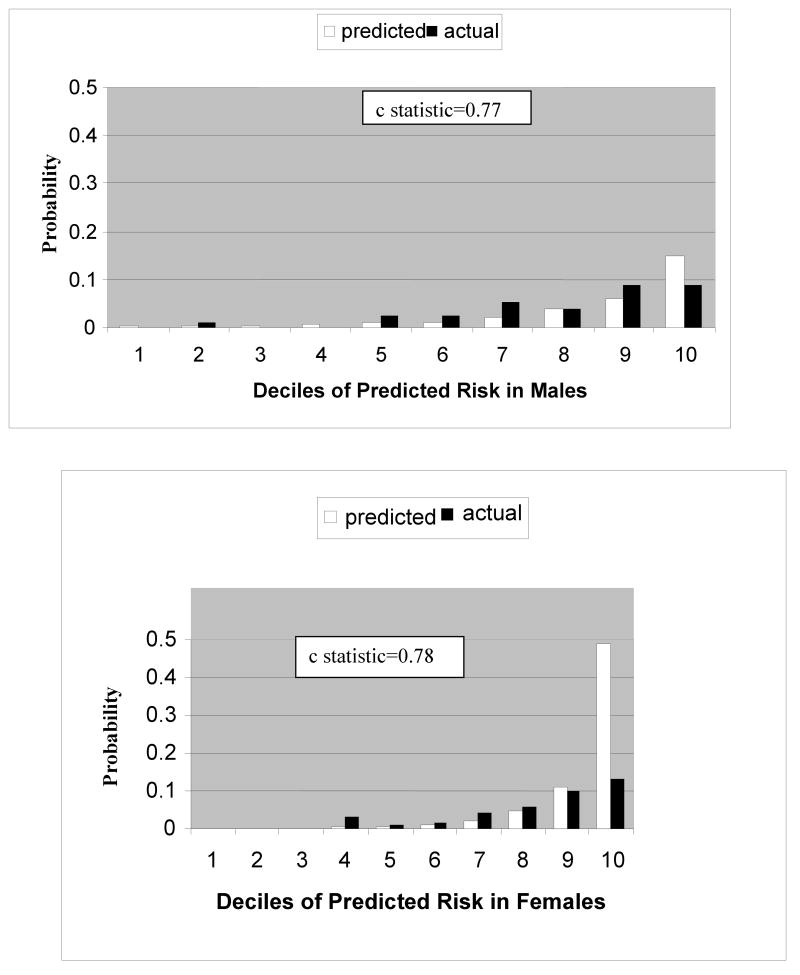
Figure 2 shows the comparison between expected and actual events applying the newly developed type1 diabetes CHD prediction model to data from the EURODIAB Study. The outcome of interest is the same ‘hard’ events (fatal or nonfatal MI, Q-wave, CHD Death) although fatal cases were based solely on death certificate information. In males, the model performed well with a c statistic of 0.77. Events were slightly overpredicted in the tenth decile. The female model also performed well with a c statistic was 0.78, however the model overpredicted events in the tenth decile.
Figure 2.
8 year prediction of hard CHD events in the EURODIAB PCS population using the Pittsburgh CHD in Type 1 Diabetes Risk Model
Discussion
We have developed and validated a CHD prediction model for type 1 diabetes using data from an epidemiologically representative cohort. This is the first study to develop and externally validate a CHD risk prediction model specific to type 1 diabetes. This model is gender specific and contains risk factors not considered in currently existing CHD risk prediction models. In males, baseline risk factors included in the model were higher white blood cell count (WBC), micro or macro albuminuira, lower HDLc and longer diabetes duration. In females, larger waist/hip ratio, higher non-HDLc (total cholesterol – HDLc), higher systolic blood pressure, use of blood pressure medication, and longer diabetes duration were included.
The characteristics of type 1 diabetes necessitate the development of a specific model in order to have accurate estimation of CHD events in type 1 diabetes. Using existing models to predict a CHD event in type 1 diabetes will not only provide inaccurate estimates of risk, but this underestimation may result in less aggressive treatment and therefore poor control of CHD risk factors. The cluster of CHD risk factors identified through these analyses are the plausible explanation for the poor prediction of events using the Framingham and UKPDS Risk Engines previously reported [8]. These models do not consider WHR, WBC, or renal disease. We included these variables in our analyses and found they were predictive of future CHD events. This new prediction model provides a more accurate measure of risk prediction for CHD in type 1 diabetes. The risk factors identified in these models are also easily measured in clinical practice which may enhance their use.
Previous work in this population demonstrated that renal disease is a significant predictor of hard CHD [21] and the Steno group demonstrated many years ago the strong effect of renal disease by showing that those developing renal disease had an 8 fold greater risk of CHD than a matched group who did not develop proteinuria [22]. In females, our final model consisted of WHR, nonHDLc, and systolic blood pressure. Other prediction models for CHD do not include WHR, which is a marker for visceral fat, known to be an important risk factor for CHD. While these women were not obese (mean BMI=26.7 kg/m2), the location of the adipose tissue may be important in predicting their risk. Previous studies in this population also found depression, as measured by the Beck Depression Index, to be an important predictor of CHD events in women [23]. Depression did not remain in the final prediction model demonstrating the relative importance of other risk factors for predicting CHD risk in women.
Neither the male or female prediction model contained a measure of glycemic control. HbA1 was not a significant predictor either univariately or multivariately, even with duration removed. These findings are consistent with the general literature as recently reviewed [24] where only a weak association, if any, between A1C and CHD, was shown [21]. The recent results from the DCCT/EDIC trial provide a contrast to these results where prior intensive glycemic control reduced CHD [25]. It is not clear, however, what degree of improved CHD outcomes is due to better glycemic control or some other mediating mechanism (eg reduced renal disease) [25]. However, those enrolled in the DCCT/EDIC study had shorter diabetes duration had to meet the exclusion criteria of the DCCT trial (no obesity, hypertension or hypercholesterolemia), and achieved a far lower A1C level than seen in the epidemiologic studies. Further, DCCT participants were closely followed during the trial which may not be reflective of health care practices in the community. A full multivariate prediction model for CHD events in DCCT/EDIC has not been reported.
Renal disease (microalbuminuria) in this model was tested as both a dichotomous and continuous variable. Since this model was developed to be used in the clinical setting, the dichotomous variable was chosen to be used as model parameters did not differ between the models.
The characteristics found to be associated with increased CHD risk in this model also have significant clinical implications. Data from diabetes cohorts demonstrate poor adherence with recommended practice guidelines [26]. Further, more aggressive treatment of blood pressure and lipids in this population is critical as a previous analysis reported low levels of treatment and control [27].
While this is the first CHD prediction model for type 1 diabetes, there are limitations to our study. The first limitation was the small number of hard CHD events experienced over the follow-up period in only 46 subjects. However, EDC is one of the only epidemiologically representative type 1 diabetes cohorts with longitudinal data available. The subjects in the EDC cohort received care in the general medical community and thus represent the natural history of care and complications in type 1 diabetes. While the sample size is limited, the EDC cohort is the ideal cohort with which to develop this model. While EDC is primarily Caucasian (15 non whites), these analyses provide new information for the prediction of CHD in type 1 diabetes, but is only generalizable to similar populations. Further, the EDC cohort represents those with longer duration type 1 diabetes, therefore a survivor bias may exist.
An external validation study was conducted using the EURODIAB PCS population in order to examine model performance in a different cohort. The EURODIAB PCS cohort is the large clinic-based type 1 diabetes cohort. Twenty eight percent of participants were lost to follow-up, however for a large European multicenter study, this is not unexpected. Previous analyses showed that individuals who dropped out were more likely to have an atherogenic risk factor profile than participants in the follow up. This could mean that the incidence of CHD may be underestimated for both men and women. The results of our external validation indicated that the model performs well with the exception of women in the highest risk decile and should be used with caution in this group. However, because of this overprediction, we examined the factors in the equation that might be contributing to the higher probability in EURODIAB women. It appears to be driven by the sensitivity of the equation to values of systolic blood pressure independent of treatment. The EURODIAB women had a significantly higher mean blood pressure in the 10th decile compared to the other deciles and to EDC women in the 10th decile. Another limitation is that the model was derived in those with childhood onset type 1 diabetes and may not be generalizable to those diagnosed with type 1 diabetes as an adult. However, the validation of the equations in a cohort with onset up to the age of 35 suggests this limitation is not problematic.
In conclusion, we have developed and externally validated the first CHD prediction model specific to those with type 1 diabetes. This model has significant public health importance given the younger age at CHD incidence and the potentially longer duration of exposure to abnormal CHD risk factors that those with type 1 diabetes experience. Given that the elevated CHD risk is often overlooked given the younger age of these patients, a risk calculator that uses easily measured risk factors may lead to more aggressive treatment and eventual declines in the incidence of CHD in this population. Future research should explore the use of this model in the clinical setting in order to provide both patients and their health care providers with risk information.
Table 3. Baseline Characteristics for Males and Females in the EURODIAB and EDC population.
| MALES | FEMALES | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| EURODIAB | EDC | EURODIAB | EDC | P-Value | |||||
| Variable | N=1126 | % | N=304 | % | N=1202 | % | N=299 | % | |
| Age at study entry | 0.0010a 0.0001b |
||||||||
| Mean (sd) | 32.1 [9.8] | 27.1 [7.6] | 32.2[9.6] | 27.3[7.9] | |||||
| Median | 30.8 | 26.6 | 31.1 | 26.6 | |||||
| Range | 15-60.7 | 8.5-45.5 | 15.2-59.7 | 8.0-48.0 | |||||
| Age at onset of diabetes | 0.0001a 0.0001b |
||||||||
| Mean (sd) | 17.4[8.0] | 8.0{4.2] | 18.2[8.1] | 8.4[3.9] | |||||
| Median | 17.4 | 8.1 | 18.1 | 8.5 | |||||
| Range | 0.3-35.4 | 0.7-15.6 | 0.1-35.6 | 0.3-15.9 | |||||
| Diabetes Duration | 0.0001a 0.0001b |
||||||||
| Mean (sd) | 14.7[9.2] | 19.1 [7.3] | 13.9[8.9] | 18.9[7.5] | |||||
| Median | 13.4 | 18.4 | 12.8 | 17.9 | |||||
| Range | 1.0-46.5 | 7.7-37.4 | 1.0-56.0 | 7.7-35.3 | |||||
| BMI (kg/m2) | 0.031a 0.11b |
||||||||
| Mean (sd) | 23.3[2.9] | 23.6[3.1] | 23.6[2.6] | 23.5[3.4] | |||||
| Median | 23 | 23.7 | 23.5 | 22.8 | |||||
| Range | 16.1-36.3 | 13.9-33.1 | 15.6-34.3 | 15.1-37.6 | |||||
| Waist Hip ratio | 0.0001a 0.0001b |
||||||||
| Mean (sd) | 0.8[0.1 | 0.9[0.05] | 0.9[0.08] | 08[.1] | |||||
| Median | 0.8 | 0.9 | 0.9 | 0.8 | |||||
| Range | 0.5-2.0 | 0.8-1.1 | .6-1.3 | .7-1.0 | |||||
| Total Cholesterol | 0.0001a 0.0003b |
||||||||
| Mean (sd) | 210.8[43.6] | 187.8[42.3] | 200.5[42.9] | 191.7[39.8] | |||||
| Median | 205.7 | 182 | 196.4 | 184 | |||||
| Range | 105.6-478.7 | 111-391 | 89.3-474.9 | 107-371 | |||||
| HDLc | 0.0001a 0.0001b |
||||||||
| Mean (sd) | 63.1 [16.9] | 49.5[9.8] | 53.4[14.8] | 58.5[12.9] | |||||
| Median | 61.5 | 48.7 | 52.2 | 57.2 | |||||
| Range | 16.2-143.1 | 28.1-91.7 | 10.4-155.1 | 26.3-113 | |||||
| LDLc | 0.0001a 0.0001b |
||||||||
| Mean (sd) | 130.4[39.1] | 117.1 [36.6] | 127.4[37.0] | 112.6[30.9] | |||||
| Median | 124.7 | 111.6 | 124.2 | 109 | |||||
| Range | 35.9-375.3 | 47-295.3 | 29.4-278.1 | 34.2-236.4 | |||||
| NonHDLc | 0.0002a 0.0001b |
||||||||
| Mean (sd) | 147.7[44.9] | 138.3[42.9] | 147[43.5] | 133.2[40.5] | |||||
| Median | 140 | 131.2 | 142.1 | 125.5 | |||||
| Range | 5.8-442.8 | 61.0-345.9 | 30.5-414.9 | 51.6-336.1 | |||||
| Total Chol/HDLc | 0.0001a 0.0001b |
||||||||
| Mean (sd) | 3.6[1.4] | 3.9[1.2] | 4.0[1.6] | 3.4[1.1] | |||||
| Median | 3.3 | 3.7 | 3.7 | 3.2 | |||||
| Range | 1.0-20.5 | 2.2-8.9 | 1.3-21.6 | 1.9-10.6 | |||||
| Systolic Bp | 0.26a 0.0001b |
||||||||
| Mean (sd) | 117.5[16.8] | 116.3[15.1] | 122.7[16.4] | 109.4[13.7] | |||||
| Median | 115 | 113.5 | 121 | 107 | |||||
| Range | 61-209 | 76-188 | 78-192 | 77-169 | |||||
| Diastolic Bp | 0.09a 0.0001b |
||||||||
| Mean (sd) | 73.8[11.0] | 74.9[10.9] | 76.1 [11.2] | 70.1 [10.2] | |||||
| Median | 73 | 75 | 75 | 69 | |||||
| Range | 36-108 | 44-118 | 32-112 | 49-114 | |||||
| Fibrinogen | 0.0001a 0.07b |
||||||||
| Mean (sd) | 340[100] | 274.3[85.9] | 305.7[92.4] | 300.5[89.8] | |||||
| Median | 333 | 255 | 297 | 280 | |||||
| Range | 31-770 | 130-700 | 86-822 | 130-680 | |||||
| Albumin excretion rate | 0.0001a 0.0001b |
||||||||
| <20 | 802 | 71.2% | 155 | 51.0% | 780 | 64.9% | 178 | 59.5% | |
| 20-200 | 209 | 18.6% | 78 | 25.7% | 261 | 21.7% | 61 | 20.4% | |
| 200+ | 73 | 6.5% | 68 | 22.4% | 99 | 8.2% | 58 | 19.4% | |
| Antihypertensive medications | 0.16a 0.62b |
||||||||
| Yes | 88 | 7.8% | 30 | 9.9% | 101 | 8.4% | 22 | 7.4% | |
| No | 1033 | 91.7% | 257 | 84.5% | 1093 | 90.9% | 269 | 90.0% | |
EDC vs EURODIAB males
EDC vx EURODIAB females
LDLc=Low Density Lipoprotein
HDLc=High Density Lipoprotein
SBP=Systolic Blood Pressure
DBP=Diastolic Blood Pressure
BMI=Body Mass Index
WBC=White Blood Cell Count
Acknowledgments
This study was funded by National Institutes of Health DK34818, DK070725 and the American Diabetes Association Junior Faculty Award 1-05-JF-59.
The EURODIAB PCS was supported by grants from the Wellcome Trust, the European Community and Diabetes UK.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris MI. Summary. In: National Diabetes Data Group, editor. Diabetes in America. second. Bethesda: National Institutes of Health; 1995. pp. 1–14. [Google Scholar]
- 2.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59 doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 3.Dorman JS, LaPorte RE, Kuller LH, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 4.Zgibor JC, Orchard TJ. Has control of hyperlipidemia and hypertension in patients with type 1 diabetes improved over time? Diabetes. 2001;50:1049, A1255. [Google Scholar]
- 5.Wilson PWF, Castelli WP, Kannel WB. Coronary risk prediction in adults (The Framingham Heart Study) Am J Cardiol. 1987;59:91G–94G. doi: 10.1016/0002-9149(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 6.Stevens RJ, Kothari V, Adler AI, Stratton IM, Holman RR, UKPDS Group The UKPDS risk engine: a model for the risk of coronary heart disease in type 2 diabetes UKPDS 56. Clinical Science. 2001;101:671–679. [PubMed] [Google Scholar]
- 7.Zgibor J, Piatt G, Ruppert K, Orchard T, Roberts M. Deficiencies of cardiovascular risk prediction models for type 1 diabetes. Diabetes Care. 2006;29:1860–1865. doi: 10.2337/dc06-0290. [DOI] [PubMed] [Google Scholar]
- 8.Ruppert K, Roberts MS, Orchard TJ, Zgibor JC. Cardiovascular disease risk prediction in type 1 diabetes: accounting for the differences. Diabetes Research and Clinical Practice. 2007;78:234–237. doi: 10.1016/j.diabres.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Wagener DK, Sacks JM, LaPorte RE, MacGregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus: risk for diabetes among relatives of IDDM. Diabetes. 1982;31:136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Garbin MG. Psychometric properties of the Beck depression inventory: 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipiprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
- 13.Borhani NO, Kass EH, Langford HG, Payne GH, Remmington RD, Stamler J. The Hypertension Detection and Follow-up Program. Prev Med. 1976;5:207–215. [Google Scholar]
- 14.University of Minnesota
- 15.Diabetes Epidemiology Research International Mortality Study Group. LaPorte RE. Major cross-country differences in risk of dying for people with IDDM. Diabetes Care. 1991;14:49–54. doi: 10.2337/diacare.14.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Koivisto VA, Stevens LK, Mattock M, et al. Cardiovascular disease and its risk factors in IDDM in Europe. Diabetes Care. 1996;19:689–697. doi: 10.2337/diacare.19.7.689. [DOI] [PubMed] [Google Scholar]
- 17.Soedamah-Muthu SS, Chaturvedi N, Toeller M, et al. Risk factors for coronary heart disease in type 1 diabetes patients in Europe. Diabetes Care. 2004;27:530–537. doi: 10.2337/diacare.27.2.530. [DOI] [PubMed] [Google Scholar]
- 18.Allison PD. Estimating Cox Regression Models with PROC PHREG. In: Whatley J, editor. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute Inc; 1995. [Google Scholar]
- 19.Orchard T, Olson J, Erbey J, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 20.Hardin J, Hilbe J. Bootstrap Generalized Linear Models and Extensions. College Station, TX: Stata Press; 2001. [Google Scholar]
- 21.Orchard T, Olson J, Erbey J, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 22.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vasular damage. The Steno Hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM: gender differences in risk factors but not risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16:720–726. doi: 10.1161/01.atv.16.6.720. [DOI] [PubMed] [Google Scholar]
- 24.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/ EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 27.Zgibor JC, Wilson RR, Orchard TJ. Has control of hyperlipidemia and hypertension in patients with type 1 diabetes improved over time? Diabetes Care. 2005;28:521–526. doi: 10.2337/diacare.28.3.521. [DOI] [PubMed] [Google Scholar]