Abstract
Background
Pioglitazone (Pio) treatment induces weight gain in Type 2 diabetes mellitus (T2DM), which could worsen hepatic lipid accumulation, and alter adiponectin and high-sensitivity C-reactive protein (hs-CRP).
Objective
To compare changes in hepatic lipid, serum adiponectin and hs-CRP in diabetics treated with Pio (with and without weight gain) against metformin (Met) treatment, which produces weight loss.
Design
Fifty-one men and women with T2DM, naive to thiazolidinediones, entered a 16-week, open-label, parallel arm study, where participants were randomized to one of three groups: (1) Pio plus the American Diabetes Association diet (Pio+ADA); (2) Pio plus a portion control weight loss diet (Pio+PC), or (3) metformin plus ADA diet (Met+ADA).
Methods
Hepatic lipid was assessed with abdominal computed tomography (CT) and the serum adiponectin and hs-CRP by enzyme-linked immunosorbent assay at baseline and study end.
Results
Forty-eight subjects completed the study. The Pio+ADA group gained (mean±S.E.M.) 2.15±1.09 kg, while Pio+PC and Met+ADA group lost −2.59±1.25 and −3.21±0.7 kg, respectively. Pio-treated groups (Pio+ADA and Pio+PC) significantly decreased hepatic fat as indicated by increased liver density on CTscan [10.1±2.4: 11.4±1.0 Hounsfield units (HU)], compared with Met+ADA group (−2.4±3.1 HU). The Pio groups demonstrated significantly increased serum adiponectin, (8.6±1.5; 7.4±1.6 μg/ml) independent of weight change, compared to Met+ADA (−0.14±0.6 μgm/ml) group which lost weight. Serum hs-CRP decreased in groups showing weight loss (Pio+PC, −3.1±1.7 mg/l; Met+ADA, −1.5±1.2 mg/l) compared to Pio+ADA (1.8±3.0 mg/l) group that gained weight.
Conclusions
Pio treatment in T2DM significantly reduced hepatic lipid and increased adiponectin independent of weight change, while decreasing hs-CRP with weight loss.
Keywords: ALT, NAFLD, NASH, Metformin, Cardiovascular disease, Adiponectin, hs-CRP, Diabetes, Obesity, Adipose tissue
1. Background
Thiazolidinediones (TZDs) are potent agonists for the peroxisome proliferator-activated receptor-gamma (PPAR-γ receptor. PPAR-γ activation by pioglitazone (Pio) improves insulin sensitivity (Waugh, Keating, Plosker, Easthope, & Robinson, 2006) and attenuates inflammatory markers (Berg & Scherer, 2005; Giannini, Serio, & Galli, 2004; Granberry & Fonseca, 2005; Kostadinova, Wahli, & Michalik, 2005). Weight gain is one major drawback to treatment with TZDs (Fonseca, 2003; Gupta, Smith, Green-way, & Bray, 2009; Smith et al., 2005). Since weight gain is typically associated with an increase in insulin resistance, a decrease in anti-inflammatory markers and an increase in proinflammatory markers, it is unclear if full benefits of the TZD treatment are realized in the presence of weight gain. We have recently shown that Pio treatment, when combined with a portion-controlled diet that prevents the weight gain, not only prevented weight gain but also resulted in weight loss (Gupta et al., 2009).
Nonalcoholic fatty liver disease (NAFLD), often present with the metabolic syndrome (Hamaguchi et al., 2005), is also common in diabetes mellitus (Younossi, Gramlich, Matteoni, Boparai, & McCullough, 2004) and obesity (Boppidi & Daram, 2008). It is manifest as a simple elevation of serum alanine aminotransferase (ALT), triglyceride (TG) accumulation in hepatocytes (hepatic steatosis) or inflammation of hepatocytes (steatohepatitis or NASH). Nonalcoholic steatohepatitis (NASH), in a fraction of the affected individuals, leads to fibrosis and cirrhosis of the liver, hepatocellular carcinoma, and even death. Nonalcoholic fatty liver disease (NALFD) is an independent predictor for cardiovascular disease risk in metabolic syndrome (Hamaguchi et al., 2007). Despite the increasing prevalence of NAFLD and potential for adverse outcomes with NASH, interventions are limited to weight loss, exercise, and modification of associated comorbid conditions.
Weight loss reverses hepatic steatosis (Assy, Hussein, & Abassi, 2007). TZDs decrease serum ALT and modulate hepatic steatosis and steatohepatitis (Khashab & Chalasani, 2007), but it is unclear whether the treatment associated weight gain offsets these effects to some degree. Adiponectin, which is secreted by adipose cells, is reduced in metabolic syndrome, diabetes, and obesity and is increased by weight loss and TZD treatment (Nedvídková, Smitka, Kopský, & Hainer, 2005; Swarbrick & Havel, 2008). Whether this effect is modulated by the weight gain associated with TZD treatment is also unknown. In contrast, high-sensitivity C-reactive protein (hs-CRP) is increased in many disease states, including diabetes mellitus and obesity (Musunuru et al., 2008; Ridker & Morrow, 2003). In this study, we compared the changes in hepatic lipid and serum adiponectin and hs-CRP in obese Type 2 diabetics treated with Pio (with and without weight gain) against metformin (Met) treatment, which is known to be associated with weight loss. We report the change in the ALT, liver lipid deposition, serum adiponectin, and serum hs-CRP along with the alterations in waist circumference, systolic and diastolic blood pressure, fasting serum glucose, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TGs).
2. Research design and methods
2.1. Study participants
Men and women, aged 35–75 years with Type 2 diabetes mellitus (T2DM) never treated with a TZD were recruited. They each signed an informed consent approved by the institutional review board after reading it and having their questions answered. T2DM was diagnosed in one of three ways: (1) a confirmed fasting plasma glucose of >126 mg/dl on two occasions; (2) a glucose >200 mg/dl 2 h after a 75-g glucose load; or (3) on current treatment with a single oral antidiabetic drug other than a TZD. Patients could be treated with diet, Met or sulfonylureas and had to be willing to be randomized to one of the three arms of the trial. Fasting plasma glucose at entry had to be <200 mg/dl. Use of adequate contraceptive control was required for women. This could include oral contraceptives, tubal ligation, hysterectomy, or postmenopausal status, as defined by more than 6 months without a menstrual cycle and follicle stimulating hormone level of >40 mIU/ml. Patients on a stable dosage of medication for chronic medical conditions were included. Patients were excluded if they had significant renal, cardiac, liver, lung, or neurological disease; although controlled hypertension with a baseline blood pressure was less than 140/90 mmHg on medications was acceptable. Patients with prior use of one of the two available thiazolidinediones (rosiglitazone or Pio), patients receiving β-blockers, patients currently pregnant, smokers, and subjects who abused alcohol or drugs were also excluded. If liver function tests at baseline (aspartate transaminase, alanine transaminase) were greater than 2.5 times the upper limit of normal, the subjects were not enrolled. Metal objects that would interfere with the measurement of visceral fat by CT, such as implanted rods or surgical clips, prevented patients from participating. In addition, patients taking drugs known to affect energy metabolism or body weight, such as orlistat, sibutramine, ephedrine, or corticosteroids, were excluded.
2.2. Clinical protocol
A total of 51 subjects meeting all criteria were randomized into this 16-week clinical trial (ClinicalTrials. gov NCT00219440). They were randomly assigned to one of the three treatment groups: (1) A group treated with Pio plus standard dietary advice from the American Diabetes Association (Pio+ADA); (2) a group treated with Pio who received the portion-controlled weight loss diet (Pio+PC); and (3) a group treated with Met and the same dietary advice that was given to the first group (Met+ADA). Patients were started on Pio at a dose 30 mg/day or Met at a dose of 500 mg/day. The hemoglobin A1c target was <7.0%. If, after 8 weeks, the hemoglobin A1c level was >7.0% or the fasting plasma glucose level was >100 mg/dl, the dosage of Pio was increased to 45 mg/d. This occurred in only one participant. The dose of Met was increased by 500 mg every week, based upon subject tolerance. A maximum dose of 2 g/d was taken as 1000 mg twice a day. As a safety criterion, individuals with an increase in HbA1c >11% or an increase in the fasting plasma glucose >240 mg/dl were to be treated with sulfonylurea and/or insulin. No participant met this criterion. The participants were randomly assigned to the three treatment groups. Their prestudy treatment profile was as follows: 17 subjects on no medications (three, eight, and six, respectively, were randomized to Pio+ADA, Pio+PC, and Met+ADA groups), 10 subjects on sulfonylurea (three, three and four, respectively, were randomized to Pio+ADA, Pio+PC, and Met+ADA groups), and 21 subjects on Met (eight, seven, and six, respectively, were randomized to Pio+ADA, Pio+PC, and Met+ADA groups). Two subjects in the Met+ADA group, by random selection, continued to receive the same dose of Met (2 g/day) that they had been receiving prior to the study. All participants were seen in our outpatient clinic 2, 4, 8, 12, and 16 weeks after randomization. A fasting weight, pulse rate, blood pressure, fingerstick glucose, medication compliance, change in medications, and adverse events were assessed by the clinic staff.
2.3. Diet and lifestyle
Energy requirements for each individual were determined using the WHO/FAO calculation and multiplying this value by an activity factor of 1.3 (Lin et al., 2003). A diet that was 500 kcal/d less than this figure was developed for each participant. In the portion-controlled group, one can of Glucerna, which provided 290 kcal, and black coffee or tea were used for breakfast and, again, for lunch. The remainder of the calories needed to meet each individual’s calculated energy needs was provided in the evening meal that was based on foods preferred by the participant in consultation with the dietitian. For the other two groups, dietary advice was based on the recommendations of the American Diabetes Association at the same calorie level. All subjects were instructed to perform physical exercise of their choice for 30 min/day, five times a week. American Heart Association Step 1 instructions were provided to all participants. These diet and lifestyle advice were provided to all the participants as pamphlets at the start of the study. No specific follow-up protocol was followed to mirror clinical practice environment.
2.4. Measurement of hepatic lipid
A multi-slice computed tomographic scan of the abdomen was done at baseline and at the end of the study. Liver density minus the spleen density at these time points is reported for the change in liver lipid deposition in the three treatment groups. CT measured hepatic density [Hounsfield units (HU)] was used as a direct measure of hepatic fat and was corrected for the spleen density (liver-spleen). Prior studies comparing this method to hepatic quantitative proton magnetic resonance spectroscopy on a 1.5-T magnetic resonance imaging revealed a high concordance (R2=0.81; unpublished observations, Larson-Myer, Smith and Newcomer).
2.5. Adiponectin and hs-CRP
Serum adiponectin and hs-CRP were measured in all the 48 subjects at baseline and at the end of the study. Change in the serum concentration of adiponectin, and hs-CRP are reported.
2.6. Outcomes
The end points reported in this article are change in liver fat, serum adiponectin, and serum hs-CRP.
2.7. Statistical analyses
All analyses were performed using procedures in the SAS System for Windows Version 9.1 (SAS Institute, Cary, NC, USA, 2004). Sample size was based on the variance of response to Pio and placebo in our previous study (Smith et al., 2005). The data are summarized as means±S.D. or means±S.E.M. We used the general linear model procedure to compare treatment groups by analysis of variance with baseline data as covariates. Pairwise comparisons were performed to identify specific significant differences among the treatments. Statistical significance was reported when P≤.05, and Tukey’s method was employed to adjust P values and maintain the simultaneous global testing at the .05 level.
3. Results
Forty-eight of the 51 subjects (95%) completed the 16-week trial. Two subjects from Pio+ADA group and one subject from Met+ADA group chose not to continue the study. One subject changed his mind, one had scheduling problems, while one had to move away due to a job change. Details regarding the disposition of subjects from screening through completion have been previously published (Gupta et al., 2009). Participants in each of the three groups were well balanced for age, gender, body mass index (BMI), blood pressure, glycemic, and lipid parameters.
Table 1 compares the demographic and laboratory characteristics for all participants in the three groups. The average participant at baseline was a middle-aged (mean±S.D.; 57±9 years) obese (weight 96.8±15 kg; BMI 35.7±6.2 kg/m2) diabetic with central obesity (waist circumference 111±13 cm; visceral adipose tissue of 5.9±2.47 kg and total body fat of 38.9±12.2 kg or 37.5±9%). Women had an average waist circumference of 110±4 cm, visceral adipose tissue of 5.0±0.5 kg, body fat of 42.4±10.2 kg or 44±1%, while the men had a waist circumference of 107±6 cm and 31.7±13.0 kg or 30.7% body fat with 6.6±1.1 kg as visceral fat. Their fasting serum glucose was 132±24 mg/dl, insulin 19±8 μU/ml for a HOMA-IR of 6.3±3.1. Their total cholesterol, low-density lipoprotein cholesterol (LDL-C), HDL-C, and TGs were 185±34, 101±29, 46±10, and 170±83 mg/dl, respectively. Their serum adiponectin, an anti-inflammatory marker, was 6.4±3.2 μg/ml, while hs-CRP, a proinflammatory marker, was 6.7±7.0 mg/l.
Table 1.
Baseline demographic and laboratory measures
| Pio group |
Met group |
||
|---|---|---|---|
| Variable | ADA diet | PC diet | ADA diet |
| Number | 14 | 18 | 16 |
| Age (y) | 59.2±2.5 | 55.7±2.4 | 56.9±2.0 |
| Gender (M/F) | 4/10 | 6/12 | 6/10 |
| Weight (kg) | 98.5±3.4 | 95.3±4.5 | 97.8±3.8 |
| BMI (kg/m2) | 35.7±1.7 | 34.3±1.4 | 36.4±1.7 |
| Waist circumference (cm) | |||
| Men | 109±6 | 110±7 | 103±5 |
| Women | 113±3 | 102±4 | 114±5 |
| Visceral fat (kg) | |||
| Men | 6.7±1.6 | 8.0±1.3 | 5.2±0.5 |
| Women | 5.6±0.5 | 4.4±0.4 | 5.1±0.5 |
| DEXA: body fat (%) | |||
| Men | 32±3 | 32±4 | 28±3 |
| Women | 45±1 | 42±1 | 45±2 |
| DEXA: body fat (kg) | |||
| Men | 30.7±4.9 | 36.5±6.8 | 27.7±4.6 |
| Women | 43.8±2.4 | 37.9±2.7 | 46.4±3.8 |
| CT | |||
| VAT (kg) | 6.1±0.5 | 6.3±0.5 | 5.4±0.8 |
| Blood pressure (systolic/diastolic)(mm Hg) | 123±4/74±2 | 127±3/80±2 | 124±4/78±2 |
| Hemoglobin A1c (%) | 6.2±0.2 | 6.4±0.2 | 6.0±0.2 |
| Glucose (mg/dl) | 140±8 | 135±5 | 129±6 |
| Insulin (μU/ml) | 19.4±1.8 | 18.8±2.2 | 18.8±2.0 |
| HOMA-IR | 2.66±0.22 | 2.72±0.48 | 2.73±0.29 |
| Cholesterol (mg/dl) | 188±9 | 183±6 | 185±10 |
| LDL-C (mg/dl) | 98±8 | 102±6 | 102±8 |
| HDL-C (mg/dl) | 46±3 | 44±2 | 48±2 |
| Triglycerides | 176±23 | 162±17 | 173±23 |
| Adiponectin (μg/ml) | 7.1±1.0 | 5.7±0.7 | 6.6±0.8 |
| hs-CRP (mg/l) | 4.75±1.1 | 7.9±2.4 | 6.9±1.1 |
DEXA, dual energy X-ray adsorptiometry; VAT, visceral adipose tissue; HOMA-IR, homeostasis model assessment of insulin resistance. Data are presented as means±S.E.M. There is no significant treatment effect in post hoc test (with Tukey adjustment).
All study participants with T2DM were being treated by their primary care physicians for associated co-morbidities (hypertension and/or dyslipidemia) with medications that included angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers for hypertension and statins (3-hydroxy-3-methylglutaryl-coenzyme A:HMG-CoA reductase inhibitors) for dyslipidemia. They all qualified to have the metabolic syndrome by National Cholesterol Education Program, Adult Treatment Panel III (NCEP ATPIII) criteria (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). At study initiation, they displayed a good glycemic control (average HbA1c 6.2±0.8%) and had well-controlled blood pressure (average: 126±15/77±8 mm Hg) and lipids profile (except for marginally elevated triglycerides; 170±84 mg/dl).
Table 2 shows the changes from baseline for the parameters in this study. The body weight in the Pio+ADA group increased (mean±S.E.M.; +2.15±1.09 kg), compared to a weight loss of (−2.59±1.25 kg) in the Pio+PC group and (−3.21±0.7 kg) in the Met+ADA group at the end of the 16-week study. Changes from baseline in parameters of the metabolic syndrome; waist circumference, systolic and diastolic blood pressure, glucose, HDL-C, and triglycerides are shown in Table 2. Pio treatment when combined with the portion control weight loss diet significantly decreased the waist circumference (−4.72±1.63 cm) and systolic and diastolic blood pressure (−7.8±0.7/−6.4±0.6 mm Hg) while significantly increasing HDL-C (8.11±1.70 mg/dl). Serum adiponectin significantly increased (7.39±1.56 μg/ml) with a simultaneous decrease in hs-CRP (−3.08±1.72 mg/l).
Table 2.
Change from baseline metabolic parameters
| Pio group |
Met group |
||
|---|---|---|---|
| Variable | ADA diet | PC diet | ADA diet |
| Number | 14 | 18 | 16 |
| Body weight (kg) | 2.15±1.09* | −2.59±1.25# | −3.21±0.7# |
| Waist circumference (cm) | −0.06±1.08* | −4.72±1.63# | −1.45±1.01*,# |
| Systolic/diastolic blood pressure (mm Hg) | 8.0±0.9*/1±0.2* | −7.8±0.7#/−6.4±0.6# | −2.5±0.3#/−2.4±0.2# |
| Glucose (mg/dl) | −17.92±4.71 | −17.16±4.96 | −12.11±4.80 |
| HDL-C (mg/dl) | 6.20±1.94*,# | 8.11±1.70* | 1.67±0.91# |
| Triglycerides (mg/dl) | −31±8.0 | −10±6.0 | −20±5.5 |
| Serum adiponectin (μg/ml) | 8.56±1.51*,# | 7.39±1.56* | −0.14±0.60# |
| Serum hs-CRP (mg/l) | 1.78±3.04 | −3.08±1.72 | −1.47±1.17 |
Data are presented as mean±S.E.M. Different symbols (e.g., * vs. #): significant difference with P<.05 (Tukey adjustment) among treatments.
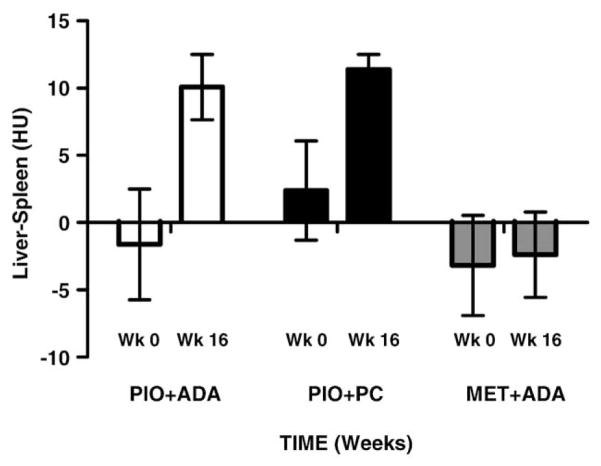
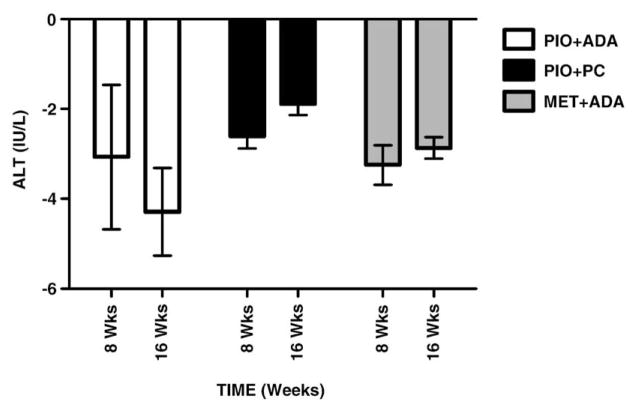
Table 3 summarizes CT measured liver minus spleen density (HU); a marker for liver fat and the time course of ALT levels. The races were distributed equally across the groups. The Caucasians were 78.5, 50, and 62.5% in the Pio +ADA, Pi0+PC and Met+ADA groups, respectively. Pio treated groups showed an increase in liver density from base line to end of study (P<.05), regardless of the weight change (Pio+ADA group: 8.5 HU, Pio+PC group: 9.1 HU). The liver density, however, did not change in the Met group which also exhibited weight loss (Met+ADA group: −5.5 HU). The spleen density did not change in any of the three groups during the study (−0.1, −0.2, and +0.3 HU, respectively). ALT levels gradually declined in all the three groups over the 16 week course of the study (P<.05 in Pio +ADA group only).
Table 3.
Liver & spleen density and time course for ALT levels
| Pio+ADA (n=14) |
Pio+PC (n=18) |
MET+ADA (n=16) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 8 | Week 16 | Week 0 | Week 8 | Week 16 | Week 0 | Week 8 | Week 16 | |
| Liver (HU) | 49.5±4.0 | 61.1±2.1 | 54.2±3.7 | 63.1±1.1 | 49.3±3.6 | 49.7±3.2 | |||
| Spleen (HU) | 51.1±0.8 | 51.0±0.7 | 51.9±0.6 | 51.7±0.6 | 52.4±0.5 | 52.1±0.6 | |||
| ALT (IU/l) | 24.0±2.3 | 20.9±1.9 | 19.7±1.3 | 22.3±2.0 | 19.7±1.8 | 20.4±1.8 | 26.5±2.9 | 23.2±2.5 | 23.6±2.7 |
| Mean±S.E.M. | |||||||||
Change in liver density and ALT levels over baseline are shown in Figs. 1 and 2, respectively. The Pio -treated groups show a statistically significant (P<.05) increased liver density when compared with the Met treated group. This is indicative of a significant decrease in liver lipid content. Liver ALT levels declined all the three groups over time, possibly indicating an improvement in liver injury.
Fig. 1.
Liver density.
Fig. 2.
Change from baseline: ALT.
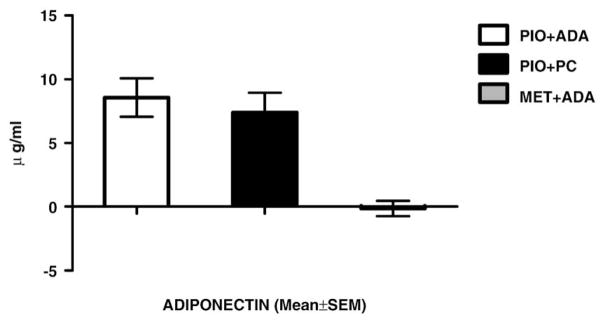
Serum adiponectin (Fig. 3) showed a statistically significant increase with Pio treatment, whether there was weight gain (Pio+ADA: 8.6±1.5 μg/ml) or weight loss (Pio+PC: 7.4±1.6 μg/ml). The Met+ADA group, which also lost weight, had no significant change in serum adiponectin (−0.14±0.06 μg/ml). Serum hs-CRP (Fig. 4) increased in the Pio+ADA group (1.8±3.0 mg/l) but decreased in both the Pio+PC (−3.1±1.7 mg/l) and Met+ ADA (−1.5±1.2 mg/l) groups.
Fig. 3.
Change from baseline: serum adiponectin.
Fig. 4.
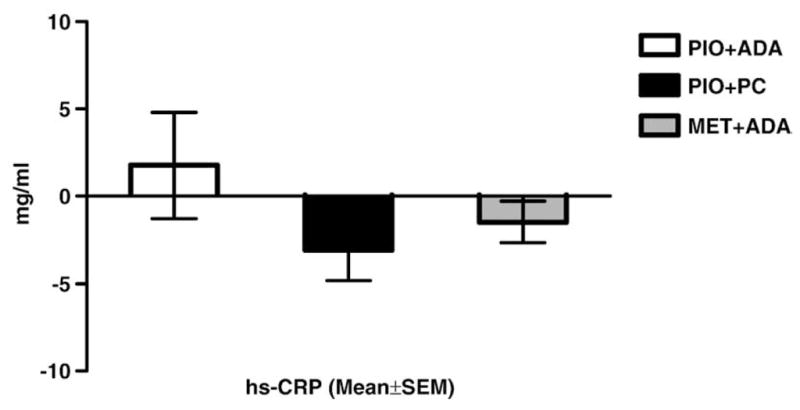
Change from baseline: serum hs-CRP.
4. Discussion
Using noninvasive computed tomographic scanned liver-spleen density increase as measure for decrease in liver fat, we show that Pio reduces liver fat in obese Type 2 diabetics. This occurred weather there was a weight gain or loss in these obese subjects with T2DM. Diabetes mellitus (Targher et al., 2007) and obesity (Weiss, 2007) are among the many conditions associated with ectopic lipid deposits in the liver, skeletal muscle (Goodpaster, Thaete, & Kelley, 2000), and heart (Montani et al., 2004). Hepatic steatosis, a feature of the nonalcoholic fatty liver disease (NAFLD), is also commonly seen in the metabolic syndrome (Björnsson, 2008). Liver biopsy has been used to assess the progression from hepatic steatosis through parenchymal inflammation, cell injury, and fibrosis (leading to cirrhosis and liver failure): nonalcoholic steatohepatitis (NASH) (Qureshi & Abrams, 2007). The severity of NAFLD is associated with increased cardiovascular disease risk in both non-obese (Sung, Ryan, & Wilson, 2008) and subjects with the metabolic syndrome (Hamaguchi et al., 2007).
In a pilot study, Pio reversed NASH in nondiabetics (Chalasani et al., 2008) and is currently in an ongoing randomized, double-masked, placebo-controlled clinical trial for comparison with vitamin E for treatment of NASH in a similar population (Promrat et al., 2004). Pio reduced hepatic steatosis in subjects with impaired glucose tolerance and diabetes mellitus (Belfort et al., 2006). Our results are consistent with the literature showing a reduction in liver fat with Pio in T2DM. We extend the literature to show that the reduction in liver fat occurred with or without a reduction in body weight. When we prevented weight gain, by controlling portion sizes with commercial products, we prevented the weight gain associated with Pio. We acknowledge that absence of a group treated with Met plus portion control diet is a weakness of this study. However, the improvement in hepatic lipid deposits during this 16-week trial was equivalent, indicating that the weight gain with Pio does not blunt its effect on hepatic lipid. Interestingly, the weight reduction associated with Met, which was comparable to the portion-controlled group treated with Pio, did not improve hepatic lipid, indicating that Pio has a much greater benefit on this variable.
Elevation of ALT levels (Kunde, Lazenby, Clements, & Abrams, 2005; Prati et al., 2002), especially in high-risk populations (Marchesini et al., 2001), is indicative of NAFLD. In the present study, ALT decreased in all treatment groups, independent of weight change. In times when the prevalence of NAFLD is on the rise (Farrell & Larter, 2006; Ruhl & Everhart, 2004) and treatment, at best, is not established (Angelico, Burattin, Alessandri, Del Ben, & Lirussi, 2007), use of Pio for treatment of hepatic steatosis may be an attractive prospect. Pio improves insulin resistance and decreases liver lipid deposits and ALT levels, independent of weight change. Decreased liver lipid deposits could also translate to a better liver perfusion and function, which is impaired in this condition (Brock & Dorman, 2007).
Serum adiponectin improved with Pio treatment, independent of weight change. Subjects treated with Met had no change in adiponectin, even though they lost as much weight as those treated with Pio combined with portion controlled diet. This increase in adiponectin, independent of weight change, may signal an improvement in adipose tissue function with Pio treatment. Serum hs-CRP concentrations seemed to reflect changes in body weight. It increased in the subjects who gained weight when treated with Pio (Pio+ADA group) but decreased in the two groups (Pio+PC and Met+ADA) that lost weight. There is a large body of data showing that weight loss is a major factor decreasing hs-CRP (Madsen et al., 2008), and our data are consistent with it.
Both NAFLD (in metabolic syndrome) and T2DM (with its comorbidities: hypertension, dyslipidemia) are associated with increased cardiovascular disease risk (Ford, 2004; Panagiotakos, Pitsavos, Skoumas, Lentzas, & Stefanadis, 2008). The underlying mechanism for the multiple simultaneous metabolic perturbations appears to be an increase in insulin resistance along with an altered systemic anti-inflammatory:proinflammatory balance (Kowalska et al., 2008). In the present study, Pio treatment when combined with portion-control weight-loss diet treated both NAFLD (reduced serum ALT and liver fat) and the metabolic syndrome (significant decrease in waist circumference, systolic and diastolic blood pressure, and a significant increase in HDL-C). The systemic anti-inflammatory:proinflammatory balance tipped towards cardiovascular disease risk reduction: significant increase in serum adiponectin accompanied by a decrease in serum hs-CRP.
4.1. Conclusion
Pio treatment in T2DM significantly reduced hepatic lipid deposition and significantly increased serum adiponectin, independent of change in body weight. Met treatment did not mimic the effects of Pio. Pio treatment when combined with weight-loss diet decreased serum hs-CRP and improved the parameters of the metabolic syndrome. Met treatment only decreased hs-CRP and did not modify the metabolic syndrome.
Acknowledgments
This study was supported by Takeda Pharmaceuticals, Lincolnshire, IL. USA. The study was designed by Drs. Bray, Greenway, Smith and Gupta. Drs. Gupta and Green-way carried out the clinical trial. Dr. Gupta drafted the manuscript, and all authors contributed to its editing.
The authors thank the participants for their contribution to this study, the Pennington Clinical Trials for their help, Brandi Armand who coordinated the study, and Susan Thomas who provided dietary counseling to the participants.
Footnotes
Supported in part by a grant from Takeda Pharmaceuticals NA.
Declaration of competing Interests: Drs. Smith and Bray have received research support for investigator initiated research from Takeda Pharmaceuticals North America. Dr. Smith has also been a consultant, received honoraria for speaking, and served on Advisory Boards for Takeda Pharmaceutical North America.
References
- Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database of Systematic Reviews (Online) 2007 January;(1):CD005166. doi: 10.1002/14651858.CD005166.pub2. [DOI] [PubMed] [Google Scholar]
- Assy N, Hussein O, Abassi Z. Weight loss induced by orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with non-alcoholic steatohepatitis. Gut. 2007 March;56(3):443–444. doi: 10.1136/gut.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. New England Journal of Medicine. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Björnsson E. The clinical aspects of non-alcoholic fatty liver disease. Minerva Gastroenterologica e Dietologica. 2008;54(1):7–18. Review. [PubMed] [Google Scholar]
- Boppidi H, Daram SR. Nonalcoholic fatty liver disease: Hepatic manifestation of obesity and the metabolic syndrome. Postgraduate Medicine. 2008;120(2):E01–E07. doi: 10.3810/pgm.2008.07.1800. [DOI] [PubMed] [Google Scholar]
- Brock RW, Dorman RB. Obesity, insulin resistance and hepatic perfusion. Microcirculation. 2007;14(4–5):339–347. doi: 10.1080/10739680701282986. [DOI] [PubMed] [Google Scholar]
- Chalasani NP, Sanyal AJ, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemporary Clinical Trials. 2008 September;30(1):88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. doi: 10.1002/hep.20973. Review. [DOI] [PubMed] [Google Scholar]
- Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. American Journal of Medicine. 2003;115(Suppl 8A):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. Review. [DOI] [PubMed] [Google Scholar]
- Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: Findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173(2):309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: Taking a look beyond antidiabetic activity. Journal of Endocrinological Investigation. 2004;27(10):982–991. doi: 10.1007/BF03347546. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in Type 2 diabetes mellitus. American Journal of Clinical Nutrition. 2000;71(4):885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- Granberry MC, Fonseca VA. Cardiovascular risk factors associated with insulin resistance: Effects of oral antidiabetic agents. American Journal of Cardiovascular Drugs. 2005;5(3):201–209. doi: 10.2165/00129784-200505030-00006. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Smith SR, Greenway FL, Bray GA. Pioglitazone treatment in Type 2 diabetes mellitus when combined with portion control diet modifies the metabolic syndrome. Diabetes, Obesity & Metabolism. 2009;11(4):330–337. doi: 10.1111/j.1463-1326.2008.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Kojima T, Takeda N, Nagata C, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World Journal of Gastroenterology. 2007;13(10):1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Kojima T, Takeda N, Nakagawa T, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Annals of Internal Medicine. 2005;143(10):722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- Khashab M, Chalasani N. Use of insulin sensitizers in NASH. Endocrinology and Metabolism Clinics of North America. 2007;36(4):1067–87. xi. doi: 10.1016/j.ecl.2007.07.006. Review. [DOI] [PubMed] [Google Scholar]
- Kostadinova R, Wahli W, Michalik L. PPAR’s in diseases: Control mechanisms of inflammation. Current Medical Chemistry. 2005;12(25):2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- Kowalska I, Straczkowski M, Nikolajuk A, Adamska A, et al. Insulin resistance, serum adiponectin, and proinflammatory markers in young subjects with the metabolic syndrome. Metabolism. 2008;57(11):1539–1544. doi: 10.1016/j.metabol.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42(3):650–656. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- Lin PH, Proschan MA, Bray GA, Fernandez CP, Hoben K, Most-Windhauser M, Karanja N, Obarzanek E DASH Collaborative Research Group. Estimation of energy requirements in a controlled feeding trial. American Journal of Clinical Nutrition. 2003:639–645. doi: 10.1093/ajcn/77.3.639. [DOI] [PubMed] [Google Scholar]
- Madsen EL, Rissanen A, Bruun JM, Skogstrand K, et al. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: A 3-year weight loss study. European Journal of Endocrinology. 2008;158(2):179–187. doi: 10.1530/EJE-07-0721. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, et al. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. International Journal of Obesity and Related Metabolic Disorders. 2004;28(Suppl 4):S58–S65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- Musunuru K, Kral BG, Blumenthal RS, Fuster V, Mora S. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nature Clinical Practice Cardiovascular Medicine. 2008;5(10):621–635. doi: 10.1038/ncpcardio1322. Electronic publication 2008 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvídková J, Smitka K, Kopský V, Hainer V. Adiponectin, an adipocyte-derived protein. Physiological Research. 2005;54(2):133–140. Review. [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Skoumas Y, Lentzas Y, Stefanadis C. Five-year incidence of Type 2 diabetes mellitus among cardiovascular disease-free Greek adults: Findings from the ATTICA study. Vascular Health and Risk Management. 2008;4(3):691–698. doi: 10.2147/vhrm.s2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Annals of Internal Medicine. 2002;137(1):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39(1):188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2007;13(26):3540–3553. doi: 10.3748/wjg.v13.i26.3540. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Morrow DA. C-reactive protein, inflammation, and coronary risk. Cardiology Clinics. 2003;21(3):315–325. doi: 10.1016/s0733-8651(03)00079-1. Review. [DOI] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE. Epidemiology of nonalcoholic fatty liver. Clinics in Liver Disease. 2004;8(3):501–19. vii. doi: 10.1016/j.cld.2004.04.008. Review. [DOI] [PubMed] [Google Scholar]
- Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: A randomized controlled trial. Metabolism. 2005;54:24–32. doi: 10.1016/j.metabol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Sung KC, Ryan MC, Wilson AM. The severity of nonalcoholic fatty liver disease is associated with increased cardiovascular risk in a large cohort of non-obese Asian subjects. Atherosclerosis. 2008 July;203(2):581–586. doi: 10.1016/j.atherosclerosis.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metabolic Syndrome and Related Disorders. 2008;6(2):87–102. doi: 10.1089/met.2007.0029. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: A review of its use in Type 2 diabetes mellitus. Drugs. 2006;66(3):340–341. [Google Scholar]
- Weiss R. Fat distribution and storage: How much, where, and how? European Journal of Endocrinology. 2007;157(Suppl 1):S39–S45. doi: 10.1530/EJE-07-0125. Review. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with Type 2 diabetes. Clinical Gastroenterology and Hepatology. 2004;2(3):262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]