Abstract
Coupled rat heart mitochondria produce externally hydrogen peroxide at the rates which correspond to about 0.8 and 0.3 per cent of the total oxygen consumption at State 4 with succinate and glutamate plus malate as the respiratory substrates, respectively. Stimulation of the respiratory activities by ADP (State 4–State 3 transition) decreases the succinate- and glutamate plus malate-supported H2O2 production 8- and 1.3-times, respectively. NH4+ strongly stimulates hydrogen peroxide formation with either substrate without any effect on State 4 and/or State 3 respiration. Rotenone-treated, alamethicin-permeabilized mitochondria catalyze NADH-supported H2O2 production at a rate about 10-fold higher than that seen in intact mitochondria under optimal (State 4 succinate-supported respiration in the presence of ammonium chloride) conditions. NADH-supported hydrogen peroxide production by the rotenone-treated mitochondria devoid of a permeability barrier for H2O2 diffusion by alamethicin treatment are only partially (~50%) sensitive to the Complex I NADH binding site-specific inhibitor, NADH-OH. The residual activity is strongly (~6-fold) stimulated by ammonium chloride. NAD+ inhibits both Complex I-mediated and ammonium-stimulated H2O2 production. In the absence of stimulatory ammonium about half of the total NADH-supported hydrogen peroxide production is catalyzed by Complex I. In the presence of ammonium about 90% of the total hydrogen peroxide production is catalyzed by matrix located, ammonium-dependent enzyme(s).
Keywords: Hydrogen peroxide production, Respiratory activity, Complex I, Matrix proteins, Ammonium, Mitochondria
1. Introduction
More than 50 years ago [1] it was recognized that hydrogen peroxide production in mitochondria accounts for up to 2% of the total oxygen consumption during controlled (State 4) respiration. Participation of respiratory chain components in the two-electron reduction of oxygen was originally demonstrated in 1966 by Jensen who observed significant antimycin-insensitive oxidation of NADH and succinate by bovine heart electron-transport particles coupled with a formation of H2O2 [2]. The NADH-ubiquinone [3–6] and ubiquinol-cytochrome c oxidoreductase (Complex III) [7–9] regions of the respiratory chain have been identified as the sites where superoxide, an immediate stoichiometric precursor of hydrogen peroxide [10], is formed. NADH-supported and ubiquinol-supported superoxide generation has also been demonstrated for isolated purified Complex I [11, 12] and Complex III [13, 14].
The rate of superoxide generation depends on both the concentration of oxygen and a reductant. Indeed, it was shown many years ago that the rate of mitochondrial hydrogen peroxide production is strongly dependent on the metabolic state: it is high in State 4, when the NADH/NAD+ and ubiquinol/ubiquinone pools are largely reduced, and is low in State 3, when the steady-state concentrations of the potential oxygen reductants are decreased [15, 16]. The degree of reduction of these components (or the components which are in equilibrium with them) and therefore the rate of superoxide/hydrogen peroxide production are expected to be under proton-motive force control, as has been experimentally demonstrated in a number of later studies on different mitochondrial preparations [6, 15–20]. This simple thermodynamic model for energy-dependent superoxide production does not hold for Complex I. The NADH-supported Complex I-catalyzed superoxide generation by inside-out submitochondrial particles shows maximal activity at low NADH concentration (~50 μM), and the reaction is strongly inhibited at higher physiologically relevant concentrations of NADH (mmolar range) [21]. Thus, an apparent contradiction seems to exist: mitochondria are capable of the NAD(P)H redox state-dependent hydrogen peroxide formation and the activity of Complex I, the major contributor to the matrix located superoxide production is inhibited by high concentrations of NADH. This apparent contradiction and other considerations (see Ref [21] for the details) led us to propose that other matrix located component(s) in addition to Complex I are significant contributors to the overall mitochondrial hydrogen-peroxide production. This proposal has been confirmed by the demonstration of NADH-supported, ammonium-stimulated generation of hydrogen peroxide by the soluble crude matrix protein fraction derived from bovine heart mitochondria [22]. Although the specific matrix located protein(s) responsible for the ammonium-sensitive NADH-supported H2O2 formation has not been identified, the quantitative evaluation of relative contributions of Complex I and matrix to the overall reaction seemed desirable. Here we will show that only about one-half of the total hydrogen peroxide produced in the mitochondrial matrix can be accounted for the Complex I-mediated reaction. Soluble matrix protein(s) are responsible for the other half of H2O2 formation. In the presence of stimulatory NH4+ the total hydrogen peroxide production drastically increases and about 90% of the NH4+-stimulated activity is accounted for by soluble matrix located protein(s). Data on limited diffusion of hydrogen peroxide across the mitochondrial membranes will also be presented and discussed.
2. Materials and Methods
Rat heart mitochondria were prepared essentially as described [23]. Fractionation procedure was conducted at 0–4° as follows. Mitochondria (10 mg protein/ml) were suspended in 0.15 M sucrose. One mM EDTA-KOH (pH 7.5, final concentration) was added and the mixture was incubated under argon flow for 15 min. The pH was adjusted to 8.6 by 1 M NH4OH and the mixture was subjected to sonication (Soniprep 150 MSE) 6 times for 30 s with 1 min intervals. The suspension was centrifuged for 15 min at 26,000 g, small precipitates were discarded, 5 mM Tris-Cl buffer (pH 7.5, final concentration) was added to the supernatant, and the mixture was centrifuged for 1 h at 200,000 g. The clear supernatant was collected and used for assays of the “matrix” protein activity. The precipitated material was suspended in 0.25 M sucrose and used as the membrane-bound (unbroken mitochondria, submitochondrial particles, outer membranes) protein activity. The standard reaction mixture for all assays was composed of 0.25 M sucrose, 10 mM KCl, 0.1 mM EDTA-KOH, and 5 mM potassium phosphate, pH 7.5 (other additions are indicated in the legends to the Tables and Figures). The respiratory activities were measured amperometrically with a Clark-type electrode. H2O2 generation was monitored at 30°C at 572–600 nm (formation of resorufin, ε572–600=54 mM−1 c−1 [24]) in the standard reaction mixture supplemented with 10 μM Amplex Red, horseradish peroxidase (2 units/ml), and superoxide dismutase from bovine erythrocytes (6 units/ml).
Protein content was determined by the biuret procedure. Amplex Red was from AnaSpec, Inc. (U.S.A.); other fine chemicals were from Sigma-Aldrich (U.S.A.). NADH-OH was kindly provided by Dr. Alexander Kotlyar (Tel Aviv University, Israel).
3. Results
As the first step of our studies the rates of total oxygen consumption and hydrogen peroxide formation during State 4 and State 3 respiration of coupled rat heart mitochondria were measured (Table 1). About 0.8% of the total oxygen consumed during succinate-supported State 4 respiration appeared in the surrounding medium as hydrogen peroxide. This value was decreased down to less than 0.03% when respiration was activated by ADP. The corresponding figures for glutamate/malate-supported H2O2 production were substantially lower (0.3 and 0.04%, respectively). Rotenone significantly decreased the succinate-supported and increased the glutamate/malate-supported generation of hydrogen peroxide thus suggesting that State 4 succinate-supported reaction proceeded via energy-dependent reverse electron transfer pathway. The data shown in Table 1 for hydrogen peroxide production correspond to the lowest values of the actual one- and two-electron reduction of oxygen in the matrix since intramitochondrial superoxide dismutases operating together with glutathione peroxidase and catalase are expected to decrease the amount of H2O2 that appears in the surrounding medium. Ammonium chloride, an activator of the matrix-catalyzed NAD(P)H-supported hydrogen peroxide production [22] stimulated both succinate- and glutamate/malate-supported generation of H2O2, whereas no effects of NH4+ on the respiratory activities in State 4 and State 3 with either substrate were seen ( data not shown). This is an expected phenomenon if the stimulatory effect of NH4+ is targeted to H2O2-producing activity other than that catalyzed by the respiratory chain components. The data presented in Table 1 suggest that the highest H2O2 production is observed when intramitochondrial pool of NAD(P)++NAD(P)H is maximally reduced. Thus, the actual maximal rates of the NADH-supported H2O2 generation by Complex I and by other enzymes can be determined in rotenone-treated mitochondria providing that externally added NADH would serve as a reductant for the intramitochondrial enzymes. This can be achieved by treatment of intact mitochondria with the pore-forming antibiotic alamethicin (in the presence of Mg2+) as has been described previously [25]. This approach also permitted the use of the potent nucleotide binding site-directed inhibitor of Complex I, NADH-OH [26] which inhibits both NADH-supported and succinate-supported (via energy-linked reverse electron transfer pathway) superoxide production by Complex I in tightly coupled submitochondrial particles [27].
Table 1.
Generation of hydrogen peroxide by coupled rat heart mitochondria (pH 7.5, 30°C)a
| Substrateb | Oxygen consumption | Hydrogen peroxide production | ||||
|---|---|---|---|---|---|---|
| (Two-electron equivalents·min−1) mg−1 × 109 |
||||||
| State 4c | State 3d | State 4c | State 3d | |||
| − NH4Cl | + NH4Cle | − NH4Cl | + NH4Cle | |||
| Succinate | 0.65±0.01 | 1.60±0.15 | 0.08±0.01 | 0.15±0.05 | ||
| + rotenoneb | 80±10 | 285±35 | 0.25±0.05 | 0.40±0.10 | 0.30±0.10 | 0.30±0.10 |
| Glutamate + Malate | 35±6 | 245±30 | 0.12±0.01 | 0.35±0.15 | 0.09±0.01 | 0.40±0.15 |
| + rotenoneb | 0 | 0 | 0.32±0.01 | 0.50±0.30 | 0.36±0.02 | 0.77+0.03 |
Averaged values from 4 experiments; 1 mg and 0.1 mg protein per ml were added when oxygen consumption and hydrogen peroxide production were assayed, respectively.
Each substrate was added at 5 mM concentration; and 5 μM rotenone was present where indicated.
No ADP was present.
400 μM ADP was added.
Ammonium chloride at 30 mM final concentration was added.
Table 2 summarizes experiments aimed to dissect the relative contributions of Complex I and other enzyme(s) to the overall NADH-supported hydrogen peroxide production by permeabilized mitochondria at different concentrations of NADH and NAD+. When both matrix enzyme(s) and Complex I were operating (no NADH-OH was added) at optimal for Complex I superoxide generation activity (50 μM NADH) the overall reaction was about 4-times stimulated by NH4+. Both basal and NH4+-stimulated activities were significantly inhibited by equal (to NADH) concentrations of NAD+. In contrast to what has been shown for Complex I-mediated superoxide generation by inside-out coupled submitochondrial particles devoid of matrix proteins [21] higher level of NADH (2 mM) did not inhibit H2O2 production. In the presence of NADH-OH (Complex I activity was blocked) mitochondria still produced H2O2 at a rate that accounted for about half of the activity seen in the absence of the inhibitor. The residual NADH-OH insensitive hydrogen peroxide production was stimulated about 6-fold by ammonium. This stimulatory effect was particularly strong (about 10-fold) when the NADH-OH insensitive reaction proceeded at high (2 mM) NADH concentration. Taken together the data shown in Table 2 suggest that: (i) at least two enzymes (one is evidently Complex I) are responsible for the overall NADH- and succinate-supported (via rotenone-sensitive energy-linked reverse electron transfer pathway) hydrogen peroxide production in mitochondria; (ii) in the absence of NH4+ Complex I at optimal non-physiological concentration of NADH is responsible for about half of the total activity and the other half becomes 6-times higher than that catalyzed by Complex I when stimulated by ammonium; (iii) both Complex I-catalyzed and ammonium-dependent H2O2 production are under the control of the NADH/NAD+ ratio.
Table 2.
The NADH-supported generation of hydrogen peroxide by permeabilized rat heart mitochondria (pH 7.5, 30°C)a
| Hydrogen peroxide production (nmol/min per mg protein) |
||||
|---|---|---|---|---|
| − NADH-OH | + NADH-OHb | |||
| − NH4Cl | + NH4Clc | − NH4Cl | + NH4Clc | |
| NADH (50 μM) | 5.1±0.4 | 19.2±2.6 | 2.4±0.2 | 15.1±0.6 |
| NADH (50 μM) + NAD+ (50 μM) | 1.0±0.2 | 3.1±0.6 | 0.7±0.1 | 3.9±1.0 |
| NADH (2 mM) | 5.3±0.2 | 33.3±2.3 | 3.9±0.5 | 32.9±0.9 |
| NADH (2 mM) + NAD+ (2 mM) | 1.2±0.1 | 2.2±0.3 | 0.9±0.1 | 1.7±0.2 |
Average values from 4 experiments. Mitochondria (20 μg/ml) were incubated for 0.5 min in the standard reaction mixture containing alamethicin (40 μg/ml), 2.5 mM MgCl2, and 5 μM rotenone. The reaction was started by the addition of NADH. Treatment with alamethicin increased the rate of externally added NADH (50 μM) oxidation by mitochondria in the absence of rotenone from 0.06 to 1.4 μmol/min per mg protein. The latter activity was more than 90% sensitive to NADH-OH.
NADH-OH (2.5 nmol/mg protein) was added after permeabilization and preincubation was continued for 1 min.
30 mM NH4Cl was presented in the assay mixture.
Comparison of the data in Tables 1 and 2 provides interesting insight into this phenomenon. The specific activities of permeabilized mitochondria in the presence of low or high NADH concentrations were much higher than those seen in intact mitochondria in the presence of succinate in State 4 or in the presence of glutamate/malate and rotenone, i.e., under the conditions where the intramitochondrial pool of NAD(P)H+NAD(P)+ is expected to be highly reduced. Endogenous reduced glutathione in intact mitochondria [28] and intramitochondrial catalase [29, 30] are expected scavengers of some part of the matrix-produced hydrogen peroxide thus decreasing external H2O2 accessible for the peroxidase used for its analytical detection. The stimulating effect of alamethicin thus could be explained at least partially by the loss of glutathione (not catalase) from permeabilized mitochondria. It also seemed possible that the inner mitochondrial membrane is not freely permeable to hydrogen peroxide. The latter possibility was directly confirmed by experiments shown in Fig. 1. Hydrogen peroxide was added to the rotenone-treated intact mitochondria after all the oxygen has been consumed in State 3 respiration with glutamate/malate, and the internal mitochondrial catalase activity was followed as an increase of oxygen concentration in the assay medium. The addition of alamethicin resulted in significant (three-fold) stimulation of hydrogen peroxide decomposition by intramitochondrial catalase. Because no evidence, whatsoever, exists for limited penetration of oxygen through the mitochondrial membranes, it is safe to interpret this results as an evidence for limited permeability of the inner membrane for hydrogen peroxide.
Fig. 1.
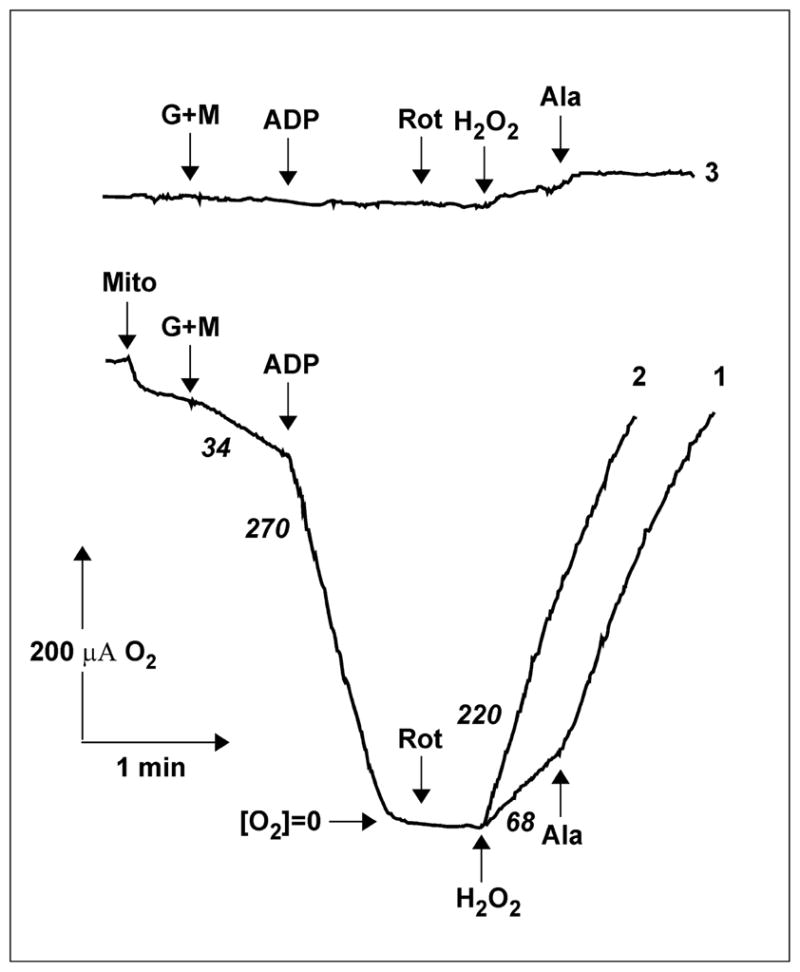
Limited permeability of mitochondrial membranes for hydrogen peroxide. Trace 1, Mitochondria (Mito) (1.8 mg/ml) were added to the standard reaction mixture in a closed vessel with oxygen-sensitive electrode and oxygen consumption and production were followed. The additions were: G+M, glutamate and malate 5 mM each; ADP 2 mM; Rot, rotenone 5 μM; H2O2 1 mM; Ala, alamethicin 40 μg/ml and MgCl2 2.5 mM. Trace 2, alamethicin and MgCl2 were added just before hydrogen peroxide. Trace 3, control, no mitochondria were added. The numbers (in italics) at the traces show the rates of oxygen consumption or production in two-electron equivalents per min per mg protein.
Next, the quantitative distribution of the hydrogen peroxide producing enzymatic activities in mitochondrial compartments was addressed. Alamethicin- and rotenone-treated mitochondria were used as the starting material and the specific activities of the fractions obtained after ultrasonic disruption and high speed centrifugation were measured (Table 3). The highest specific NADH-OH-insensitive H2O2 producing activity was recovered in the soluble fraction, which was increased up to about 50 nmol/min per mg protein in the presence of NH4+. A relatively low specific activity was found in the precipitated material. The membrane-associated activity was only slightly stimulated by ammonium and it was only partially sensitive to NADH-OH. The residual ammonium-sensitive and NADH-OH-insensitive activity recovered in the precipitated material was evidently due to a slight contamination of the membranes by soluble matrix proteins. Fig. 2 shows the distribution of the total hydrogen peroxide producing activities of mitochondria treated as described in Table 3. About half of the total activity could be accounted for by Complex I-catalyzed H2O2 production. The other half (NADH-OH-insensitive activity) was recovered in the soluble matrix fraction. In the presence of ammonium dramatic changes both in the total activity and its distribution were seen. The total activity was greatly increased and about 90% of that could be accounted for by the matrix-catalyzed H2O2 production.
Table 3.
Fractionation of the mitochondrial hydrogen peroxide-generating activitya
| Specific activity (nmoles of H2O2/min per mg protein) | ||||
|---|---|---|---|---|
| − NADH-OH | + NADH-OHc | |||
| − NH4Cl | + NH4Cld | − NH4Cl | + NH4Cld | |
| Mitochondriab | 5.3±0.4 | 19.8±4.0 | 2.9±0.2 | 17.4±3.3 |
| 200,000 g supernatant (matrix proteins) | 7.7±2.9 | 46.1±10.6 | 6.4±2.6 | 49.7±14.1 |
| Precipitate (membrane-bound proteins) | 4.3±0.1 | 6.9±0.1 | 1.3±0.1 | 4.4±0.2 |
The results of two experiments.
Mitochondria were permeabilized (see Table 2); all assays were done with 50 μM NADH in the presence of 5 μM rotenone.
NADH-OH (2.5 nmol/mg protein) was added after permeabilization, and preincubation was continued for 1 min.
30 mM NH4Cl was presented in the assay mixture.
Fig. 2.
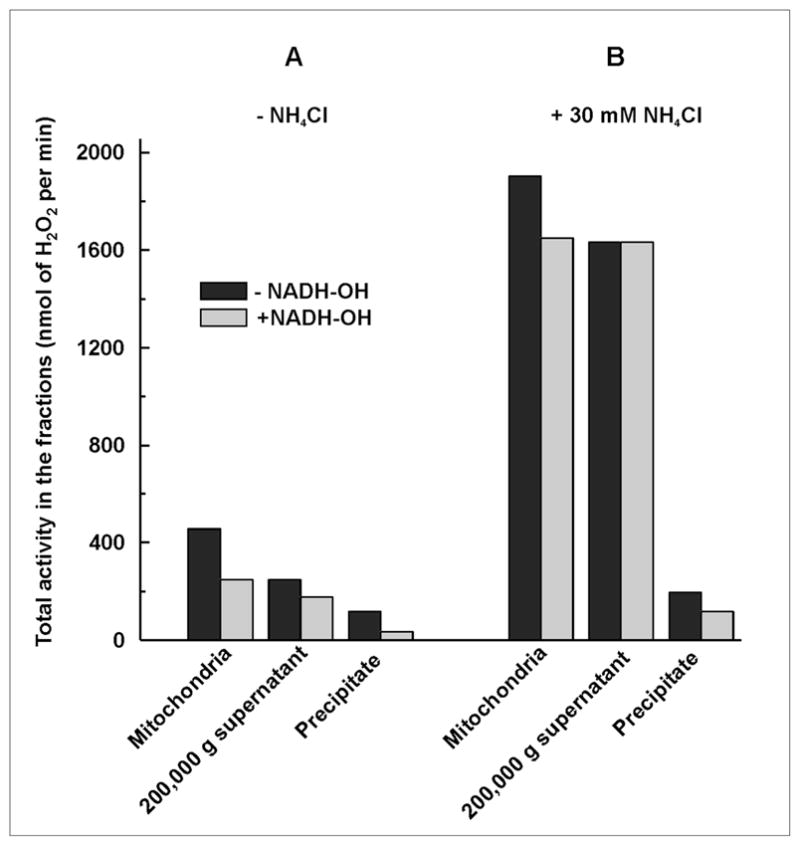
Distribution of the total NADH-supported hydrogen peroxide generating activities between the membrane-bound and matrix proteins in rat heart mitochondria. Mitochondria were treated, fractionated, and assayed as described in Table 3.
4. Discussion
The major aim of this study was to evaluate quantitatively the relative contributions of Complex I and matrix-associated proteins to the overall production of hydrogen peroxide by mitochondria. Despite a vast literature on the subject we experienced difficulties in finding articles where the absolute specific rates and percentage of oxygen reduction to water and to hydrogen peroxide (via superoxide) have been directly compared during State 4 and State 3 respiration with different substrates, except for the old classical report [31] on H2O2 production by pigeon heart mitochondria and more recent data for rat and human brain mitochondria respiring at saturating oxygen concentration [32]. Interestingly, our average values for the succinate-supported hydrogen peroxide formation by intact mitochondria in State 4 at pH 7.5 (0.65 nmoles per min per mg of protein) are the same as reported by Chance et al., for pigeon heart mitochondria using the same conditions (0.7 nmol/min per mg protein) [31]. These coincident values disagree with several reports on the difference between the rates of hydrogen peroxide production by mitochondria from heart [33, 34] and other organs [35] from long- (pigeon) and short-life span (rat) vertebrates of similar size. It should be emphasized that in the vast majority of published data the highest rates of external H2O2 production by mitochondria are seen during State 4 succinate-supported respiration. As exemplified by the data in Table 1, this activity is decreased by rotenone and by State 4–State 3 transition (see also [15, 16]) thus suggesting that the degree of NAD(P)+ reduction via energy-linked reverse electron transfer is the critical factor for the rate of hydrogen peroxide formation. On the other hand the reverse electron transfer activity is, perhaps, the most sensitive indicator of coupling tightness for any particular preparation of mitochondria. We believe that comparison of hydrogen peroxide production by mitochondria from different organs (or animals) without normalizing the measured activity to their coupling efficiency should be interpreted with caution.
Complex III that presumably generates H2O2 via superoxide formation at the ubisemiquinone level at the Qo-site facing the intermembranous space [20] contributes in about 40% to the maximal externally detected hydrogen peroxide formation as is evident from the comparison of the succinate-supported H2O2 formation in the absence and presence of rotenone (0.65 and 0.25 nmol/min per mg protein, respectively, Table 1).
Under conditions where the Complex I-mediated reaction is prevented by NADH-OH significant H2O2 production greatly stimulated by ammonium was observed (Table 2). Both total and NADH-OH-insensitive fractions of the externally detected hydrogen peroxide production were increased by permeabilization of mitochondria. It is generally believed that the inner mitochondrial membrane is freely permeable to hydrogen peroxide in contrast to its precursor, the superoxide anion. Our data (Table 2 and Fig. 1) show that this is not the case. To our knowledge this is the first demonstration of limited diffusion of hydrogen peroxide across the mitochondrial membranes. The existence of H2O2 gradients across plasma and peroxisomal membranes of Jurkat T-cells has been detected by Antunes and Cadenas [36]. Aquaporin-mediated facilitated diffusion of hydrogen peroxide across the cellular membrane of yeast has recently been reported [37] (see also [38] for the review). Limitation of hydrogen peroxide diffusion across the mitochondrial membrane as demonstrated here raises two important questions: what is the H2O2 concentration gradient, if any, between the mitochondrial matrix space and the cytosol, and is the external production of hydrogen peroxide by mitochondria under control by specific protein-carrier like aquaporins? Further detailed studies on H2O2 permeation across the outer and inner mitochondrial membranes are evidently needed. The specific rates of the intramitochondrial hydrogen peroxide production (in matrix) as measured using intact mitochondria reported here (Table 1) and likely in many other papers seem to be underestimated because of limited diffusion of hydrogen peroxide across the membrane (Fig. 1). We believe that the specific activities of hydrogen peroxide formation measured in permeabilized mitochondria (Table 2) are more realistic. If the specific activity of about 5 nmol/min per mg protein measured with 2 mM NADH in the presence of rotenone (without activating ammonium) is the value to approximate hydrogen production at State 4 respiration, the percentage of electron flow in one-/two-electron reduction of oxygen would be as high as about 6%. In the presence of activating ammonium about an equal amount of the reducing equivalents from NADH are utilized by the respiratory chain in State 4 (35 natom O2/min per mg protein, Table 1) and by the enzyme(s) catalyzing hydrogen peroxide production (33 nmol H2O2/min per mg protein, Table 2).
Here we confirmed and extended our previous data [22] showing that a substantial fraction of mitochondrial superoxide/hydrogen peroxide generation is formed by soluble matrix-associated protein(s). This is expected to be especially true when external or endogenously formed NH4+ is present. Although the apparent Ka for the activating effect of NH4+ is well above the physiologically relevant concentration [22] the actual intramitochondrial concentration of ammonium (formed in glutamate dehydrogenase, AMP-deaminase, and glycine-cleavage reactions) in a variety of metabolic states is not known.
The data presented here and elsewhere [21, 22] show that the specific sites of superoxide anion/H2O2 production are located in the hydrophilic matrix space: FMN bound to hydrophilic part of Complex I [11] and matrix associated NAD(P)H+NAD(P)+-dependent protein(s). Reactive oxygen species, including those produced by mitochondria, are believed to be important players in development of a number of pathologies and aging, and also in normal cellular signaling (see different aspects of the problem in reviews [39–44]). Vigorous attempts to use mitochondria-targeted antioxidants have been recently undertaken to prevent oxidative stress-related diseases and senescence [45, 46]. These mitochondria-addressed drugs are lipophilic ubi- [45] or plastoquinone [46] moieties covalently linked with positively charged triphenylphosphonium, and they are expected to be accumulated in the inner mitochondrial membrane, thus acting as lipophilic antioxidants. In light of the findings reported here that the major part of reactive oxygen species are formed in hydrophilic matrix it would be of interest to compare the effects of hydrophilic mitochondria-targeted antioxidants with those described for the lipophilic ones [45, 46].
The last point to be briefly discussed concerns the nature of the soluble NAD(P)H-dependent, ammonium-stimulated hydrogen peroxide producing enzyme(s) in mitochondrial matrix. At present we are not able to answer this question confidently. Some results recently obtained by our group (V. Grivennikova, A. Kareyeva, A. Vinogradov, manuscript in preparation) can be summarized as follows. (i) SDS-electrophoretically pure protein (one band with apparent molecular mass of 50 kDa) with a specific activity of about 1 μmol/min per mg protein in the presence of ammonium chloride is responsible for the matrix-associated NADH-dependent hydrogen peroxide production. (ii) Mass-spectrometry analysis shows significant homology of this protein and bovine heart dihydrolipoyl dehydrogenase. In light of earlier data on significant heterogeneity of dihydrolipoyl dehydrogenases in bovine heart mitochondria [47, 48] it is not unreasonable to suggest that the protein in question is one of the multiple forms of that enzyme or other flavoproteins similar to it.
Acknowledgments
This study was supported by grant 08-04-00594 to ADV and grant 09-04-00505 to VGG of the Russian Foundation for Fundamental Research and by NIH Fogarty International Research grant 5R03TW007825-03 to ADV and G. Cecchini (Molecular Biology Division, VA Medical Center, 4150 Clement Street, San Francisco, CA 94121, and Department of Biochemistry & Biophysics, University of California, San Francisco, CA 94158, USA).
We are grateful to Dr. A. Kotlyar for his kind gift of NADH-OH and to Mr. D. Kalashnikov for his help in preparation of mitochondria.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. Biochim Biophys Acta. 1966;122:157–166. doi: 10.1016/0926-6593(66)90057-9. [DOI] [PubMed] [Google Scholar]
- 3.Hinkle PC, Butow RA, Racker E, Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome b region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem. 1967;242:5169–5173. [PubMed] [Google Scholar]
- 4.Takeshige K, Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1979;180:129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamoorthy AIG, Hinkle P. Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J Biol Chem. 1988;263:17566–17575. [PubMed] [Google Scholar]
- 7.Ksenzenko MYu, Konstantinov AA, Khomutov GB, Tikhonov AN, Ruuge EK. Effect of electron transfer inhibitors on superoxide generation in the cytochrome bc1 site of the mitochondrial respiratory chain. FEBS Lett. 1983;155:19–24. doi: 10.1016/0014-5793(83)80200-2. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Trumpower B. Superoxide anion generation by the cytochrome bc1 complex. Arch Biochem Biophys. 2003;419:198–206. doi: 10.1016/j.abb.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria. Central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 10.Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 11.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galkin A, Brandt U. Superoxide radical formation by pure complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica. J Biol Chem. 2005;280:30129–30135. doi: 10.1074/jbc.M504709200. [DOI] [PubMed] [Google Scholar]
- 13.Forquer I, Covian R, Bowman MK, Trumpower BL, Kramer DM. Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J Biol Chem. 2006;281:38459–38465. doi: 10.1074/jbc.M605119200. [DOI] [PubMed] [Google Scholar]
- 14.Dröse S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- 15.Loschen G, Flohé L, Chance B. Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 16.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 18.Votyakova TV, Reynolds IJ. ΔΨm-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 19.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 20.Rottenberg H, Covian R, Trumpower BL. Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J Biol Chem. 2009;284:19203–19210. doi: 10.1074/jbc.M109.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikova VG, Cecchini G, Vinogradov AD. Ammonium-dependent hydrogen peroxide production by mitochondria. FEBS Lett. 2008;583:1287–1291. doi: 10.1016/j.febslet.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Jacobus WE, Saks VA. Creatine kinase of heart mitochondria: changes in its kinetic properties induced by coupling to oxidative phosphorylation. Arch Biochem Biophys. 1982;219:167–178. doi: 10.1016/0003-9861(82)90146-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 25.Gostimskaya IS, Grivennikova VG, Zharova TV, Bakeeva LE, Vinogradov AD. In situ assay of the intramitochondrial enzymes: use of alamethicin for permeabilization of mitochondria. Anal Biochem. 2003;313:46–52. doi: 10.1016/s0003-2697(02)00534-1. [DOI] [PubMed] [Google Scholar]
- 26.Kotlyar AB, Karliner JS, Cecchini G. A novel strong competitive inhibitor of complex I. FEBS Lett. 2005;579:4861–4866. doi: 10.1016/j.febslet.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 27.Grivennikova VG, Kotlyar AB, Karliner JS, Cecchini G, Vinogradov AD. Redox-dependent change of nucleotide affinity to the active site of the mammalian complex I. Biochemistry. 2007;46:10971–10978. doi: 10.1021/bi7009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nohl H, Hegner D. Evidence for the existence of catalase in the matrix space of rat-heart mitochondria. FEBS Lett. 1978;89:126–130. doi: 10.1016/0014-5793(78)80537-7. [DOI] [PubMed] [Google Scholar]
- 30.Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, Freeman BA. Detection of catalase in rat heart mitochondria. J Biol Chem. 1991;266:22028–22034. [PubMed] [Google Scholar]
- 31.Chance B, Boveris A, Oschino N, Loschen G. The nature of the catalase intermediate in its biological function. In: King TE, Mason HS, Morrison M, editors. Oxidases and Related Redox Systems. University Park Press; Baltimore, London, Tokyo: 1973. pp. 350–353. [Google Scholar]
- 32.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 33.Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- 34.Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 35.Barja G, Herrero A. Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J Bioenerg Biomembr. 1998;30:235–243. doi: 10.1023/a:1020592719405. [DOI] [PubMed] [Google Scholar]
- 36.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 37.Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 38.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 40.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 41.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 43.Maklashina E, Ackrell BA. Is defective electron transport at the hub of aging? Aging Cell. 2004;3:21–27. doi: 10.1111/j.1474-9728.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B, Gutteridge JHC. Free radicals in biology and medicine. 4. Oxford University Press; 2007. [Google Scholar]
- 45.James AM, Cochemé HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 46.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, Zorov DB. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Lusty CJ, Singer TP. Lipoyl dehydrogenase. Free and complexed forms in mammalian mitochondria. J Biol Chem. 1964;239:3733–3742. [PubMed] [Google Scholar]
- 48.Kenney WC, Zakim D, Hogue PK, Singer TP. Multiplicity and origin of isoenzymes of lipoyl dehydrogenase. Eur J Biochem. 1972;28:253–260. doi: 10.1111/j.1432-1033.1972.tb01908.x. [DOI] [PubMed] [Google Scholar]


