Abstract
Elevated C-reactive protein (CRP) is associated with an increased risk of cardiovascular disease. Physical activity has been inversely associated with CRP. However, the clinical trials examining the effect of exercise training have produced conflicting results.
Purpose
The purpose of this study was to examine the influence an exercise training program on CRP in postmenopausal women.
Methods
Sedentary, overweight/obese, postmenopausal women with elevated systolic blood pressure (120.0 to 159.9 mm Hg) (N= 464) were randomized into 1 of 4 groups: a non-exercise control or 1 of 3 aerobic exercise groups; exercise energy expenditure of 4, 8, or 12 kcal/kg/week (KKW), for 6 months at a training intensity of 50% of peak VO2.
Results
Complete data was available of 421 participants and mean baseline CRP was 5.7 (5.5) mg/L with no significant differences across groups. While VO2 increased in a dose response manner, there were no significant changes in CRP in any of the exercise intervention groups compared to the control group. Change in fitness was not associated with change in CRP, whereas change in weight was significantly associated with change in CRP.
Conclusion
Despite increasing fitness, six months of aerobic exercise training did not improve CRP. However, improvements in CRP were associated with reductions in weight.
Keywords: exercise, inflammation, female, weight
INTRODUCTION
Elevated C-reactive protein (CRP) is a well-recognized clinical marker of inflammation that is predictive of stroke, atherosclerosis, and associated with an increased incidence of myocardial infarction and cardiovascular mortality (22). Epidemiological data suggests that physical activity levels are inversely associated with markers of inflammation including CRP (6, 8, 12). In contrast, exercise training trials have not consistently demonstrated exercise to reduce CRP (2). However, previous exercise and CRP studies had a number of important methodological weaknesses including short duration, low mean baseline CRP, small number of participants, and poor exercise compliance. As a result the effectiveness of exercise training to reduce CRP concentrations remains an unresolved issue.
Postmenopausal women exhibit elevated concentrations of inflammatory markers such as CRP and interleukin-6 (IL-6), and are at a higher risk for cardiovascular disease compared to premenopausal women (21, 24). The Dose-Response to Exercise in postmenopausal Women (DREW) study was designed to examine the health benefits of 50%, 100%, and 150% of the NIH Consensus Panel physical activity recommendation in sedentary, overweight or obese, postmenopausal women with elevated blood pressure (16, 19). The results from the primary outcomes, cardiorespiratory fitness and blood pressure, have been reported, but due to the large undertaking and costs associated with conducting the DREW trial, a number of important secondary outcomes were included a priori in the study design including changes in CRP. In DREW, participant retention was excellent (92%), non-exercise activity was monitored and the exercise training was supervised with outstanding adherence (~97% for completers) (7). Thus, the DREW study represents a unique opportunity to examine the effect of different doses of exercise on CRP. We hypothesized that a higher dose of physical activity (100% and 150% of the NIH Consensus Panel recommendation would result in a significant reduction in circulating CRP.
METHODS
Study Design
DREW is a randomized, single-center, dose-response exercise training trial of sedentary, overweight or obese postmenopausal women with elevated blood pressure. The study was reviewed annually by an Human Subjects review committee (The Cooper Institute, Dallas, TX) and was comprised of a non-exercise control group and three exercise training groups exercising at incremental doses (50%, 100%, and 150%) of the NIH Consensus Panel physical activity recommendation on cardiorespiratory fitness (19) . The primary outcomes for DREW included peak aerobic capacity and resting blood pressure. We have published a complete description of the DREW design, methods, and primary outcomes (7, 16).
Study Subjects
Participants were recruited from the community using a wide variety of techniques including newspaper, radio, television, mailers, community events, and email distributions. All subjects signed a statement of informed consent in accordance with the Human Subjects review committee (The Cooper Institute, Dallas, TX). In brief, DREW study participants were sedentary (exercising < than 20 minutes; <3 d/wk; < 8000 steps/d assessed over the course of 1 week), overweight or obese (BMI; 25.0 to 43.0 kg/m2), and had a systolic blood pressure of 120.0 to 159.9 mm Hg. We excluded women who had a history of stroke, heart attack, or any serious medical condition that prevented participants from adhering to the protocol or exercising safely. Following baseline testing, participants were randomized into respective treatment groups. After an initial run-in period, we randomized 464 postmenopausal women (45-75 y) to 1 of 3 exercise training groups or a non-exercise control for a 6-month intervention period. Cardiovascular exercise training consisted of having women expend 4, 8, or 12 kcal/kg/week (KKW). We asked the non-exercise training group to maintain their current level of activity during the trial period. Exercising women participated in 3-4 supervised exercise sessions per week on a semi-recumbent cycle ergometer and treadmill at a heart rate associated with 50% of each woman’s peak VO2 (16). As previously reported, the 4-KKW exercise group obtained 72.2 (12.3) minutes per week over 2.6 (0.3) sessions, the 8-KKW obtained 135.8 (19.5) minutes per week during 2.8 (0.4) sessions, and the 12-KKW group performed 191.7 minutes per week during 3.1 (0.5) sessions. The average METs during the cycle ergometer training was similar across groups at approximately 3.8 METs. During the treadmill training, the METs were 3.1 (0.6), 3.3 (0.6) and 3.5 (0.8) across the 4 KKW, 8 KKW and 12 KKW groups, respectively (16). Adherence to the prescribed exercise training was 97%. Diet was assessed by the Food Intake and Analysis System semiquantitative food frequency questionnaire Version 3.0, Austin: University of Texas, Houston School of Public Health; 1996).
Blood Analysis
Venous blood samples were obtained at baseline and after 6 months. Samples were spun within 3 minutes of collection at 4°C and 1200 g, and stored at −70°C. Analyses were run in batches containing both pre- and post-intervention samples using the same assay kit. CRP concentrations were measured using a high-sensitivity assay on a Prospect nephelometer (Dade Division of Baxter Healthcare Corporation, Delaware, Maryland). The coefficient of variation for C-reactive protein in this analysis was 1.0 % (6). Blood lipid profiles, fasting glucose and insulin concentrations, and muscle and hepatorenal indices were analyzed with a Dimension RXL analyzer (Oxford, CT, USA) in a laboratory that is certified by the College of American Pathologists and meets the quality control standards of the CDC Lipid Standardization Program.
Statistical Analysis
Since the DREW trial was not specifically designed to examine CRP, we calculated power for hypothesized changes in CRP in response to exercise training. For all power calculations, the exercise versus control group comparison was based on the two-sample t-test for change-score differences, with equal group size and 5% significance (two-sided). For CRP, average change values and variability were based on observations from published reports (15, 25). Power was calculated for 30% change in CRP (compared to no change in the control group), which were derived from previous reports of CRP being reduced 31% and 35% in response to exercise training in uncontrolled studies (15, 25). Assuming 90 participants in each group, we calculated power to be 0.99 to detect differences in CRP between the experimental and control groups of 30%. Thus, given the large sample size, the DREW trial is more than adequately powered to examine the effect of exercise on CRP based on the available literature.
Descriptive baseline characteristics of groups were tabulated as means and SDs or as percentages. Spearmen correlations were used to assess associations between CRP and select variables. Median CRP change values were compared between groups using the Kruskal-Wallis test. Differences in CRP among the randomization groups were tested by ANOVA with adjustment for select prespecified covariates. For statistically significant ANOVAs (p<.05), all pairwise comparisons among the randomization groups were tested using Tukey studentized range adjustment. Results are presented as adjusted least-squares means with confidence intervals. In order to eliminate values that might be linked to an acute inflammatory process, participants with changes in CRP concentration that were more than 3 standard deviations outside the mean group change were identified and the primary analysis was repeated. All reported P values are two-sided. All analyses were performed using SAS version 9.0 (Cary, NC).
RESULTS
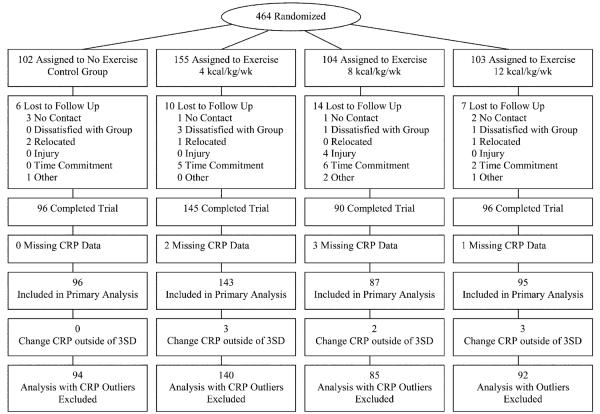
Of the 464 randomized participants, 427 returned for follow-up testing (92.0%). The return for follow-up across randomized groups ranged from 86.5% to 94.1%. Of the 427 participants completing the study protocol, 421 had plasma available for both baseline and follow-up CRP measurements (Figure 1).
Figure 1.
Participant flow diagram.
We present the baseline characteristics of our study participants in Table 1. Overall, our study population had a mean (SD) age of 57.3 (6.4) years, a BMI of 31.8 (3.8) kg/m2, and was 34.6 % non-Caucasian. While baseline systolic blood pressure was high (139.7 [13.0]) in our sample, baseline LDL-C, HDL-C, triglycerides and fasting glucose were within clinically acceptable ranges. The mean baseline CRP was 5.7 (5.5) mg/L with no significance differences across groups. Forty-five percent of the study participants reported using hormone therapy at baseline and women using hormone therapy had significantly higher concentrations of CRP compared to women not taking hormone therapy(6.7 (5.7) versus 4.8 (5.2) mg/L, p<0.001).
Table 1.
Baseline Participant Characteristics*
| Randomization Groups |
|||||
|---|---|---|---|---|---|
| All | Control | 4 kcal/kg/wk | 8 kcal/kg/wk | 12 kcal/kg/wk | |
| Characteristics | (n = 421) | (n = 96) | (n = 143) | (n =87) | (n = 95) |
| Age, y | 57.3 ± 6.4 | 57.3 ± 5.9 | 58.0 ± 6.5 | 56.8 ± 6.6 | 56.6 ± 6.5 |
| Ethnicity, % | |||||
| Caucasian | 64.4 | 65.6 | 60.8 | 59.8 | 72.6 |
| African American | 29.2 | 25 | 32.9 | 32.2 | 25.3 |
| Hispanic/other | 6.4 | 9.4 | 6.3 | 8.0 | 2.1 |
| Current cigarette smoking, % | 5.9 | 6.3 | 6.6 | 3.4 | 8.4 |
| Blood pressure medication, % | 28.5 | 25.0 | 27.3 | 32.1 | 30.5 |
| Cholesterol medication, % | 16.3 | 16.7 | 20.3 | 14.9 | 11.6 |
| Thyroid medication, % | 15.5 | 15.6 | 12.5 | 17.2 | 17.9 |
| Anti-depressant medication, % | 18.5 | 16.7 | 18.9 | 18.4 | 20.0 |
| Current hormone therapy, % | 45.4 | 51.0 | 43.3 | 42.5 | 45.2 |
| Weight, kg | 84.5 ± 11.9 | 85.9 ± 12.4 | 83.7 ± 11.5 | 85.7 ± 12.6 | 83.5 ± 11.2 |
| Body mass index, kg/m2† | 31.8 ± 3.8 | 32.2 ± 3.9 | 31.5 ± 3.7 | 32.4 ± 4.0 | 31.2 ± 3.5 |
| Waist circumference, cm | 101.0 ± 11.8 | 102.8 ± 12.0 | 100.0 ± 11.2 | 102.5 ± 11.9 | 99.4 ± 11.9 |
| Relative Peak VO2, kg/ml/min | 15.6 ± 2.8 | 15.6 ± 2.9 | 15.5 ± 2.9 | 15.2 ± 2.2 | 16.1 ± 2.9 |
| Peak absolute VO2, L/min | 1.3 ± 0.2 | 1.3 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 |
| LDL-C, mg/dL | 118.4 ± 26.7 | 118.1 ± 26.6 | 116.6 ± 27.4 | 118.6 ± 25.1 | 120.3 ± 27.3 |
| HDL-C, mg/dL | 57.3 ± 14.2 | 56.9 ± 14.0 | 57.6 ± 14.5 | 57.0 ± 15.4 | 57.9 ± 13.7 |
| Blood pressure, mm Hg | |||||
| Systolic | 139.7 ± 13.0 | 141.7 ± 12.0 | 138.8 ± 13.3 | 140.3 ± 13.4 | 138.2 ± 13.0 |
| Diastolic | 80.9 ± 8.5 | 80.8 ± 7.8 | 80.7 ± 9.0 | 81.4 ± 8.2 | 80.9 ± 8.7 |
| Triglycerides, mg/dL | 130.2 ± 64.4 | 135.2 ± 68.5 | 128.6 ± 59.4 | 132.0 ± 60.0 | 126.0 ± 71.5 |
| Fasting glucose, mg/dL | 94.9 ± 9.1 | 96.4 ± 10.5 | 94.1 ± 8.7 | 94.9 ± 8.8 | 95.0 ± 8.5 |
| C-Reactive Protein (mg/L) | |||||
| Mean | 5.7 ± 5.5 | 6.0 ± 5.8 | 5.3 ± 5.3 | 6.4 ± 6.1 | 5.0 ± 4.8 |
| Median (IQR) | 4.0 (1.8, 7.6) | 3.9 (1.5, 8.9) | 3.5 (1.6, 7.4) | 4.9 (2.9,8.0) | 3.4 (1.6, 6.7) |
LDL-C indicates low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range, and VO2, volume of oxygen consumed. SI conversions: To convert LDL-C, and HDL-C to mmol/L multiply by 0.0259; To convert triglycerides to mmol/L multiply by 0.0113; To convert glucose to mmol/L multiply by 0.0555
Continuous variables presented as means (SD) and dichotomous variables presented as count (percentage) unless otherwise noted.
Calculated as weight in kilograms divided by height in meters squared
Table 2 summarizes the spearman correlations between baseline CRP and baseline characteristics within all participants and by hormone therapy sub-groups. In all three groups, CRP was positively correlated to body mass, BMI, and waist circumference. Triglycerides were associated in the whole group and within the HR users. HDL-C (negatively) was associated with CRP non-HR group only. Insulin was associated with CRP in the whole group and within the non-HR users. During the intervention, 82.72% of participants did not change their HRT/non HRT status. A small percentage (4.5%, n=19) of participants began taking HRT and 12.8% (n=54) stopped taking HRT during the intervention. Neither starting nor stopping HRT had a significant effect on CRP. For example, the change in CRP in the group that began taking HRT was −0.4 mg/L (−2.5 to 1.7 mg/L) and in those that stopped taking HRT, the mean change in CRP was −0.89 mg/L (−2.2 to 0.4 mg/L). It is important to note that this small number of participants that changed HRT status does not provide adequate power to access HRT induced changes in CRP.
Table 2.
Spearman Correlations Between Baseline CRP and Descriptive Variables by Hormone Therapy Status
| All | No HR | HR | |
|---|---|---|---|
| Characteristics | n =421 | n=230 | n=191 |
| Age, y | −0.03 | −0.10 | 0.03 |
| Peak absolute VO2, L/min | −0.01 | 0.07 | −0.07 |
| Weight, kg | 0.27 † | 0.28 † | 0.30 † |
| Body mass index, kg/m2 | 0.31 † | 0.35† | 0.33 † |
| Waist circumference, cm | 0.23 † | 0.19 * | 0.28 † |
| Blood pressure, mm Hg | |||
| Systolic | 0.11 | 0. 05 | 0.14 |
| Diastolic | −0.01 | −0.01 | −0.01 |
| LDL-C, mg/dL | 0.01 | 0.07 | −0.03 |
| HDL-C, mg/dL | −0.08 | −0.16 * | −0.05 |
| Triglycerides, mg/dL | 0.20 † | 0.08 | 0.27† |
| Fasting glucose, mg/dL | 0.09 | 0.12 | 0.06 |
| Insulin (μU/ml) | 0.16 ** | 0.29 † | 0.12 |
CRP indicates C-reactive protein; LDL-C indicates low-density lipoprotein cholesterol; HDL-C indicates high-density lipoprotein cholesterol; and VO2 volume of oxygen consumed.
P < 0.001
P < 0.05
P < 0.01
The change in relative VO2 was −0.29 (−0.63, 0.06), 0.73 (0.44, 1.01), 1.33 (0.97, 1.69), 1.51 (1.16, 1.85) ml/kg/min and the change in absolute VO2 was −0.04 (−0.07, −0.01), 0.04 (0.02, 0.06), 0.08 (0.05, 0.11) 0.10 (0.08, 0.13) L/min across the across the control, 4, 8 and 12 KKW, respectively. For change in absolute and relative VO2 measures, all exercise groups were significantly different from control with the trend highly significant (p< 0.001). The change in weight across the control, 4, 8 and 12 KKW groups was −1.0 (−1.7,−.3), −1.3 (−1.9, −0.7), −1.8 (−2.5,−1.0), −1.3 (−2.0,−0.6) kg, respectively, with no differences between any group. The change in waist circumference across the control, 4, 8, and 12 KKW groups was −0.05 (−1.5, 1.4), −2.8 (−4.0,−1.6), −2.7 (−4.3, −1.2), −2.5 (−4.0, −1.0) cm respectively with all exercise groups experiencing a significant reduction in waist circumference when compared to controls
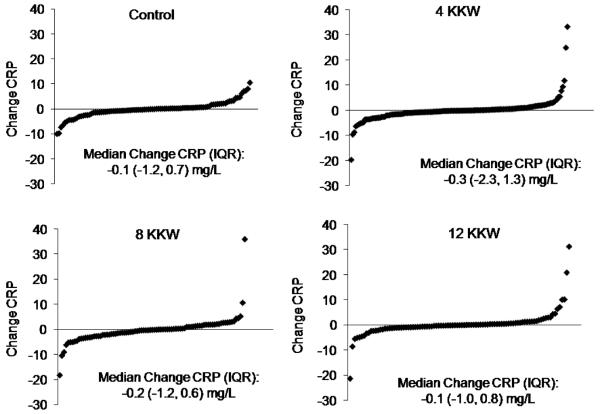
Figure 2 depicts the distribution of the CRP change values in the control and exercise groups. The shape of the distribution of change values was similar between groups, and there were no differences in median (IQR) CRP change between any of the groups (p=0.6). Each of the exercise groups had individuals with mean change in CRP values that were more than 3 standard deviations (change in CRP more or less than ±14.0 mg/L, respectively) from the mean change in CRP.
Figure 2.
The distribution of the change in CRP values in the control and exercise groups. There were no differences in median (IQR) CRP change between any of the groups (p=0.6).
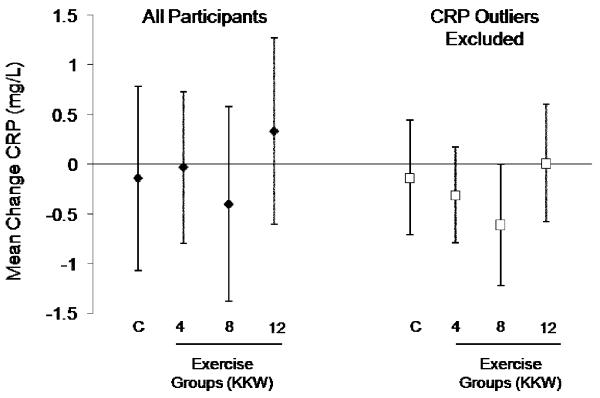
As depicted in Figure 3, there were no significant changes in CRP in any of the exercise groups. Analysis after removing individuals (n=8) with changes in CRP > 3 SD had no substantial effect on change in CRP across the exercise groups. To eliminate any potential effect of hormone therapy on exercise induced changes in CRP, we repeated the analyses but limited the sample to those not use hormone therapy at baseline or follow-up (n=230). The results from this sub-group did not differ from the full cohort as none of the exercise groups had significant changes in CRP compared with the control group.
Figure 3.
Change in CRP by study group: control, exercise energy expenditure of 4, 8, or 12 kcal/kg/week (KKW). The left set of bars represents findings for all participants and the right set of data represent findings with individuals with changes in CRP > 3 standard deviations from the mean removed. There were no significant changes in CRP in any of the exercise groups compared with the control group in either of the analyses.
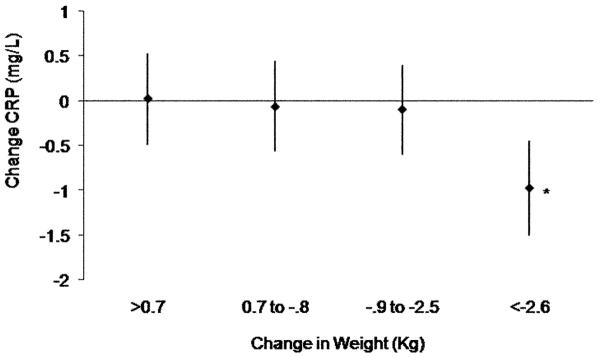
We examined the Spearman correlations between change in CRP and measures of fitness and body composition. Change in CRP was associated with change in body weight (0.15; P< 0.001) and waist circumference (0.12; P=0.01), but not peak absolute VO2 (L/min). Change in CRP was not associated with change in blood pressure. To further examine these associations, we looked at changes in CRP across quartiles of change in weight. The mean CRP change adjusted for baseline CRP and randomization group was significantly (p<0.02 for each) different in the 4th quartile (most weight loss) compared to the 1st, 2nd and 3rd quartiles of weight (Figure 4). The mean weight loss in the 4th quartile was 5.9 (3.0) kg and the mean change in CRP was 1.0 (2.8) mg/L.
Figure 4.
Changes in CRP (adjusted for baseline CRP and randomization group) across quartiles of change in weight. * P<0.02 in all other groups.
DISCUSSION
The primary finding of our study was that 6-months of exercise training did not improve CRP in previously sedentary, overweight and obese, postmenopausal women. These findings are in direct contrast to many cross-sectional reports, including our own, that have reported physical activity or cardiorespiratory fitness to be inversely associated with CRP. However, this adequately powered exercise intervention trial has produced conflicting results with respect to the ability of exercise to reduce CRP. In exercise training studies using smaller sample sizes and shorter intervention periods, exercise training has been shown to reduce CRP concentrations (14, 17). However, a recent meta-analysis reported that aerobic interventions that produced improvements in fitness by as much as 12% failed to reduce CRP (13). Some have hypothesized that positive changes in CRP are related to exercise intensity and mode of exercise; (4, 11, 18, 26) however, recent evidence by Huffman et al. have provided evidence to the contrary (11) by examining 6-months of aerobic exercise training at two different intensities (40-55% or 65-80% peak VO2) in a population at high risk for cardiovascular disease. Despite showing a decrease in adiposity and improvement in insulin sensitivity, aerobic exercise at either intensity was insufficient to positively modulate CRP concentration (11).
In a recent review involving 28 lifestyle (diet and/or exercise) and 5 surgical interventions (i.e., gastric banding), it was concluded that significant weight loss must occur in order for there to be a significant reduction in CRP (23). Our findings support this hypothesis, because despite having excellent exercise compliance and seeing a dose response increase in fitness across exercise groups, we observed minimal changes in weight combined with no change in CRP. However, we found the amount of weight change to be associated with change in CRP, and when individuals were categorized into quartiles of weight loss, the quartile with the greatest weight loss had the only within group significant change in CRP and had a significant reduction in CRP compared to all other quartiles.
At baseline, circulating CRP concentrations in women taking HRT were 40.3% higher when compared to women who were not taking HRT. These observations are consistent with the current literature (3, 9, 27). Baseline concentrations of CRP in the HRT group were significantly correlated with body mass index, waist circumference, systolic blood pressure and fasting triglycerides.
The limitations of our study deserve mention. It is important to note that DREW was not specifically designed to examine the effect of exercise training on CRP. The study population was limited to sedentary, overweight or obese, postmenopausal women at moderate risk for CVD and consequently, our results may not be similar in women of other ages or men. The current analysis is unable to account for potential genetic differences amongst our study participants as there is an increasing line of evidence examining the genetic control of CRP (10). For example, a variation in the CRP gene promoter (+1444C>T) in the 3’UTR has been associated with higher concentrations of circulating CRP (5). Further, a functional variant in the TNF-α gene, namely the presence of the TNFA2 allele, increases the potential for higher circulating concentrations of serum CRP (1). Thus, genetic control of CRP and CRP-regulating genes may provide additional information regarding individual assessment and potential to respond to an exercise program. Diet also appears to play a role in the production of CRP (20). Although we were not able to control the nutrient intake during the intervention period, analysis of dietary intake showed that there were no differences in mean energy intake (p = .56) for between-group differences.
The primary strengths of the DREW study are that it is a well designed and stringently controlled exercise dose trial with all exercise sessions being completed under laboratory conditions. Further, each exercise session was extensively monitored for energy expenditure, heart rate, and steps taken outside of the structured exercise prescription (7). In conclusion, in a group of previously sedentary, post-menopausal women, six months of aerobic exercise training did not improve CRP. However, reductions in weight were associated with improvements in CRP.
ACKNOWLEDGEMENTS
This work was performed at The Cooper Institute, and the staff is especially commended for their efforts. We thank The Cooper Institute Scientific Advisory Board and the DREW participants. This work was supported by NIH grants # HL66262 & HL071900. We thank Life Fitness for providing exercise equipment. The results of this study do not constitute endorsement by ACSM.
Funding: This work was supported by NIH grants # HL66262 & HL071900.
Footnotes
CONFLICT OF INTEREST None
Clinical Trial Registration Information: clinicaltrials.gov (Identifier NCT 00011193)
REFERENCES
- 1.Araujo F, Pereira AC, Mota GF, Mdo R Latorre, Krieger JE, Mansur AJ. The influence of tumor necrosis factor -308 and C-reactive protein G1059C gene variants on serum concentration of C-reactive protein: evidence for an age-dependent association. Clin Chim Acta. 2004 Nov;349(1-2):129–34. doi: 10.1016/j.cccn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault BJ, Cote M, Cartier A, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009 May 21; doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenfeld Z, Boulman N, Leiba R, et al. High C-reactive protein levels are associated with oral hormonal menopausal therapy but not with intrauterine levonorgestrel and transdermal estradiol. Scand J Clin Lab Invest. 2007;67(3):257–63. doi: 10.1080/00365510601113241. [DOI] [PubMed] [Google Scholar]
- 4.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4(1):19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brull DJ, Serrano N, Zito F, et al. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003 Nov 1;23(11):2063–9. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- 6.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002 Nov 1;22(11):1869–76. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 7.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007 May 16;297(19):2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 8.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004 Jul;52(7):1098–104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper BC, Burger NZ, Toth MJ, Cushman M, Sites CK. Insulin resistance with hormone replacement therapy: associations with markers of inflammation and adiposity. Am J Obstet Gynecol. 2007 Feb;196(2):123, e1–7. doi: 10.1016/j.ajog.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danik JS, Ridker PM. Genetic determinants of C-reactive protein. Curr Atheroscler Rep. 2007 Sep;9(3):195–203. doi: 10.1007/s11883-007-0019-2. [DOI] [PubMed] [Google Scholar]
- 11.Huffman KM, Samsa GP, Slentz CA, et al. Response of high-sensitivity C-reactive protein to exercise training in an at-risk population. Am Heart J. 2006 Oct;152(4):793–800. doi: 10.1016/j.ahj.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005 May 17;45(10):1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 13.Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006 Nov;55(11):1500–7. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005 Apr;54(4):533–41. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000 Jan;21(1):21–4. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 16.Morss GM, Jordan AN, Skinner JS, et al. Dose Response to Exercise in Women aged 45-75 yr (DREW): design and rationale. Med Sci Sports Exerc. 2004 Feb;36(2):336–44. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 17.Nassis GP, Papantakou K, Skenderi K, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005 Nov;54(11):1472–9. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond) 2007 Jun;31(6):996–1003. doi: 10.1038/sj.ijo.0803534. [DOI] [PubMed] [Google Scholar]
- 19.Physical activity and cardiovascular health. NIH consensus development panel on physical activity and cardiovascular health. JAMA. 1996 Jul 17;276(3):241–6. [PubMed] [Google Scholar]
- 20.Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007 Apr;26(2):163–9. doi: 10.1080/07315724.2007.10719598. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 22.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007 Jan 8;167(1):31–9. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Sites CK, Toth MJ, Cushman M, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002 Jan;77(1):128–35. doi: 10.1016/s0015-0282(01)02934-x. [DOI] [PubMed] [Google Scholar]
- 25.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999 May 12;281(18):1722–7. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 26.Stewart LK, Flynn MG, Campbell WW, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007 Oct;39(10):1714–9. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 27.Zegura B, Guzic-Salobir B, Sebestjen M, Keber I. The effect of various menopausal hormone therapies on markers of inflammation, coagulation, fibrinolysis, lipids, and lipoproteins in healthy postmenopausal women. Menopause. 2006 Jul-Aug;13(4):643–50. doi: 10.1097/01.gme.0000198485.70703.7a. [DOI] [PubMed] [Google Scholar]