Abstract
Postmenopausal women (PMW) are at greater risk for salt-sensitive hypertension and insulin resistance than premenopausal women. Peroxisome proliferator activated receptor-gamma (PPARγ) agonists reduce blood pressure (BP) and insulin resistance in humans. As in PMW, ovariectomy (OVX) increases salt sensitivity of BP and body weight in Dahl salt sensitive (DS) rats. This study addressed whether rosiglitazone (ROSI), a PPARγ agonist, attenuates salt-sensitive hypertension in intact (INT) and OVX DS rats, and if so, whether insulin resistance, nitric oxide (NO), oxidative stress, and/or renal inflammation were contributing mediators. Telemetric BP was similar in OVX and INT on low salt diet (0.3% NaCl), but was higher in OVX than INT on high salt (8% NaCl). ROSI reduced BP in OVX and INT on both low and high salt diet, but only attenuated salt sensitivity of BP in OVX. Nitrate/nitrite excretion (NOx; index of NO) was similar in INT and OVX on low salt diet, and ROSI increased NOx in both groups. High salt diet increased NOx in all groups but ROSI only increased NOx in OVX rats. OVX females exhibited insulin resistance, increases in body weight, plasma leptin, cholesterol, numbers of renal cortical macrophages, and renal MCP-1 and osteopontin mRNA expression compared to INT. ROSI reduced cholesterol and macrophage infiltration in OVX, but not INT. In summary, PPAR-gamma activation reduces BP in INT and OVX females, but attenuates the salt sensitivity of BP in OVX only likely due to increases in NO and in part to reductions in renal resident macrophages and inflammation.
Keywords: ovariectomy, menopause, hypertension, inflammation, oxidative stress
Introduction
In women the prevalence of hypertension increases after menopause. In addition, the incidence of salt sensitivity of blood pressure (BP) is greater following both surgical and natural menopause (18). Increases in BP following menopause are also associated in many cases with increases in body weight and insulin resistance.
Current data from female Dahl Salt-sensitive (DS) rats indicate that after 15 weeks of age, ovariectomy (OVX) is accompanied by an increase in BP, when compared to intact females, regardless of whether rats are maintained on high (11; 28) or low salt diets (10; 28), suggesting a protective role for female hormones in preventing BP increases in this rat strain. OVX increases body weight in female DS rats, and even after pair-feeding, OVX rats have significantly greater body weight (11). Whether OVX female DS exhibit other metabolic disturbances, such as insulin resistance, in addition to increased body weight, that could impact BP has not been addressed.
Renal inflammation has been shown to play a role in the development of hypertension in several animal models (20; 21), including male DS rats (17) and ovariectomized female rats (1). There is also evidence that increases in reactive oxygen species (ROS) are important mediators of hypertension in male rats (23). However, our own data do not support a role for oxidative stress in control of blood pressure in female rats, such as spontaneously hypertensive rats or females given angiotensin (Ang) II (23). Whether oxidative stress and renal inflammation play roles in mediating salt sensitivity of BP in OVX DS rats has not been determined.
Peroxisome proliferator activated receptor gamma (PPARγ) agonists are well-known insulin-sensitizers and lower glucose in individuals with type II diabetes mellitus (16). In addition, PPARγ agonists reduce BP in humans (15; 19) and animal models of hypertension, such as male DS rats (3), and have anti-inflammatory and antioxidant properties. However, whether PPARγ agonists will attenuate salt-sensitive hypertension in females has not been studied.
Therefore, the present study addressed whether rosiglitazone (ROSI), a PPARγ agonist, would attenuate salt-sensitive hypertension in intact (INT) and/or ovariectomized (OVX) Dahl salt sensitive (DS) rats, and if so, whether insulin resistance, oxidative stress, and/or inflammation were contributing mediators.
Methods
Animals
Female DS rats (Rapp strain) were purchased from Harlan Sprague-Dawley, Inc (Indianapolis, IN) at 7 weeks of age. Ovariectomy (OVX) was performed by the vendor at 6 weeks of age in half of the rats. Animals were maintained on tap water and low salt, low nitrate/nitrite, phytoestrogen-free diet (AIN 76A, 0.3% sodium chloride, Harlan Sprague-Dawley) ad libitum in 12-hour:12-hour light/dark cycle, temperature-controlled rooms. The protocols complied with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Measurement of Blood Pressure
At 8 weeks of age rats were implanted with radiofrequency transmitters (Transoma Medicals, St. Paul, MN), using TA11PA-C40 transducers (Transoma Medical), as we previously described (24). Rats were placed in individual cages over an RLA-3000 radiotelemetry receiver (Transoma Medicals). Mean arterial pressure (MAP) recordings were obtained during 10-second sampling periods every 7 minutes and are expressed as 24-hour averages.
Rats were allowed to recover 2 weeks after telemeter implantation, and then MAP was recorded throughout the study, starting at 10 weeks of age. Baseline MAP was recorded for 1 day in intact (INT) and OVX female DS rats, and thereafter, they were assigned to four groups (n=6–8 per group): INT and OVX rats receiving low salt diet alone (INT-Control and OVX-Control); INT and OVX rats receiving low salt diet containing ROSI (100mg/kg diet; equivalent to a dose of approximately 5–6 mg/kg body weight) (INT-ROSI and OVX-ROSI). MAP was recorded for 1 week, and then all rats were challenged for 1 week to high salt diet (AIN 76A + 8% sodium chloride) in the presence or absence of ROSI.
Food intake was measured daily. Body weight was measured every other day. Rats were housed in metabolic cages for 24 h urine collections at the end of the first week (low salt) and the end of the high salt diet period. Blood samples and kidneys were collected at the end of the experiment for further studies, as described below.
Blood Glucose, Plasma Estradiol, Insulin, Leptin and Cholesterol
At the end of the study on high salt diet and after an overnight fast, blood glucose was determined using a glucometer (Accucheck Advantage; Roche). Plasma estradiol was measured by radioimmunoassay (Ultrasensitive estradiol kit, Diagnostic Systems Laboratories, Inc. Webster, TX), as we previously described (24). Plasma insulin and leptin were measured by radioimmunoassay, according to the manufacturer’s recommendations using commercially available kits (Linco Research, St. Charles, MO). Plasma cholesterol was measured using an enzymatic colorimetric method following the manufacturer’s directions (Wako Pure Chemical Industries, Ltd., Richmond, VA).
Urinary F2-isoprostanes, nitrate/nitrite and protein
Urinary F2-isoprostanes were measured using a commercially available kit (Oxford Biomedical Research, Oxford, MI), as we previously described (24). F2-isoprostanes were normalized to urinary creatinine (CR 01, Oxford Biomedical Research). Data are presented as ng F2-isoprostanes per mg of creatinine. Nitrate/nitrite (NOx) excretion was measured as we previously described (24). Urinary protein excretion was measured using the Bradford assay (BioRad, Torrance, CA) and bovine serum albumin as standard. Data are presented as mg protein excreted/24 hrs.
Immunohistochemistry Staining for Macrophage Cells in the Kidney
Kidneys from each rat were perfused free of blood with 2% heparin in 0.9% saline, removed, weighed and placed in 10% buffered formalin. Kidneys were embedded in paraffin, cut into 5 micron serial sections, and placed on glass slides. Macrophage presence was detected by immunohistochemistry, using anti-CD68 (ED-1) antibodies (Chemicon MAB1435, Chemicon International Inc., Temecula, CA, 1:50). Control slides were incubated with non-immune serum. Slides were analyzed by an observer blinded to the groups (LR). Glomerular and peritubular ED-1 positive cells were counted and averaged per 30 glomeruli and 30 high power fields (40X), respectively. Data are expressed as number of glomerular and peritubular ED-1 positive cells.
Measurement of Renal Cortical Macrophage Chemoattractant Protein-1 (MCP-1) and Osteopontin mRNA Expression
Renal cortical total RNA was extracted, reverse transcribed and gene expression quantified by real time PCR, as we previously described (15). MCP-1 primers were (sense: TAGCATCCACGTGCTGTCTC, antisense: CCGACTCATTGGGATCATCT, product size: 91 bp). Osteopontin primers were previously described by Blasi and colleagues (2). GAPDH mRNA levels were used for standardization.
Statistical Analyses
Data are expressed as mean±SEM. For comparisons among groups, a two-way analysis of variance (ANOVA) was used, followed by the Student-Newman-Keuls post-hoc test using Prism 5.0 version (GraphPad). Significance was defined at P<0.05.
Results
Body Weight, Food Intake and Plasma Estradiol
The initial body weight was higher in OVX compared to INT rats (265.3±2.6 vs. 228±1.5 g; OVX vs. INT, respectively, p<0.001). Over the course of the experiment, all animals gained weight, but OVX rats gained more weight than INT females, and ROSI-treated animals gained more weight than controls (% change: INT control, +5.9±1.1; INT + ROSI, +13.1±1.1; OVX control, +10.9±0.1; OVX + ROSI, +19.5±0.7 % (p<0.05 INT vs. OVX; p<0.05, INT or OVX vs. INT + ROSI or OVX + ROSI). Despite the differences in weight gain, food intake on high salt diet was not different among the experimental groups (INT-Control: 56.1 ± 2.1, INT-ROSI: 53.7 ± 2.1, OVX-Control: 57.0 ± 0.7, OVX-ROSI: 57.9 ± 1.2 g/kg BW). As expected, OVX significantly reduced plasma estradiol levels compared to INT, and ROSI did not alter estradiol levels in INT or OVX (Table 1).
Table 1.
Blood glucose, plasma estradiol, insulin, leptin and cholesterol, and urinary F2-isoprostanes levels.
| INT-Control | INT-ROSI | OVX-Control | OVX-ROSI | |
|---|---|---|---|---|
| Estradiol (pg/ml) | 25.9±2.4 | 38.5±5.6 | 6.3±0.7* | 6.7±0.3* |
| Insulin (uU/ml) | 6.7±0.6 | 7.6±0.6 | 11.7±1.4* | 14.1±2.0* |
| Leptin (ng/ml) | 0.78±0.07 | 0.74±0.07 | 2.17±0.35* | 1.97±0.17* |
| Cholesterol (mg/dl) | 79.0±3.6 | 89.4±2.5 | 124.6±7.7* | 109.0±5.1*† |
| Glucose (mg/dl) | 70.6±5.1 | 70.6±4.1 | 61.4±1.9 | 59.1±2.1 |
| Urinary F2-isoprostanes (ng/mg creatinine) | 7.3±0.5 | 6.0±0.6 | 3.4±0.1* | 2.9±0.1* |
Data shown are mean±SEM.
p<0.05 compared to INT same treatment condition;
p<0.05, compared to Control same condition.
Blood Pressure
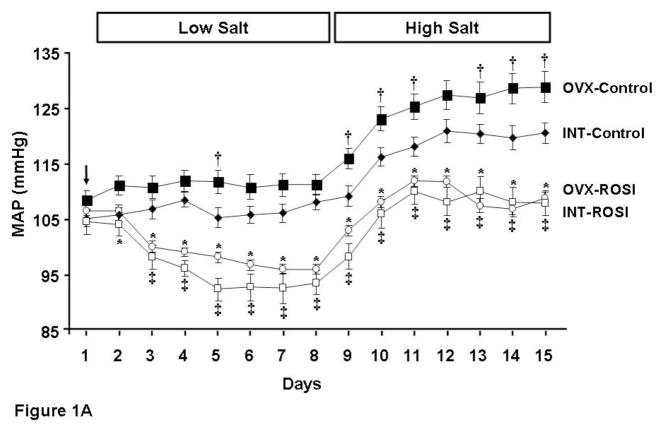
MAP was recorded for 1 week on low salt diet and 1 week on high salt diet (Figure 1A). On low salt diet MAP was not different between INT-Control and OVX-Control rats, and ROSI reduced MAP to a similar extent in both INT and OVX rats (approximately 15 mm Hg). With high salt diet, MAP increased in all rats (Figure 1A). Salt sensitivity of BP was greatest in OVX rats (increase of approximately 18 mm Hg compared to 13 mm Hg in INT; see Figure 1B). The absolute MAP was attenuated with ROSI in both INT and OVX rats and was not different between groups (Figure 1A). However, salt sensitivity of BP was only attenuated with ROSI in OVX (Figure 1B).
Figure 1. Mean arterial pressures (MAP) in intact (INT) and ovariectomized (OVX) female Dahl Salt-sensitive rats given control diet (Control) or diet containing rosiglitazone (ROSI).
1A: Baseline MAP was measured for 24 h (Day 1). Then rats (n=6–8 per group) were assigned to receive for 1 week either control low salt (0.3% NaCl) diet (INT-Control and OVX-Control) or low salt diet with rosiglitazone (INT-ROSI and OVX-ROSI). On day 9 all the rats were switched for 1 week to either control high salt (8% NaCl) diet (INT-Control and OVX-Control) or high salt diet with rosiglitazone (INT-ROSI and OVX-ROSI). Data are expressed as mean arterial pressure (MAP) as measured by radiotelemeter devices placed in the abdominal aorta. Black closed diamonds, INT-Control; black closed squares, OVX-Control; white closed squares, INT-ROSI; white closed circles, OVX-ROSI. The arrow indicates baseline MAP. †, p<0.05, compared to INT-Control; *, p<0.05, compared to OVX-Control; ‡, p<0.05, compared to INT-Control. 1B: Delta MAP last day on high salt diet (HS) minus last day on low salt diet (LS). *, p<0.05, compared to INT-Control and INT-ROSI; †, p<0.05, compared to OVX-Control.
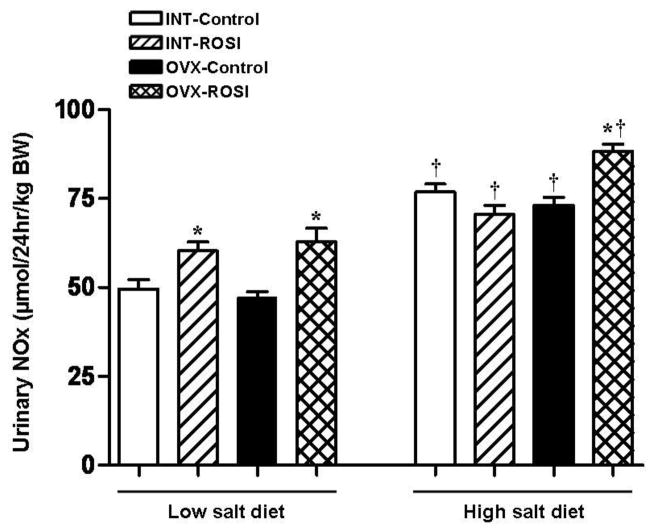
As an index of oxidative stress, F2-isoprostane excretion was measured in rats on high salt diet. F2-isoprostane excretion was higher in INT than OVX rats (Table 1), and was not affected by ROSI in either group. As an index of nitric oxide production, urinary nitrate/nitrite excretion was measured in rats at the end of the first week of ROSI and at the end of the experiment. As shown in Figure 2, at the end of the first week of ROSI with rats on low salt diet, NOx was similar in OVX-control and INT-control. With ROSI, there was an increase in NOx in both OVX and INT. At the end of the study on high salt diet, NOx increased in all groups and to similar levels in INT- and OVX-control rats. ROSI had no further effect on NOx in INT rats, but increased NOx in OVX rats.
Figure 2. Urinary protein excretion in intact (INT) and ovariectomized (OVX) female Dahl Salt-sensitive rats given high salt diet alone (Control) or high salt diet containing rosiglitazone (ROSI).
24 h protein excretion was significantly reduced in INT and OVX rats given ROSI. *, p<0.05 compared to Control same condition.
Plasma insulin, glucose, leptin, cholesterol
Plasma leptin and insulin, measured at the end of high salt diet in fasted rats, were elevated in OVX compared to INT, and were not changed by ROSI. Despite higher insulin levels in OVX, fasting glucose was similar to or slightly lower in OVX compared to INT, suggesting that OVX females were insulin resistant. ROSI had no effect on fasting glucose in OVX or INT. Plasma cholesterol levels were significantly higher in OVX than INT, and were slightly, yet significantly, reduced by ROSI only in OVX (Table 1).
Markers of renal inflammation and injury
As shown in Figure 3, on high salt diet, urinary protein excretion was similar in OVX and INT rats, and ROSI reduced proteinuria in both groups. As shown in Figure 4, macrophage infiltration was greater in high salt fed OVX than INT and decreased with ROSI in OVX only. Renal cortical mRNA expressions of MCP-1 and osteopontin were significantly higher in OVX than INT rats, and ROSI had no effect in either group (see Figure 5).
Figure 3. Urinary Nitrate/nitrite (NOx) excretion in intact (INT) and ovariectomized (OVX) female Dahl Salt-sensitive rats given control diet (Control) or diet containing rosiglitazone (ROSI).
On low salt diet urinary NOx was significantly increased with ROSI in both INT and OVX rats. Compared to low salt diet, high salt diet elicited a significant increase in urinary NOx in all the experimental groups and to similar levels in INT- and OVX-control rats. ROSI had no further effect on NOx in INT rats, but increased NOx in OVX rats. *, p<0.05 compared to Control same condition. †, p<0.05, compared to low salt diet same treatment and condition.
Figure 4. Immunohistochemical analyses for ED-1-positive cells (macrophages) in intact (INT) and ovariectomized (OVX) female Dahl Salt-sensitive rats given high salt diet alone (Control) or high salt diet containing rosiglitazone (ROSI).

Glomerular ED-1-positive cells (2A) and peritubular ED-1-posivite cells (2B) were significantly higher in OVX-Control rats and were significantly reduced by treatment with ROSI only in OVX rats. Representative immunohistochemical staining for ED-1-positive cells (arrows) in OVX-Control and OVX-ROSI rats (Panel C, magnification 20X). *, p<0.05, compared to INT-Control and INT-ROSI; †, p<0.05, compared to OVX-Control.
Figure 5. Renal Cortical Macrophage Chemoattractant Protein-1 MCP-1 and Osteopontin mRNA Expression in intact (INT) and ovariectomized (OVX) female Dahl Salt-sensitive rats given control diet (Control) or diet containing rosiglitazone (ROSI).
Renal cortical MCP-1 mRNA (Panel A) and Osteopontin mRNA (Panel B) expression were significantly higher in OVX compared to INT animals and treatment with ROSI had no effect on their expression. *, p<0.05, compared to INT-Control and INT-ROSI.
Discussion
Postmenopausal women have greater salt sensitivity of blood pressure than pre-menopausal women (18). Consistent with these data, OVX of female DS rats results in greater salt sensitivity of blood pressure than in INT females (11; 12; 28). The reasons why menopause with the reduction in estradiol levels causes increases in salt-sensitive hypertension are not clear and were the focus of the present study. The present study tested whether rosiglitazone (ROSI), a PPARγ agonist, would attenuate salt-sensitive hypertension in INT and/or OVX DS rats, and if so, whether insulin resistance, oxidative stress, and/or inflammation were contributing mediators.
There are several new findings in this study: 1) ROSI reduces blood pressure in INT and OVX female DS rats regardless of salt intake, but reduced salt sensitivity of BP only in OVX rats; 2) On low salt diet, NOx was similar in INT-control and OVX-control rats that had similar BPs, and ROSI increased NOx in both groups leading to lower BP in both INT and OVX; however, on high salt diet, NOx increased only in OVX-ROSI rats compared to the other groups suggesting that the increase in NOx with ROSI protected against salt sensitivity of BP in OVX rats, but not INT; 3) OVX DS rats on high salt diet exhibit insulin resistance compared with INT females, which is consistent with studies in postmenopausal women who typically gain weight after menopause (14; 22); 4) OVX DS rats on high salt diet exhibit higher levels of renal inflammatory markers than INT; 5) despite the reductions in blood pressure with ROSI, only hypercholesterolemia, but not hyperleptinemia or hyperinsulinemia, was reduced in OVX females, which does not support a role for insulin resistance in mediating the higher BP in OVX females; 6) oxidative stress plays no role in mediating the salt-sensitive hypertension in either INT or OVX DS, since ROSI reduced blood pressure without affecting oxidative stress; 7) the ROSI-mediated reduction in renal macrophage infiltration likely contributes to the lower BP in OVX rats. The mechanism by which ROSI reduces BP in INT is not clear from the present studies.
In previous studies, Bolten and colleagues reported that ROSI only transiently reduced blood pressure, mainly diastolic blood pressure, in male DS that had been on high salt diet for 3 weeks prior to start of ROSI (3). Whether ROSI would reduce blood pressure in female DS had not been tested previous to our study. Our protocol was slightly different than that of Bolten and colleagues since the OVX and INT female DS were given ROSI prior to the start of and during the high salt diet. In the present study ROSI had a sustained effect on BP in both INT and OVX females regardless of salt intake. It is possible that the male DS in the Bolten study had renal damage at the time ROSI was started following three weeks of high salt and thus ROSI was not as effective as in the females in our study. Of course, we cannot rule out the possibility that blood pressure in the female DS is more responsive to ROSI than in males irrespective of the experimental protocol.
ROSI attenuated the salt sensitivity pressor response in OVX females, but not INT females. As shown in Figure 1B, there was an increase in BP in both INT and OVX females, but in ROSI-treated INT rats, the increase in BP was similar on high salt as in untreated INT rats on high salt, thus having no effect on the salt sensitivity of BP. In contrast, in OVX rats, ROSI attenuated the pressor response to high salt diet compared to untreated OVX protecting against the salt sensitivity of BP. Nitric oxide is likely to have played an important role in mediating the pressor response to salt in all groups. For example, on low salt diet, NOx and BP were similar in INT and OVX females. ROSI increased NOx in both INT and OVX leading to lower but similar BP in both groups. With high salt diet, BPs and NOx increased in all groups. However, untreated OVX exhibited the greatest response to salt and thus the most salt sensitivity of BP. ROSI increased the NOx leading to lower BP in OVX and attenuating the salt sensitivity of BP. In INT, high salt increased BP by similar amounts in both INT-controls and INT-ROSI, and NOx was similar between the groups. These data suggest that there was a compensatory increase in nitric oxide in response to high salt diet that was present in both groups. However, with high salt diet ROSI only significantly increased NOx in OVX rats, and thus attenuated the salt sensitivity of BP in OVX, not INT. It is somewhat difficult to explain why NOx was similar among INT-control, INT-ROSI and OVX-control and yet BPs were significantly different among the three groups. The mechanism for these differences may reflect the additional renal inflammation in the OVX-controls.
In the present study we evaluated several other mechanisms by which ROSI could reduce BP in female DS rats. Obesity and metabolic syndrome are major risk factors for the development of hypertension and other adverse cardiovascular events (9). Menopause is associated with weight gain and leads to increased incidence of metabolic syndrome compared with premenopausal women (14; 22). OVX DS rats exhibit weight gain compared to INT females, which is one reason ROSI was tested as a possible antihypertensive in this study. Although not obese per se, OVX females had greater body weight and increased plasma insulin, leptin and cholesterol, compared to INT rats. Peroxisome proliferator activated receptors (PPARs) are transcription factors that belong to a nuclear receptor superfamily and regulate gene transcription upon ligand binding. Three PPARs have been identified to date, PPARα, PPARβ and PPARγ (26). ROSI is a PPARγ agonist that has been widely used for treatment of type 2 Diabetes Mellitus, which is commonly associated with weight gain and insulin resistance, because it improves peripheral utilization of glucose. Thus we anticipated that ROSI would have a positive effect on parameters characteristic of metabolic syndrome and reduce BP.
However, although ROSI reduced BP in both INT and OVX female DS, there was no change in plasma leptin, insulin or fasting glucose in the respective groups. In fact, ROSI increased body weight in both OVX and INT compared with their respective controls, and had no effect on plasma insulin or leptin. There was a reduction in plasma total cholesterol in OVX females treated with ROSI. Thus increased lipids, but not insulin resistance, may contribute to salt sensitivity of blood pressure in OVX female DS, but are not likely to be important in INT females.
Oxidative stress is thought to be a major factor in mediating hypertension in males of many models of hypertension (24). In male DS rats given a high salt diet, antioxidants, such as tempol and apocynin, reduce blood pressure (13). In the present study we found that urinary F2-isoprostanes were lower in salt treated OVX-controls that had higher BP than in INT-controls. Furthermore, ROSI had no effect on either group. In addition, NOx was similar between INT-control and OVX-control rats on high salt diet despite the higher BP in OVX-controls, and ROSI only increased NOx in OVX despite reducing BP in both INT and OVX rats, thus not supporting a role for oxidative stress in the BP of OVX or INT rats. These data are consistent with our previous studies showing that neither increasing oxidative stress with molsidomine or decreasing oxidative stress with antioxidants play a role in controlling BP in female rats. For example, increasing oxidative stress will increase blood pressure in male SHR, but not in females, and by the same token, decreasing oxidative stress will decrease blood pressure in male SHR, but not females (23). Similar findings have been made by other investigators in females of other models of hypertension (4; 6). We also have unpublished data that female DS rats, INT or OVX, do not exhibit a depressor response to antioxidant treatment (Lopez-Ruiz and Reckelhoff, unpublished observations). Thus the effect of ROSI to attenuate salt-sensitive hypertension in INT and OVX females in the present study is likely independent of oxidative stress.
ROSI has also been shown to reduce systemic and tissue inflammation (7; 10). In the present study we found that OVX DS rats on high salt diet had a greater infiltration of macrophages into their kidneys than did INT females. With ROSI, there was no effect on macrophage numbers in kidneys of INT rats, but there was a significant reduction of macrophages in kidneys of OVX rats. In previous studies in male DS rats, other investigators found that renal macrophage infiltration and inflammatory responses play an important role in mediating salt-sensitive hypertension, and that chronic treatment with mycophenolate mofetil (MMF), an anti-inflammatory drug, attenuated salt-sensitive hypertension (17). Although intrarenal macrophage infiltration was attenuated by ROSI in OVX females, it was not due to reductions in expression of macrophage chemotactic molecules, MCP-1 or osteopontin. However, since activation of PPARγ has been shown to directly inhibit macrophage function and stimulate macrophage apoptosis (5), it is possible that a direct effect on macrophage function played a role in the attenuation of salt sensitivity of blood pressure in OVX female DS rats. On the other hand, it is not likely that inhibition of macrophage activity strongly contributed to salt-sensitive hypertension in INT females since they had significantly lower renal macrophage levels compared to OVX, and ROSI reduced the BP in INT but not the number of resident macrophages in their kidneys. Urinary protein excretion was reduced with ROSI in both INT and OVX females on high salt, but this was likely due to the reduction in BP with ROSI in both groups.
Our data suggest that different mechanisms may be responsible for the ROSI-induced reductions in BP in INT and OVX female DS. The question remains as to why. Our data support the concept that the basis for the difference may be due to estrogen or estrogen-mediated receptor activation and/or signal transduction. Several studies have shown cross-talk between PPARγ agonists and estrogen receptors (ER) (8; 25), and estradiol inhibits PPARγ transactivation in breast cancer cells expressing ERα but not ER (27). Therefore, it is tempting to speculate that ROSI reduces salt-sensitive hypertension in OVX rats in part by activating ERα, just as estradiol would, and by increasing nitric oxide and attenuating intrarenal macrophage infiltration. In INT rats higher estradiol levels would interfere with renal PPARγ activation explaining the lack of attenuation of the pressor response to high salt compared to OVX. Future studies will be needed to address this hypothesis.
In conclusion, the present study shows that the PPARγ agonist ROSI reduces BP in INT and OVX female DS rats on both high and low salt diets, but attenuated the salt sensitivity of BP in OVX females only. The effect of ROSI was independent of oxidative stress in both groups. In addition, modulation of insulin resistance did not play a role in reducing BP in ROSI-treated OVX. In OVX females the mechanism by which ROSI attenuated salt-sensitive hypertension is likely mediated by increased nitric oxide production, and in part by inhibition of intrarenal macrophage infiltration, and thus renal inflammation and to reduced cholesterol. The mechanism by which ROSI reduces the BP in INT female DS on high salt diet, but does not attenuate the salt sensitivity of BP, is not clear.
Perspectives and Significance
BP in postmenopausal women increases with advancing age. Salt sensitivity of BP also increases with age in postmenopausal women. Our data suggest that ROSI may protect against the increase in BP in young postmenopausal women. However, with aging it is likely that there is an increase in endothelial dysfunction and thus if the mechanism by which ROSI attenuates salt sensitive hypertension is mediated by nitric oxide, it is likely that ROSI may not be as protective in aging postmenopausal women as in younger women.
Acknowledgments
The authors would like to thank Huimin Zhang and Stephanie Evans for excellent technical support. J. C. Sartori-Valinotti is recipient of American Heart Association, Greatest Southeast Affiliate Postdoctoral Fellowship (0725561B). Licy L. Yanes is recipient of American Heart Association Scientist Development Grant (0830239N). This work was supported by HL51971, HL69194, and HL66072 (to JFR) and HL085907 (to MJR) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 2.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 3.Bolten CW, Payne MA, McDonald WG, Blanner PM, Chott RC, Ghosh S, Arhancet GB, Staten NR, Gulve EA, Sullivan PM, Hromockyj AE, Colca JR. Thiazolidinediones inhibit the progression of established hypertension in the Dahl salt-sensitive rat. Diab Vasc Dis Res. 2007;4:117–123. doi: 10.3132/dvdr.2007.029. [DOI] [PubMed] [Google Scholar]
- 4.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2. Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med. 2008;5 (Suppl A):S65–S75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens. 2007;25:1263–1271. doi: 10.1097/HJH.0b013e3280acac60. [DOI] [PubMed] [Google Scholar]
- 7.Efrati S, Berman S, Chachashvili A, Cohen N, Siman-Tov Y, Averbukh Z, Weissgarten J. Rosiglitazone treatment attenuates renal tissue inflammation generated by urinary tract obstruction. Nephrology (Carlton ) 2009 doi: 10.1111/j.1440-1797.2008.01032.x. [DOI] [PubMed] [Google Scholar]
- 8.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guize L, Pannier B, Thomas F, Bean K, Jego B, Benetos A. Recent advances in metabolic syndrome and cardiovascular disease. Arch Cardiovasc Dis. 2008;101:577–583. doi: 10.1016/j.acvd.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–684. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 11.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;42:1157–1163. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- 12.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 13.Hisaki R, Fujita H, Saito F, Kushiro T. Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats. Am J Hypertens. 2005;18:707–713. doi: 10.1016/j.amjhyper.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 14.Innes KE, Selfe TK, Taylor AG. Menopause, the metabolic syndrome, and mind-body therapies. Menopause. 2008;15:1005–1013. doi: 10.1097/01.gme.0b013e318166904e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komajda M, Curtis P, Hanefeld M, Beck-Nielsen H, Pocock SJ, Zambanini A, Jones NP, Gomis R, Home PD. Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study) Cardiovasc Diabetol. 2008;7:10. doi: 10.1186/1475-2840-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinowski JM, Bolesta S. Rosiglitazone in the treatment of type 2 diabetes mellitus: a critical review. Clin Ther. 2000;22:1151–1168. doi: 10.1016/s0149-2918(00)83060-x. [DOI] [PubMed] [Google Scholar]
- 17.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 18.Myers J, Morgan T. The effect of sodium intake on the blood pressure related to age and sex. Clin Exp Hypertens A. 1983;5:99–118. doi: 10.3109/10641968309048813. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson PM, Hedblad B, Donaldson J, Berglund G. Rosiglitazone reduces office and diastolic ambulatory blood pressure following 1-year treatment in non-diabetic subjects with insulin resistance. Blood Press. 2007;16:95–100. doi: 10.1080/08037050701396652. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 22.Rossi R, Nuzzo A, Origliani G, Modena MG. Metabolic syndrome affects cardiovascular risk profile and response to treatment in hypertensive postmenopausal women. Hypertension. 2008;52:865–872. doi: 10.1161/HYPERTENSIONAHA.108.110478. [DOI] [PubMed] [Google Scholar]
- 23.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 24.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 25.Talbert DR, Allred CD, Zaytseva YY, Kilgore MW. Transactivation of ERalpha by Rosiglitazone induces proliferation in breast cancer cells. Breast Cancer Res Treat. 2008;108:23–33. doi: 10.1007/s10549-007-9575-y. [DOI] [PubMed] [Google Scholar]
- 26.Touyz RM, Schiffrin EL. Peroxisome-proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vasc Pharmacol. 2006;45:19–28. doi: 10.1016/j.vph.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Kilgore MW. Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol. 2002;194:123–133. doi: 10.1016/s0303-7207(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 28.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009 Apr;296(4):F771–9. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]