Abstract
Keratinocytes in the skin play an important role in innate immune responses by secreting chemokines. This study aimed to determine if keratinocyte cell lines can be used for studies of innate immune mechanisms. Human primary keratinocytes and the HaCaT, CCD 1106 KERTr (KERTr) and HEK001 cell lines were treated with a panel of Toll-like receptor (TLR)-ligands. Expression of IL-8, CCL20, CXCL9 and CXCL10 was determined. All three cell lines expressed TLR1-6 and TLR9. KERTr cells responded to the same TLR-ligands as primary keratinocytes. Overall HEK001 responded similarly, but appeared to be relatively more sensitive to flagellin. This was in agreement with increased expression of TLR5. The expression profiles were most distinct in HaCaT cells. Furthermore, our data confirms and extends previously reported TLR7 and TLR8 independent IL-8 secretion by keratinocytes after Imiquimod treatment. The different cell lines represent complementary tools for molecular studies of innate immunity of the skin.
Keywords: Human, Keratinocytes, Innate Immunity of the skin, Chemokines, TLRs
Background
The innate immune response is the first line of host defence. Recognition of different classes of microorganisms involves signalling through specific receptors, one major group being the Toll-like receptors (TLRs). Following pathogen detection the TLRs mediate activation of innate and adaptive immune responses through modulation of gene expression (1). Keratinocytes play an essential role in orchestrating immune responses by producing chemokines following TLR engagement (2, 3). These chemokines in turn stimulate migration of leukocytes to the site of injury or infection. TLRs recognise highly conserved motifs from different microorganisms including lipopolysaccharide (LPS/recognized by TLR4), peptidoglycan (PGN/TLR2), lipoteichoic acid (LTA/TLR2), double-stranded RNA (TLR3), flagellin (TLR5), CpG motifs in DNA (TLR9) and yeast zymosan (TLR2/6) (4). TLR1, TLR2 and TLR4 are constitutively expressed by keratinocytes throughout the epidermis, while TLR3 and TLR5 expression is primarily restricted to the basal layer of keratinocytes (5, 6).
The TLR7-ligand Imiquimod (an imidazoquinoline amine analogue to guanosine) is widely used in the treatment of skin disorders such as warts, actinic keratosis and basal cell carcinoma (7). TLR7 and TLR8 are expressed by immune cells such as monocytes and dendritic and B cells (8) but not keratinocytes (2, 9, 10). Curiously, it has been reported that Imiquimod, independent of TLR7, stimulates cytokine production by keratinocytes (11, 12) possibly through inhibition of adenylyl cyclase and adenosine receptor signalling (13).
Questions addressed
Cell lines are invaluable tools for molecular studies of innate immune mechanisms, including signalling pathways activated by the TLRs. HaCaT, a spontaneously occurring keratinocyte cell line, has been widely used as a model of keratinocyte function. However, reports have shown that HaCaT cells exhibit limitations for some applications (14). The less used CCD 1106 KERTr (KERTr) and HEK001 cell lines were generated through transformation with the human papillomavirus 16 (HPV-16) E6/E7 proteins. The KERTr cells have been used in studies of UVB induced apoptosis in keratinocytes (15). HEK001 cells express ICAM-1 and keratin 14, but not keratin 10. The latter suggests these cells are proliferating basal cells (16). HEK001 cells can take up flurbiprofen and indomethacin (17). In this study we examined if HaCaT, KERTr and HEK001 cells lines respond to TLR-ligands in manners similar to primary keratinocytes.
Experimental design
Human primary keratinocytes and the HaCaT, KERTr (CRL-2309) and HEK001 (CRL-2404) cell lines were treated with a panel of TLR-ligands (Supplement-1). TLR and keratin mRNA expression was examined as described elsewhere ((18) and Supplement-1). ELISAs were used to determine levels of IL-8 (PeproTech, Rocky Hill, NJ), CCL20, CXCL9 and CXCL10 (R&D Systems, Minneapolis, MN). Data are shown as average values and standard deviations (SD) from one representative experiment of at least three experiments. Data were analyzed using the Student's t test.
Results
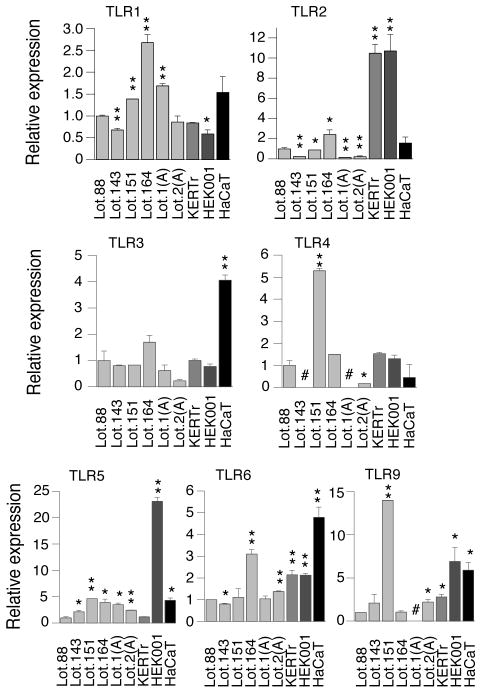
It has been demonstrated that experiments using primary cells can be influenced by the age and differentiation stage of the cells, variations in isolation techniques as well as donor differences (19). Likewise, as depicted on Figure 1, TLR mRNA expression varies between different batches of primary cells. The TLR7 and TLR8 mRNAs were not detected in any of the examined keratinocytes (data not shown). Given the variation in TLR mRNA expression between batches of primary cells expression of the TLRs in the three cell lines was in general similar. Noticeable differences included: higher expression of TLR2 in KERTr and HEK001, TLR3 in HaCaT and TLR5 in HEK001.
Figure 1.
TLR mRNA expression in primary keratinocytes and the KERTr, HEK001 and HaCaT cell lines. Total RNA was isolated from 6 independent batches of primary keratinocytes and KERTr, HEK001 and HaCaT cells. TLR mRNA levels were determined using real-time RT-PCR and the comparative CT method. TLR mRNA levels were normalized against levels of GAPDH mRNA and are graphically represented relative to the levels in the primary keratinocyte Lot 88. *, P < 0.05 (compared to primary keratinocytes Lot 88). **, P < 0.01 (compared to primary keratinocytes Lot 88). #, Not determined.
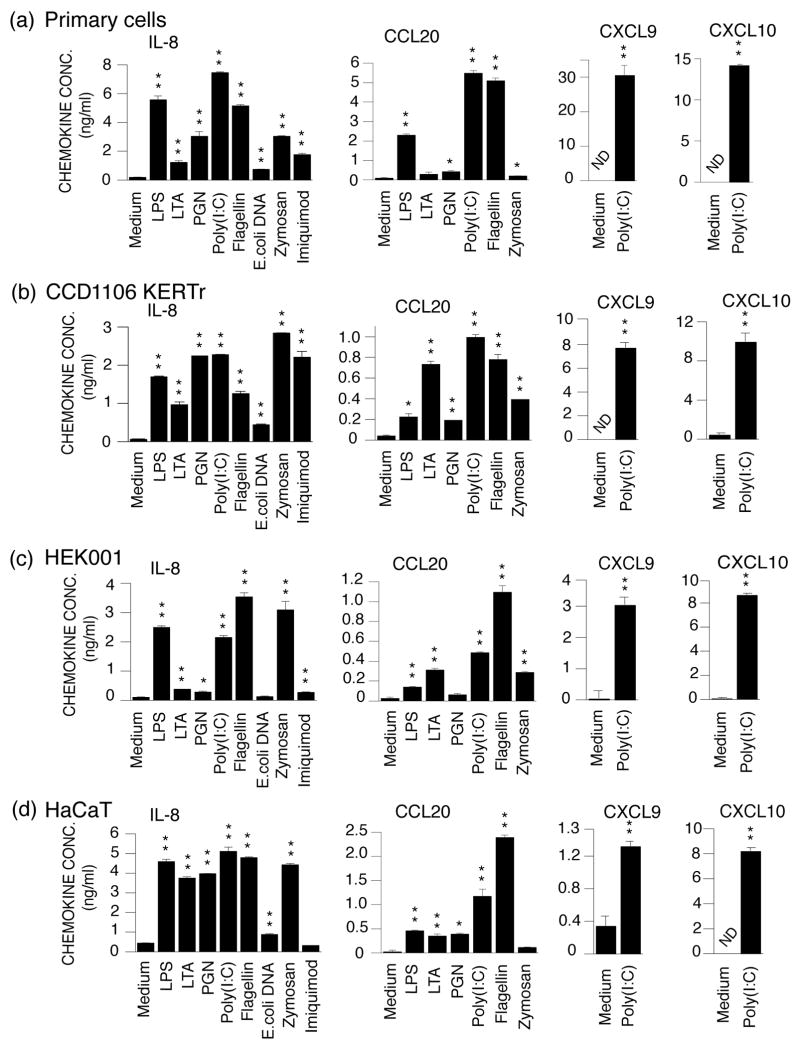
In agreement with previous observations (2, 3) primary keratinocytes secreted IL-8, CCL20, CXCL9 and/or CXCL10 following TLR-ligand stimulation (Fig. 2a). KERTr cells reacted to the same TLR-ligands in a very similar manner (Fig. 2b). HEK001 responded to all TLR ligands except TLR9 (Fig. 2c). Curiously, these cells were more responsive to flagellin than poly(I:C) (Fig. 2c) unlike primary keratinocytes and KERTr cells which produced the highest cytokines levels after poly(I:C) treatment (Fig. 2a and b). High levels of IL-8 were detected in HaCaT medium in response to all TLR-ligands except DNA and Imiquimod (Fig. 2d). Although CXCL10 levels reached approximately 8 ng/ml (P < 0.01) after poly(I:C) treatment, we could not detect CXCL9 secretion using standard culture conditions (not shown). When monolayers were treated 24 hours after reaching confluence a modest induction of CXCL9 could be detected in poly(I:C) treated cells (Fig. 2d).
Figure 2.
IL-8, CCL20, CXCL9 and CXCL10 production in primary keratinocytes, KERTr, HEK001 and HaCaT cell lines after stimulation with TLR-ligands. Primary keratinocytes (a) and the keratinocyte cell lines KERTr (b), HEK001 (c) and HaCaT (d) were treated with medium only, LPS, LTA, PGN, poly(I:C), flagellin, E. coli DNA or zymosan for 24 hours. Levels of IL-8, CCL20, CXCL9 and CXCL10 in the culture medium were determined using ELISA. *, P < 0.05 (compared to cells treated with medium only). **, P < 0.01 (compared to cells treated with medium only). ND, not detected.
Conclusions
In the present study we have characterized TLR expression and activation in the HaCaT, KERTr and HEK001 cell lines. Overall the cell lines responded to TLR ligands in manners very similar to that of primary keratinocytes. However, there were some interesting differences. While primary and KERTr cells produced more IL-8 and CCL20 when treated with poly(I:C) than with flagellin (Fig. 2a and b) the opposite applied to the HEK001 cells (Fig. 2c). This difference is possibly due to the observed higher expression of TLR5 in HEK001 cells than in primary and KERTr cells (Fig. 1). The relatively elevated expression of TLR5 in HEK001 is in agreement with the basal cell characteristics of these cells ((keratin 14 but not keratin 10 expression (16)) and previously reported restriction of TLR5 expression to the basal layer of keratinocytes in the epidermis (5, 6). Other differences, e.g. differential induction of CCL20 and IL-8 by LPS and LTA, cannot directly be explained by the TLR levels and may be related to the more than 30 signalling factors involved in regulating TLR induced gene expression (1, 18).
Imiquimod stimulates leukocyte secretion of cytokines with indirect anti-viral and anti-tumour properties through TLR7 (20). However, a TLR7-independent regulatory loop involving adenosine receptors up-regulates expression of the same cytokines (13). Our results confirmed the presence of TLR7/TLR8 independent signalling in primary keratinocytes, KERTr and HEK001 cells. Hence studies of Imiquimod's functional effect(s) must be designed and interpreted with caution.
In summary our data strongly suggest that in addition to primary keratinocytes and HaCaT cells, the KERTr and HEK001 cell lines are highly suitable for studies of drug responses and innate immune mechanisms in keratinocytes and the skin.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant AR053672, the Transdisciplinary Program in Translational Medicine and Therapeutics at the University of Pennsylvania, and a National Scientist Development grant from the American Heart Association.
References
- 1.Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001;6:733–742. doi: 10.1046/j.1365-2443.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- 2.Lebre MC, van der Aar AM, van Baarsen L, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Chen Q, Shen Y, Liu W. Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll-like receptor 2. Exp Dermatol. 2009;18:603–610. doi: 10.1111/j.1600-0625.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 5.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 6.Begon E, Michel L, Flageul B, et al. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol. 2007;17:497–506. doi: 10.1684/ejd.2007.0264. [DOI] [PubMed] [Google Scholar]
- 7.Tyring S, Conant M, Marini M, Van Der Meijden W, Washenik K. Imiquimod; an international update on therapeutic uses in dermatology. Int J Dermatol. 2002;41:810–816. doi: 10.1046/j.1365-4362.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008;21:69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 9.Mempel M, Voelcker V, Kollisch G, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121:1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 10.Kollisch G, Kalali BN, Voelcker V, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schon MP, Schon M. Imiquimod: mode of action. Br J Dermatol. 2007;157 2:8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 12.Kono T, Kondo S, Pastore S, et al. Effects of a novel topical immunomodulator, imiquimod, on keratinocyte cytokine gene expression. Lymphokine Cytokine Res. 1994;13:71–76. [PubMed] [Google Scholar]
- 13.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 14.Maas-Szabowski N, Starker A, Fusenig NE. Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-alpha. J Cell Sci. 2003;116:2937–2948. doi: 10.1242/jcs.00474. [DOI] [PubMed] [Google Scholar]
- 15.Kim PK, Weller R, Hua Y, Billiar TR. Ultraviolet irradiation increases FADD protein in apoptotic human keratinocytes. Biochem Biophys Res Commun. 2003;302:290–295. doi: 10.1016/s0006-291x(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 16.Sugerman PB, Bigby M. Preliminary functional analysis of human epidermal T cells. Arch Dermatol Res. 2000;292:9–15. doi: 10.1007/pl00007461. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Tsuji H, Kato Y, Sai Y, Kubo Y, Tsuji A. Characterization of the transdermal transport of flurbiprofen and indomethacin. J Control Release. 2006;110:542–556. doi: 10.1016/j.jconrel.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Sanmiguel JC, Olaru F, Li J, Mohr E, Jensen LE. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell Signal. 2009;21:685–694. doi: 10.1016/j.cellsig.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boukamp P, Breitkreutz D, Stark HJ, Fusenig NE. Mesenchyme-mediated and endogenous regulation of growth and differentiation of human skin keratinocytes derived from different body sites. Differentiation. 1990;44:150–161. doi: 10.1111/j.1432-0436.1990.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 20.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.