SUMMARY
Objective
Endothelial adhesion molecules like E-selectin play an important role in leukocyte recruitment and development of atherosclerotic plaque. E-selectin is increased in obesity, yet little is known regarding the specific factors contributing to elevated E-selectin in obesity and whether tumor necrosis factor alpha (TNF-alpha) increases E-selectin in vivo in this population. The objectives of this study were to: 1) determine the body composition, metabolic and inflammatory factors associated with increased E-selectin, and 2) determine the role of TNF-alpha in the physiological regulation of E-selectin by antagonism of TNF-alpha with etanercept among obese subjects.
Methods
E-selectin levels, body composition, metabolic parameters and inflammatory cytokines were assessed in 51 obese subjects and 37 non-obese healthy controls. Obese subjects were randomized to etanercept 50 mg weekly or placebo for four weeks. Changes in E-selectin were compared between treatment groups.
Results
Obese subjects had higher E-selectin than non-obese controls (47.4 [32.7 – 58.8] vs. 27.2 [20.3 – 42.1] ng/mL, obese vs. non-obese, p<0.0001). E-selectin was significantly associated with multiple body composition measures and metabolic parameters, along with specific measures of TNF-alpha activation, including soluble tumor necrosis factor receptors 1 (p=0.03) and 2 (p=0.02). In multivariate modeling, visceral adipose tissue, but not other measures of body composition, remained significantly associated with E-selectin. Among obese subjects, treatment with etanercept significantly decreased E-selectin (−5.7 ± 8.7 vs. 0.5 ± 6.0 ng/mL, etanercept vs. placebo, p=0.005).
Conclusions
E-selectin is increased in obesity, in relationship to increased visceral adiposity and markers of TNF-alpha activation. TNF-alpha antagonism with etanercept reduces E-selectin in obese subjects, providing evidence that the systemic circulatory release of E-selectin is regulated at least in part by TNF-alpha in obesity.
Keywords: obesity, inflammation, tumor necrosis factor-alpha, E-selectin
INTRODUCTION
Obesity is a widespread condition afflicting nearly a third of Americans1 and conferring an increased risk of morbidity and mortality from cardiovascular diseases2. Obese individuals have a higher level of tonic, subclinical inflammation than normal-weight subjects, as reflected in elevated circulating levels of cytokines including TNF-alpha, IL-6, and CRP3–5. Although it is known that patients with chronic inflammatory diseases such as rheumatoid arthritis and lupus have a higher rate of atherosclerosis6, 7, it is unclear if elevated inflammatory cytokines in obesity play a direct role in atherogenesis.
Inflammatory cytokines may affect atherogenesis through the upregulation of endothelial adhesion markers such as E-selectin. A member of the selectin family of transmembrane glycoproteins, E-selectin is constitutively expressed on endothelial cells. It promotes leukocyte adherence to the endothelium8, facilitating eventual endothelial transmigration and development of atherosclerotic plaque9. In vitro studies demonstrate that TNF-alpha upregulates E-selectin8, 10, 11 and also triggers enzymatic cleavage of the extracellular portion of the protein, with subsequent release of soluble E-selectin11, 12. Soluble E-selectin is increased in obesity13, 14, but little is known regarding the effects of TNF-alpha on E-selectin in vivo among obese subjects.
In this study we sought to investigate the factors associated with increased E-selectin in obesity using detailed measures of body composition and inflammatory indices in both obese and non-obese subjects. To further explore these relationships, we used a prior experimental model in which we randomized obese subjects to etanercept or placebo15 to investigate both the effect of etanercept on E-selectin and the relationship of change in E-selectin to changes in other inflammatory indices and markers of TNF-alpha activation. We demonstrate that E-selectin is increased in obese subjects in relationship to visceral fat and show that inhibition of TNF-alpha with etanercept leads to a reduction in E-selectin, providing in vivo evidence that TNF-alpha is one of the physiological regulators of E-selectin in obesity.
SUBJECTS AND METHODS
Study population
Thirty-seven non-obese subjects between the ages of 18 and 55 with a BMI ≤ 26 kg/m2 were studied as healthy control subjects and compared with 51 obese subjects (BMI > 30 kg/m2), also between the ages of 18 and 55 with similar gender distribution. Obese subjects demonstrated metabolic abnormalities and met the modified World Health Organization (WHO) criteria of metabolic syndrome16, but none had a specific medical condition known to be associated with increased inflammation, other than obesity itself. Exclusion criteria for obese subjects included known coronary artery disease, diabetes mellitus, chronic infection such as HIV, chronic autoimmune disease such as rheumatoid arthritis, and use of anti-hyperglycemic medications, immunosuppressants, niacin, or fibrates15.
All subjects gave written informed consent, and the study was approved by the Human Research Committee of the Massachusetts General Hospital (MGH), the Committee on the Research of Human Subjects at the Massachusetts Institute of Technology (MIT), and the Food and Drug Administration (FDA) (IND BB-IND no. 11463 to SKG). We previously reported the effects of etanercept on inflammatory indices, including CRP, IL-6, fibrinogen, and adiponectin in this obese cohort15. In the current study, we used stored serum to investigate the effects of etanercept on E-selectin. In addition, we compared baseline levels of E-selectin prior to etanercept administration in the obese subjects with levels obtained in a recently recruited group of healthy non-obese controls.
Study procedures
Both non-obese and obese subjects underwent morning visits after a 12-hour overnight fast. Height, metabolic weight, and BMI were determined at each visit. Blood samples were drawn in the morning, precluding confounding by the known circadian variation in E-selectin levels17. Subcutaneous and visceral adipose tissue (SAT and VAT, respectively) were measured by single-slice abdominal CT scan at the L4 pedicle as previously described15, 18. Following the baseline visit, obese subjects were randomized to receive either 50 mg subcutaneous weekly etanercept or identical placebo for four weeks. All tests obtained at the baseline visit before randomization were repeated on day 25, three days after the fourth and final dose of the study drug15.
The MGH Research Pharmacy performed the randomization based on sequential enrollment numbers using a permuted block algorithm and kept the randomization code. Randomization was stratified by sex. All investigators, study staff, and subjects were blinded to drug assignment throughout the study.
Laboratory methods
Blood samples were stored at −80°C. All samples from the same patient were run in duplicate in the same assay. E-selectin (ng/ml), was measured with an enzyme-linked immunosorbent assay (R & D Systems Inc., Minneapolis, MN). The intraassay coefficient of variation (CV) was 5.8% and the interassay CV was 7.9%. The laboratory methodologies for measurements of lipids, tumor necrosis factor alpha (TNF-alpha), soluble tumor necrosis factor receptor 1 (sTNFR1), soluble tumor necrosis factor receptor 2 (sTNFR2), c-reactive protein (CRP), interleukin-6 (IL-6), and fibrinogen were as previously described15.
Statistical analysis
As most baseline metabolic and inflammatory parameters were not normally distributed, baseline comparisons between obese and non-obese subjects were made with the Wilcoxon Rank Sums test, and baseline correlations were performed using Spearman's Rho. Multivariate analysis was performed using standard least squares regression modeling. Sensitivity analyses assessing for potentially collinear body composition parameters in multivariate modeling did not affect the results. Results are reported as median [intraquartile range (IQR)] unless otherwise indicated. In the intervention study, change from baseline was compared using Student's t-test between obese subjects randomized to etanercept or placebo, and univariate associations between changes in inflammatory markers during treatment were assessed using the Pearson correlation coefficient. Statistical significance was defined as a 2-tailed p value of <0.05. Statistical analysis was performed using JMP for SAS (SAS Institute Inc, Cary, NC).
RESULTS
Comparison of clinical characteristics in non-obese and obese cohorts
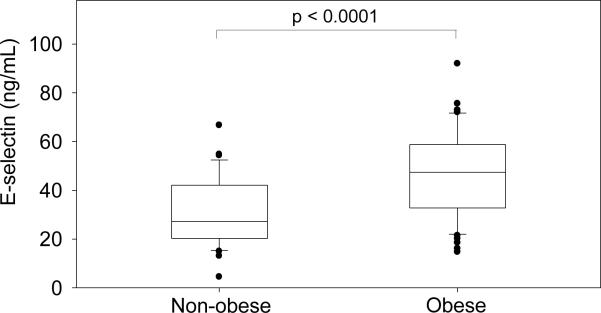
Baseline characteristics of obese and non-obese subjects are shown in Table 1. Age, gender, race, and current smoking were not significantly different between groups (Table 1). As expected, obese subjects had higher fasting glucose, insulin, triglyceride, and systolic and diastolic blood pressure, as well as lower HDL. Compared with non-obese subjects, obese subjects had significantly higher levels of E-selectin (47.4 [32.7 – 58.8] vs. 27.2 [20.3 – 42.1] ng/mL obese vs. non-obese, p<0.0001, Figure 1).
Table 1.
Baseline clinical characteristics in obese vs. non-obese
| Non-obese (N=37) | Obese (N=51) | p-value* | |
|---|---|---|---|
| Age (y) | 44 [36 – 50] | 47 [39 – 53] | 0.14 |
|
| |||
| Race(n) | 0.15 | ||
| White | 25 | 33 | |
| African American | 7 | 16 | |
| Other | 5 | 2 | |
|
| |||
| Gender (% M/F) | 54/46 | 53/47 | 1.0 |
|
| |||
| Current smoking (%) | 19 | 16 | 0.77 |
|
| |||
| BMI (kg/m2) | 23 [22 – 24] | 34 [32 – 41] | <0.0001 |
|
| |||
| Waist circumference (cm) | 82 [75 – 86] | 113 [109 – 122] | <0.0001 |
|
| |||
| Systolic BP (mmHg) | 119 [108 – 128] | 128 [120 – 139] | 0.001 |
|
| |||
| Diastolic BP (mmHg) | 71 [68 – 78] | 83 [73 – 90] | <0.0001 |
|
| |||
| Fasting glucose (mg/dL) | 84 [79 – 89] | 94 [87 –100] | <0.0001 |
|
| |||
| Fasting insulin (uU/mL) | 2.6 [1.9 – 3.7] | 13.3 [9.3 – 24.0] | <0.0001 |
|
| |||
| HDL (mg/dL, fasting) | 56 [48 – 63] | 40 [33 – 45] | <0.0001 |
|
| |||
| Fasting Triglyceride (mg/dL) | 64 [40 – 95] | 165 [103 – 232] | <0.0001 |
|
| |||
| E-selectin (ng/mL) | 27.2 [20.3 – 42.1] | 47.4 [32.7 – 58.8] | < 0.0001 |
Data are presented as median [intraquartile range] unless otherwise indicated. Abbreviations: BMI, body mass index.
p-value for comparison at baseline obtained by the Wilcoxon Rank Sums test, except for race (p-value by Pearson Chi-Square) and gender and current smoking (p-value by 2-tail Fisher's Exact Test).
Figure 1.
Baseline E-selectin levels by weight category. p-value by Wilcoxon Rank Sum Test
Relationship of E-selectin, body composition, metabolic, and inflammatory markers
In the combined cohort of obese and non-obese subjects, E-selectin concentrations were significantly associated with BMI (rho = 0.52, p < 0.0001), waist-to-hip ratio (rho = 0.43, p < 0.0001), SAT (rho = 0.45, p < 0.0001), VAT (rho = 0.61, p < 0.0001), fasting glucose (rho = 0.40, p = 0.0001), fasting insulin (rho = 0.60, p < 0.0001), and triglycerides (rho = 0.44, p < 0.0001), Table 2. In addition, there was a significant inverse association between E-selectin and HDL (rho = −0.33, p = 0.002). There was not a significant relationship between E-selectin and age (Table 2), current smoking (p-value by Wilcoxon Rank Sums test = 0.28), or gender (p value by Wilcoxon Rank Sums test = 0.34).
Table 2.
Baseline associations between E-selectin and body composition and metabolic parameters
| Spearman's rho | p-value | |
|---|---|---|
| Age | 0.16 | 0.13 |
| BMI | .52 | <.0001 |
| WHR | .43 | <.0001 |
| VAT | .61 | <.0001 |
| SAT | 0.45 | <0.0001 |
| Fasting glucose | 0.40 | 0.0001 |
| Fasting insulin | 0.60 | <0.0001 |
| Total cholesterol | 0.13 | 0.22 |
| LDL | 0.07 | 0.51 |
| HDL | −0.33 | 0.002 |
| Triglyceride | 0.44 | <0.0001 |
| TNF-alpha* | 0.16 | 0.27 |
| sTNFR1* | 0.31 | 0.03 |
| sTNFR2* | 0.32 | 0.02 |
| CRP* | 0.24 | 0.09 |
| IL-6* | 0.28 | 0.048 |
| Fibrinogen* | 0.32 | 0.02 |
Data available in the obese cohort only.
Abbreviations: BMI, Body Mass Index; WHR, waist-to-hip ratio; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; TNF-alpha, tumor necrosis factor alpha; sTNFR1, soluble tumor necrosis factor alpha receptor 1; sTNFR2, soluble tumor necrosis factor alpha receptor 2; CRP, c-reactive protein; IL-6, interleukin 6.
In the obese subjects, for whom other markers of systemic inflammation were also measured, E-selectin was significantly and positively associated with fibrinogen (rho = 0.32, p = 0.02), IL-6 (rho = 0.28, p = 0.048), sTNFR1 (rho = 0.31, p = 0.03), and sTNFR2 (rho = 0.32, p = 0.02), Table 2.
In multivariate analysis with E-selectin as the dependent variable and age, BMI, VAT, SAT, and waist-to-hip ratio (WHR) as independent variables, VAT (p = 0.005) but not other measures of body composition remained significantly related to E-selectin (r2 = 0.42, p < 0.0001 for model) (Table 3A). In a separate multivariate analysis using VAT and metabolic syndrome parameters, including fasting glucose, fasting insulin, HDL, triglyceride, SBP, and DBP, as independent variables, VAT (p < 0.0001) was the only significant predictor of E-selectin levels (r2 = 0.44, p < 0.0001 for model, Table 3B).
Table 3A.
Multivariate effects of body composition measurements on E-selectin*
| β-estimate | p-value | |
|---|---|---|
| Age | −0.07 | 0.72 |
| Gender (Male vs. Female) | 1.6 | 0.45 |
| BMI (kg/m2) | 1.4 | 0.06 |
| Waist-to-hip ratio | −34.1 | 0.32 |
| Visceral adipose tissue (cm2) | 0.1 | 0.005 |
| Subcutaneous adipose tissue (cm2) | −0.03 | 0.19 |
Multivariate analysis performed using standard least squares regression.
R2 for model 0.42; p-value for model < 0.0001.
Table 3B.
Multivariate effects of metabolic syndrome parameters on E-selectin*
| β-estimate | p-value | |
|---|---|---|
| Visceral adipose tissue (cm2) | 0.1 | <0.0001 |
| Systolic blood pressure (mmHg) | −0.2 | 0.18 |
| Diastolic blood pressure (mmHg) | 0.0 | 0.90 |
| HDL (mg/dL) | 0.0 | 0.85 |
| Triglyceride (mg/dL) | 0.0 | 0.68 |
| Fasting glucose (mg/dL) | 0.1 | 0.58 |
| Fasting insulin (uU/mL) | 0.2 | 0.30 |
Multivariate analysis performed using standard least squares regression.
R2 for model 0.44; p-value for model < 0.0001.
Effect of Etanercept on E-selectin in obese subjects
Among the 51 obese subjects, 26 were randomized to placebo and 25 to etanercept treatment. E-selectin and other baseline clinical characteristics, including age, gender, race, statin use, anti-hypertensive use, and current smoking, were not significantly different between the two groups (Table 4). BMI was slightly lower in the placebo group (34 [32 – 37] vs. 38 [33 – 43], placebo vs. etanercept, p = 0.04, Table 4).
Table 4.
Baseline characteristics of obese subjects randomized to placebo or Etanercept treatment
| Placebo (n=26) | Etanercept (n=25) | p value* | |
|---|---|---|---|
| Age (y) | 49 [42 – 53] | 45 [39 – 54] | 0.76 |
|
| |||
| Gender (%M/F) | 54/46 | 52/48 | 1.00 |
|
| |||
| Race | 0.32 | ||
| White (N) | 17 | 16 | |
| African American (N) | 9 | 7 | |
| Other (N) | 0 | 2 | |
|
| |||
| Statin use (N) | 9 | 6 | 0.54 |
|
| |||
| Anti-hypertensive use (N) | 16 | 12 | 0.40 |
|
| |||
| Current smoking (%) | 15 | 16 | 1.00 |
|
| |||
| BMI (kg/m2) | 34 [32 – 37] | 38 [33 – 43] | 0.04 |
|
| |||
| E-selectin (ng/mL) | 44.0 [33.1 – 53.1] | 54.9 [30.7 – 70.2] | 0.10 |
Data are presented as median [intraquartile range] unless otherwise indicated. Abbreviations: BMI, body mass index.
For continuous variables, p-value for comparison at baseline obtained by the Wilcoxon Rank Sums test. For race, p-value by Pearson Chi-Square. For gender, menopausal status, statin use, anti-hypertensive use, and current smoking, p-value by 2-tail Fisher's Exact Test.
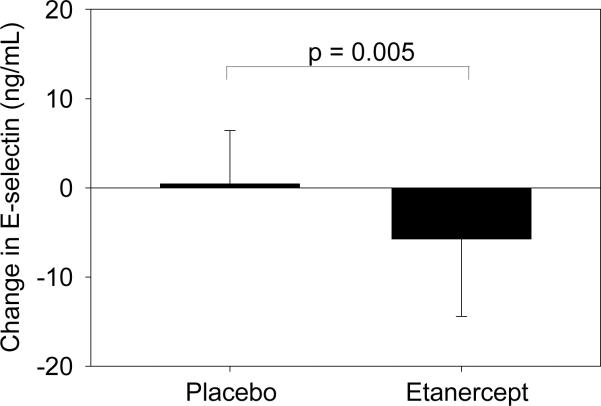
Treatment of obese subjects with etanercept significantly decreased levels of E-selectin relative to placebo (−5.7 ± 8.7 vs. +0.45 ± 6.0 ng/ml, etanercept vs. placebo, p = .005) (Figure 2). No changes in weight or body composition parameters were seen. Levels of TNF-alpha and sTNFR2 increased and CRP decreased significantly in the etanercept group compared with the placebo group15. Effects of etanercept on E-selectin remained significant when controlling for baseline BMI in multivariate modeling. Etanercept was well-tolerated, without any side effects related to infections or other adverse effects.
Figure 2.
Change in E-selectin in placebo vs. etanercept groups after 4-week treatment with etanercept. p-value by Student's t-test.
The change in E-selectin over time was inversely associated with changes in TNF-alpha (r = −0.34, p = 0.02) and sTNFR2 (r = −0.33, p = 0.02). In addition, the change in E-selectin was positively associated with the change in CRP (r = 0.36, p = 0.009) (Table 5).
Table 5.
| Baseline | Treatment Effect (Etanercept vs. Placebo) | r* | p-value* | ||
|---|---|---|---|---|---|
| Etanercept (N=25) | Placebo (N=26) | ||||
| TNF-alpha (pg/mL) | 1.7 [1.3 – 2.3] | 1.4 [1.2 – 1.8] | 96.8† | −0.34 | 0.015 |
|
| |||||
| sTNFR1 (ng/mL) | 1.3 [1.0 – 1.5] | 1.2 [1.0 – 1.6] | 0.0 | 0.21 | 0.14 |
|
| |||||
| sTNFR2 (ng/mL) | 2.8 [2.2 – 3.6] | 2.6 [2.0 – 3.3] | 7.9† | −0.33 | 0.019 |
|
| |||||
| CRP (mg/L) | 7.3 [4.5 – 10.2] | 5.4 [2.2 – 7.1] | −2.9† | 0.36 | 0.009 |
|
| |||||
| IL-6 (ng/L) | 3.3 [1.8 – 6.0] | 3.6 [2.0 – 7.0] | −0.8 | 0.22 | 0.12 |
Baseline values are Median [Intraquartile Range]. Baseline values for etanercept vs. placebo groups are not statistically different (p>0.05 for all comparisons using Wilcoxin Rank Sums test).
p < 0.05 for comparison of change in etanercept vs. placebo groups
r is Pearson correlation coefficient for univariate regression between change in E-selectin and change in the inflammatory marker. p-value for this univariate regression is also provided.
DISCUSSION
E-selectin, constitutively expressed on endothelial cells, is a transmembrane glycoprotein which binds carbohydrate ligands on leukocytes, causing leukocyte rolling and facilitating endothelial adhesion and transmigration8. TNF-alpha and other cytokines upregulate E-selectin gene expression8, 10, 19 and trigger enzymatic cleavage near the protein's membrane insertion point, with subsequent circulatory release of soluble E-selectin (sE-selectin)11, 12. Levels of sE-selectin are thus thought to reflect systemic endothelial cell activation, though not necessarily endothelial cell dysfunction12.
Previous studies have shown that serum E-selectin levels are elevated in obesity and decrease with weight loss14, 20, 21. We confirmed this relationship between E-selectin and BMI in our cohort, and, interestingly, found that visceral adiposity may be more strongly associated with E-selectin than indices of total fat such as BMI or of subcutaneous fat. Both BMI and SAT were significant in univariate regression with E-selectin but were no longer significant in multivariate modeling including VAT. The importance of visceral fat as a predictor of E-selectin levels has also been suggested by a recent study of morbidly obese subjects20, whereas, in a cohort of type 2 diabetics, BMI was a stronger predictor of E-selectin than VAT13. We also demonstrate that E-selectin is associated with fasting insulin, glucose, triglyceride, and HDL levels, but these associations were no longer significant in a multivariate model including VAT. E-selectin has been associated with hyperinsulinemia22, 23 and hyperlipidemia22 in previous studies, although these relationships may be mediated largely by excess body weight23. Although other studies have demonstrated that E-selectin levels are higher in smoking24, we did not find this association in our cohort.
We investigated the relationship between TNF-alpha and E-selectin and show in a human model of obesity that E-selectin is positively associated with sTNFR1and sTNFR2 levels. Soluble forms of TNFR1 and TNFR2 are produced by cleavage of the extracellular portion of the transmembrane receptors, which occurs both constitutively and in response to TNF-alpha and other cytokines25–27. Thus, circulating levels of sTNFR1 and sTNFR2 reflect systemic inflammation and may be proportional to levels of membrane bound receptors. Studies have confirmed that activity of both TNFR1 and TNFR2 are necessary for the upregulation of E-selectin by TNF-alpha19. Because causality can not be determined definitively in a cross-sectional study, we sought to further test the relationship between E-selectin and the TNF family by determining whether systemic treatment of obese subjects with a specific TNF-alpha antagonist, etanercept, would decrease E-selectin levels.
Treatment of obese subjects with etanercept significantly reduced E-selectin levels. The decrease in E-selectin was significantly associated with an increase in levels of TNF-alpha and sTNFR2. The directionality of change in TNF-alpha and sTNFR2 levels can be explained by drug-specific effects. Etanercept, a fusion protein between the recombinant human p75 TNF-alpha receptor 2 and the Fc fragment of human IgG1, binds TNF-alpha in the circulation, reducing its biological activity. Since etanercept sequesters TNF-alpha and prolongs its half life, circulating TNF-alpha levels rise with therapy. Moreover, as etanercept is detected in the assay for sTNFR2, measured sTNFR2 levels also rise with therapy. Thus, the significant association between decreases in E-selectin and increases in TNF-alpha and sTNFR2 supports the hypothesis that systemic TNF-alpha antagonism with etanercept influences E-selectin levels. In addition, decreases in E-selectin with etanercept were significantly associated with decreases in other serum inflammatory markers (as shown in Table 5), suggesting a relationship between a reduction in E-selectin and other markers of subclinical inflammation in obesity. Of note, the reduction in E-selectin with etanercept occurred after only 4 weeks of treatment, suggesting rapid, acute effects of TNF-antagonism on E-selectin. These changes in E-selectin occurred in the absence of any changes in weight and VAT. Taken together our data suggest a schema whereby increased VAT may contribute to increased E-selectin through activation of the TNF family, but antagonism of TNF is sufficient to reduce E-selectin, independent of reduction in VAT. Thus unlike the studies of weight loss in which both weight and inflammation change simultaneously, the current model allows us to isolate the effects of TNF-alpha antagonism on E-selectin. While it is known that in patients with overt, inflammatory arthritides, TNF-alpha antagonism decreases E-selectin28, 29, our data demonstrate a similar effect in subjects with no known cause for inflammation apart from obesity. The significance of visceral adipose tissue rather than subcutaneous or total adipose tissue in determining levels of E-selectin in our cohort may reflect disproportionate secretion of inflammatory cytokines by visceral fat compared with subcutaneous fat. In particular, visfatin, which upregulates TNF-alpha production, is predominantly produced by visceral adipose tissue and has been shown in vitro to increase soluble E-selectin through activation of the NF-κB pathway30. Further research is needed to clarify the specific mechanisms by which increased visceral fat may contribute to increased E-selectin.
The clinical significance of our findings depends on whether E-selectin simply serves as a marker of inflammation or whether it plays a role in human pathophysiology. A preponderance of evidence suggests the latter. The prevailing, inflammation-centric model of atherogenesis suggests that E-selectin and other leukocyte adhesion molecules help lure monocytes from the bloodstream to the tunica intima of affected vessels, where they acquire characteristics of tissue macrophages. These cells then internalize oxidized lipoprotein molecules, giving rise to foam cells that form the fatty core of an atheromatous plaque31, 32. Intriguingly, a polymorphism of the E-selectin gene in humans is linked with a higher risk of early-onset atherosclerosis33. Thus reductions in E-selectin via manipulation of the TNF-alpha axis in obesity may have clinical relevance. Given the potential side effect profile with etanercept, it is unlikely that this strategy will be pursued to reduce inflammation in obesity, but our data provide preliminary proof of the principle that such a strategy might be useful. Other strategies, including inhibition of nuclear factor kappa B with salsalate34, 35, may be safer and equally effective, but have not been tested with respect to E-selectin.
Our study has a number of limitations. Although we performed both cross-sectional and interventional studies to assess the effects of TNF-alpha on E-selectin, we cannot be sure that the antagonism of TNF acts directly to downregulate E-selectin, or whether this effect is mediated through other pathways. Further studies are necessary to definitively conclude that TNF-alpha is directly causal in the pathway to increased E-selectin in obesity. Nonetheless, we see striking reductions in a number of inflammatory cytokines and endothelial markers, including E-selectin, after short-term antagonism of TNF, thus suggesting the potential utility of treating the subclinical inflammation of obesity in addition to the weight itself.
Acknowledgements
The authors would like to thank the patients for their commitment to the study, as well as the nursing staff of the Massachusetts Institute of Technology Clinical Research Center and the Massachusetts General Hospital Clinical Research Center for their dedicated patient care.
Funding: Funding was provided by Amgen in the form of an investigator initiated research grant and NIH M01-RR-01066 and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. NIH funding also provided by K24 DK064545 to S.G. There are no financial conflicts to disclose.
Footnotes
Clinical Trials.gov identifier: NCT00409318
Competing interests/financial disclosure: Nothing to declare.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Uchegbu EC, Kopelman PG. Cardiovascular risks in obesity. Journal of Endocrinological Investigation. 2002;25:915–8. doi: 10.1007/BF03344056. [DOI] [PubMed] [Google Scholar]
- 3.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–65. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 4.Festa A, D'Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 5.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance and endothelial dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 6.Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. American Journal of Medicine. 2008;121:S3–8. doi: 10.1016/j.amjmed.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, Sammaritano L, Devereux RB, Schwartz JE, Levine DM, Salmon JE. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Annals of Internal Medicine. 2006;144:249–56. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 8.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opinion on Therapeutic Targets. 2007;11:1473–91. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter AM, Grant PJ. Vascular homeostasis, adhesion molecules, and macrovascular disease in non-insulin-dependent diabetes mellitus. Diabetic Medicine. 1997;14:423–32. doi: 10.1002/(SICI)1096-9136(199706)14:6<423::AID-DIA421>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. Journal of Immunology. 1996;156:2558–65. [PubMed] [Google Scholar]
- 11.Wyble CW, Hynes KL, Kuchibhotla J, Marcus BC, Hallahan D, Gewertz BL. TNF-alpha and IL-1 upregulate membrane-bound and soluble E-selectin through a common pathway. Journal of Surgical Research. 1997;73:107–12. doi: 10.1006/jsre.1997.5207. [DOI] [PubMed] [Google Scholar]
- 12.Roldan V, Marin F, Lip GY, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Journal of Thrombosis and Haemostasis. 2003;90:1007–20. doi: 10.1160/TH02-09-0083. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Sera Y, Abe Y, Tominaga T, Horikami K, Hirao K, Ueki Y, Miyake S. High serum concentrations of soluble E-selectin correlate with obesity but not fat distribution in patients with type 2 diabetes mellitus. Metabolism. 2002;51:932–4. doi: 10.1053/meta.2002.33354. [DOI] [PubMed] [Google Scholar]
- 14.Ferri C, Desideri G, Valenti M, Bellini C, Pasin M, Santucci A, De Mattia G. Early upregulation of endothelial adhesion molecules in obese hypertensive men. Hypertension. 1999;34:568–73. doi: 10.1161/01.hyp.34.4.568. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Archives of Internal Medicine. 2006;166:902–8. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Maple C, Kirk G, McLaren M, Veale D, Belch JJ. A circadian variation exists for soluble levels of intercellular adhesion molecule-1 and E-selectin in healthy volunteers. Clinical Science (Lond) 1998;94:537–40. doi: 10.1042/cs0940537. [DOI] [PubMed] [Google Scholar]
- 18.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. American Journal of Clinical Nutrition. 1982;36:172–7. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 19.Chandrasekharan UM, Siemionow M, Unsal M, Yang L, Poptic E, Bohn J, Ozer K, Zhou Z, Howe PH, Penn M, DiCorleto PE. Tumor necrosis factor alpha (TNF-alpha) receptor-II is required for TNF-alpha-induced leukocyte-endothelial interaction in vivo. Blood. 2007;109:1938–44. doi: 10.1182/blood-2006-05-020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontiroli AE, Frige F, Paganelli M, Folli F. In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obesity Surgery. 2009;19:745–50. doi: 10.1007/s11695-008-9626-4. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Ohshima A, Inoue M, Ohto N, Nakasuga K, Kaji Y, Maruyama T, Nishioka K. Weight reduction decreases soluble cellular adhesion molecules in obese women. Clinical and Experimental Pharmacology and Physiology. 2002;29:399–404. doi: 10.1046/j.1440-1681.2002.03672.x. [DOI] [PubMed] [Google Scholar]
- 22.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, Lamarche B, Bergeron N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. Journal of Clinical Endocrinology and Metabolism. 2005;90:6454–9. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 23.Pontiroli AE, Pizzocri P, Koprivec D, Vedani P, Marchi M, Arcelloni C, Paroni R, Esposito K, Giugliano D. Body weight and glucose metabolism have a different effect on circulating levels of ICAM-1, E-selectin, and endothelin-1 in humans. European Journal of Endocrinology. 2004;150:195–200. doi: 10.1530/eje.0.1500195. [DOI] [PubMed] [Google Scholar]
- 24.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. American Journal of Cardiology. 2002;89:1117–9. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 25.Lantz M, Gullberg U, Nilsson E, Olsson I. Characterization in vitro of a human tumor necrosis factor-binding protein. A soluble form of a tumor necrosis factor receptor. Journal of Clinical Investigation. 1990;86:1396–1402. doi: 10.1172/JCI114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lantz M, Malik S, Slevin ML, Olsson I. Infusion of tumor necrosis factor (TNF) causes an increase in circulating TNF-binding protein in humans. Cytokine. 1990;2:402–6. doi: 10.1016/1043-4666(90)90048-x. [DOI] [PubMed] [Google Scholar]
- 27.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine and Growth Factor Reviews. 1996;7:231–40. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 28.Cordiali-Fei P, Trento E, D'Agosto G, Bordignon V, Mussi A, Ardigo M, Mastroianni A, Vento A, Solivetti F, Berardesca E, Ensoli F. Effective therapy with anti-TNF-alpha in patients with psoriatic arthritis is associated with decreased levels of metalloproteinases and angiogenic cytokines in the sera and skin lesions. Annals of the New York Academy of Science. 2007;1110:578–89. doi: 10.1196/annals.1423.062. [DOI] [PubMed] [Google Scholar]
- 29.Mastroianni A, Minutilli E, Mussi A, Bordignon V, Trento E, D'Agosto G, Cordiali-Fei P, Berardesca E. Cytokine profiles during infliximab monotherapy in psoriatic arthritis. British Journal of Dermatology. 2005;153:531–6. doi: 10.1111/j.1365-2133.2005.06648.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee WJ, Wu CS, Lin H, Lee IT, Wu CM, Tseng JJ, Chou MM, Sheu WH. Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-kappaB pathway. International Journal of Obesity (Lond) 2009;33:465–72. doi: 10.1038/ijo.2009.24. [DOI] [PubMed] [Google Scholar]
- 31.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. Atherosclerosis is an inflammatory disease. American Heart Journal. 1999;138:S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel K, Felix S, Kleber FX, Brachold R, Menke T, Schattke S, Schulte KL, Glaser C, Rohde K, Baumann G, et al. E-selectin polymorphism and atherosclerosis: an association study. Human Molecular Genetics. 1994;3:1935–7. doi: 10.1093/hmg/3.11.1935. [DOI] [PubMed] [Google Scholar]
- 34.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin [see comments] Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 35.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]