Abstract
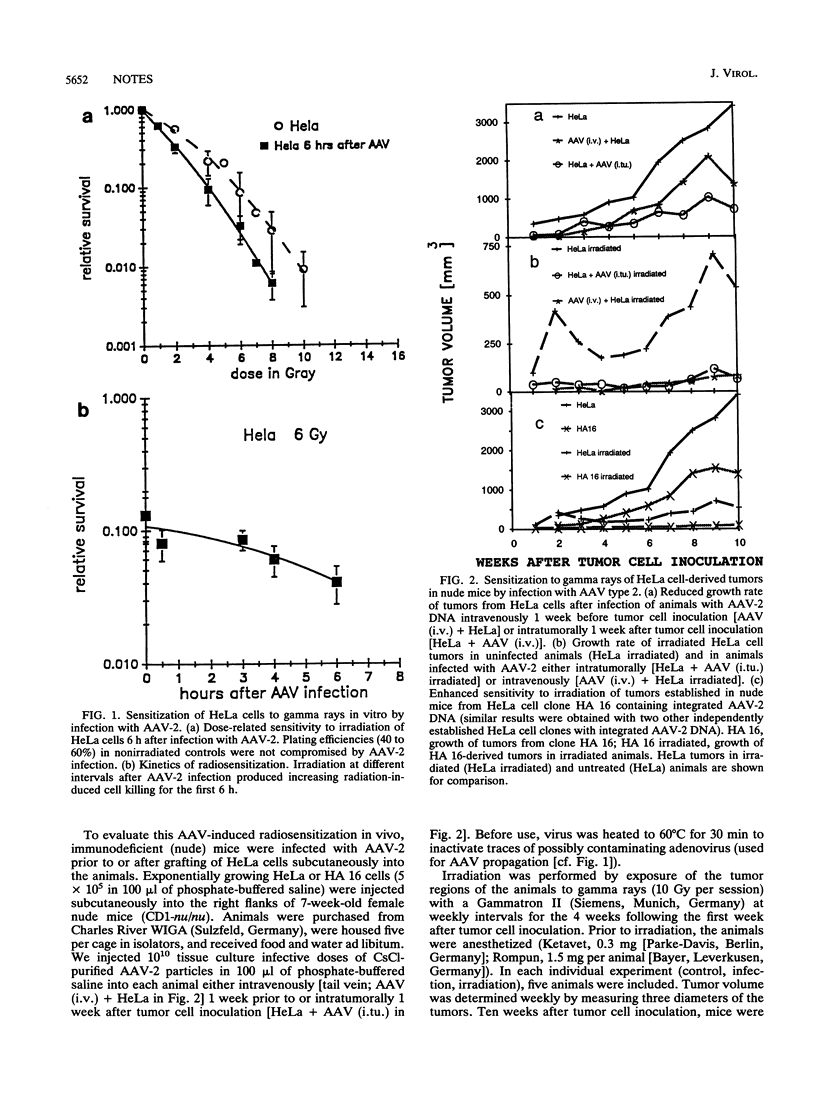
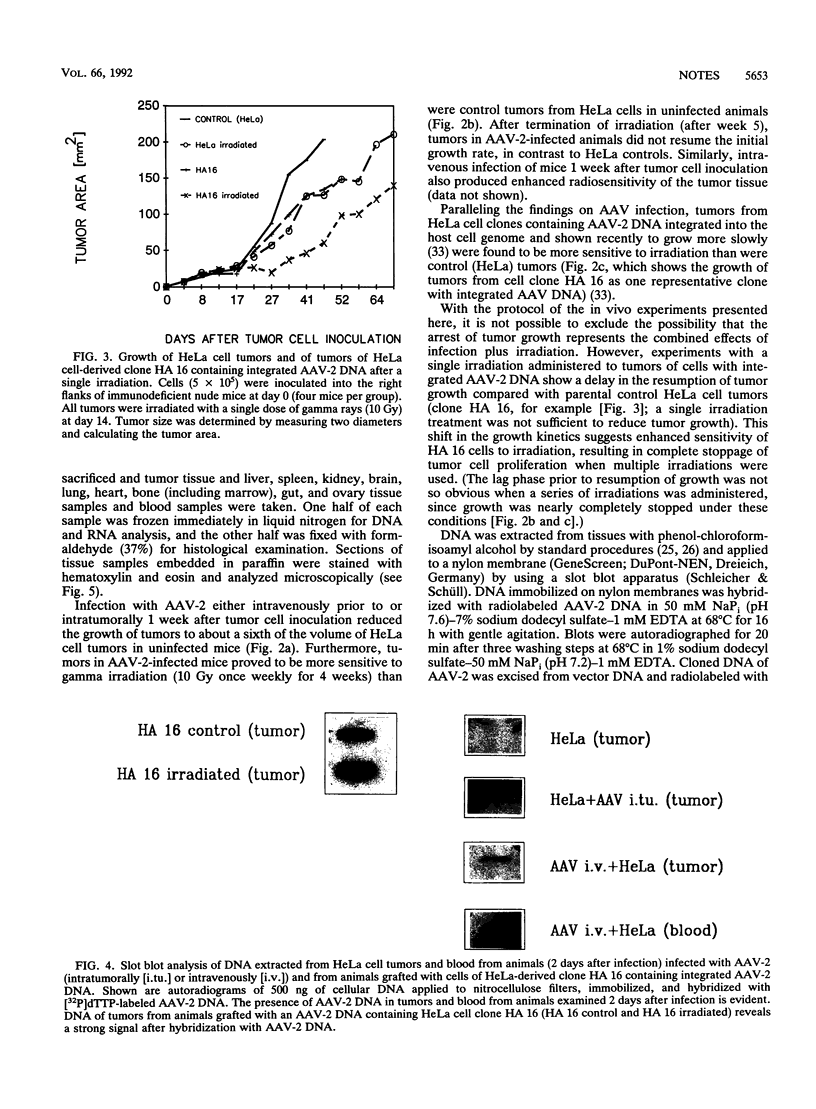
Infection with the helper virus-dependent human parvovirus adeno-associated virus (AAV) is known to interfere with cellular transformation in vitro and oncogenesis in vivo. Here we report on sensitization to gamma irradiation by AAV infection of cells in culture and of tumors established from HeLa cells grafted into immunodeficient (nude) mice: infection of HeLa cells with AAV type 2 enhanced cell killing and reduced plating efficiency after irradiation compared with uninfected cells. Similarly, HeLa cell tumors in nude mice displayed a reduced growth rate and were more sensitive to gamma irradiation when the animals were infected with AAV type 2 prior to or after tumor cell inoculation. Since no pathogenicity is known for AAV, the ability of this virus to render radiotherapy of human tumor cells more efficient may up open novel approaches in cancer treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantel-Schaal U. Infection with adeno-associated parvovirus leads to increased sensitivity of mammalian cells to stress. Virology. 1991 May;182(1):260–268. doi: 10.1016/0042-6822(91)90669-3. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Bohenzky R. A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Hauswirth W. W. Adeno-associated viruses. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R., Kibrick S., Swan I. C. Effect of adeno-associated virus on cancer expression by herpesvirus-transformed hamster cells. J Natl Cancer Inst. 1975 Oct;55(4):957–959. doi: 10.1093/jnci/55.4.957. [DOI] [PubMed] [Google Scholar]
- Georg-Fries B., Biederlack S., Wolf J., zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984 Apr 15;134(1):64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Bürkle A., Stephan S., zur Hausen H. The adeno-associated virus rep gene suppresses herpes simplex virus-induced DNA amplification. J Virol. 1990 Jun;64(6):3012–3018. doi: 10.1128/jvi.64.6.3012-3018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., Yalkinoglu A. O., Zur Hausen H. Selective DNA-amplification induced by carcinogens (initiators): evidence for a role of proteases and DNA polymerase alpha. Int J Cancer. 1985 Jul 15;36(1):85–91. doi: 10.1002/ijc.2910360114. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., zur Hausen H. Selective killing of carcinogen-treated SV40-transformed Chinese hamster cells by a defective parvovirus. Virology. 1984 Jul 30;136(2):439–441. doi: 10.1016/0042-6822(84)90180-6. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L. The adeno-associated virus Rep78 gene inhibits cellular transformation induced by bovine papillomavirus. Virology. 1989 Sep;172(1):253–261. doi: 10.1016/0042-6822(89)90127-x. [DOI] [PubMed] [Google Scholar]
- Katz E., Carter B. J. Effect of adeno-associated virus on transformation of NIH 3T3 cells by ras gene and on tumorigenicity of an NIH 3T3 transformed cell line. Cancer Res. 1986 Jun;46(6):3023–3026. [PubMed] [Google Scholar]
- Khleif S. N., Myers T., Carter B. J., Trempe J. P. Inhibition of cellular transformation by the adeno-associated virus rep gene. Virology. 1991 Apr;181(2):738–741. doi: 10.1016/0042-6822(91)90909-u. [DOI] [PubMed] [Google Scholar]
- Kirschstein R. L., Smith K. O., Peters E. A. Inhibition of adenovirus 12 oncogenicity by adeno-associated virus. Proc Soc Exp Biol Med. 1968 Jul;128(3):670–673. doi: 10.3181/00379727-128-33095. [DOI] [PubMed] [Google Scholar]
- Klein-Bauernschmitt P., zur Hausen H., Schlehofer J. R. Induction of differentiation-associated changes in established human cells by infection with adeno-associated virus type 2. J Virol. 1992 Jul;66(7):4191–4200. doi: 10.1128/jvi.66.7.4191-4200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Drake S., Stahmann J., Mumford D. M. Antibodies to adeno-associated satellite virus and herpes simplex in sera from cancer patients and normal adults. Am J Obstet Gynecol. 1976 Sep 1;126(1):100–104. doi: 10.1016/0002-9378(76)90472-5. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Houlditch G. S., Mumford D. M. Influence of adeno-associated satellite virus on adenovirus-induced tumours in hamsters. Nat New Biol. 1973 Jan 10;241(106):44–46. doi: 10.1038/newbio241044b0. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Duckworth D. H., Berns K. I. Inhibition of adenovirus-transformed cell oncogenicity by adeno-associated virus. Virology. 1981 Sep;113(2):521–533. doi: 10.1016/0042-6822(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Cornelis J. J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991 Aug;33(3):233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Heilbronn R., Georg-Fries B., zur Hausen H. Inhibition of initiator-induced SV40 gene amplification in SV40-transformed Chinese hamster cells by infection with a defective parvovirus. Int J Cancer. 1983 Nov 15;32(5):591–595. doi: 10.1002/ijc.2910320512. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Heilbronn R. Infection with adeno-associated virus type 5 inhibits mutagenicity of herpes simplex virus type 1 or 4-nitroquinoline-1-oxide. Mutat Res. 1990 Aug;244(4):317–320. doi: 10.1016/0165-7992(90)90079-y. [DOI] [PubMed] [Google Scholar]
- Schmitt J., Schlehofer J. R., Mergener K., Gissmann L., zur Hausen H. Amplification of bovine papillomavirus DNA by N-methyl-N'-nitro-N-nitrosoguanidine, ultraviolet irradiation, or infection with herpes simplex virus. Virology. 1989 Sep;172(1):73–81. doi: 10.1016/0042-6822(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Sprecher-Goldberger S., Thiry L., Lefébvre N., Dekegel D., de Halleux F. Complement-fixation antibodies to adenovirus-associated viruses, cytomegaloviruses and herpes simplex viruses in patients with tumors and in control individuals. Am J Epidemiol. 1971 Oct;94(4):351–358. doi: 10.1093/oxfordjournals.aje.a121330. [DOI] [PubMed] [Google Scholar]
- Walz C., Schlehofer J. R. Modification of some biological properties of HeLa cells containing adeno-associated virus DNA integrated into chromosome 17. J Virol. 1992 May;66(5):2990–3002. doi: 10.1128/jvi.66.5.2990-3002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Callaham M. F., Huberman E. Perturbation of the cell cycle by adeno-associated virus. Virology. 1988 Dec;167(2):393–399. [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalkinoglu A. O., Heilbronn R., Bürkle A., Schlehofer J. R., zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988 Jun 1;48(11):3123–3129. [PubMed] [Google Scholar]
- de la Maza L. M., Carter B. J. Inhibition of adenovirus oncogenicity in hamsters by adeno-associated virus DNA. J Natl Cancer Inst. 1981 Dec;67(6):1323–1326. [PubMed] [Google Scholar]





