Abstract
The goal of this study was to determine whether functional changes in cortical control of swallowing are evident in early Alzheimer’s disease (AD), before dysphagia (swallowing impairment) is evident. Cortical function was compared between an early AD group and a group of age-matched controls during swallowing. Swallowing oropharyngeal biomechanics examined from videofluoroscopic recordings were also obtained to more comprehensively characterize changes in swallowing associated with early AD. Our neuroimaging results show that the AD group had significantly lower BOLD response in many cortical areas that are traditionally involved in normal swallowing (i.e. pre and postcentral gyri, Rolandic and frontal opercula). There were no regions where the AD group recruited more brain activity than the healthy controls during swallowing and only 13% of all active voxels were unique to the AD group, even at this early stage. This suggests that the AD group is not recruiting new regions, nor are they compensating within regions that are active during swallowing. In videofluoroscopic measures, the AD group had significantly reduced hyo-laryngeal elevation than the controls. Although, swallowing impairment is usually noted in the late stages of AD, changes in cortical control of swallowing may begin long before dysphagia becomes apparent.
Keywords: deglutition, Alzheimer’s disease, neurophysiology, neuroimaging, videofluoroscopy
Introduction
Dysphagia, or swallowing impairment, is a growing concern in Alzheimer’s Disease (AD). It frequently leads to aspiration pneumonia, a common cause of death in this population [1], particularly in the later stage of AD [1–4]. Patients with early to mid-stage AD also show changes in swallowing physiology that may put them at risk for malnutrition, dehydration, or aspiration pneumonia. Dysphagia in early-stage AD is characterized by delayed onset of the pharyngeal swallow and reduced lingual movement [5], while moderate AD adds difficulty with oral preparation of the bolus, pharyngeal clearance, upper esophageal sphincter opening and visible aspiration on videofluoroscopy [6].
Cortical regions that are involved in normal swallowing are affected by AD, including the insula/inferior frontal gyrus pars operculum [7, 8] and the anterior cingulate cortex [9, 10]. Other studies have reported activity in the antero-medial temporal lobe during normal swallowing [11, 12], a cortical region that is significantly atrophied in AD [13].
In individuals with early AD and in age-matched healthy adults, the structures in the head and neck that are important for swallowing move at different temporal durations depending upon the bolus consistency that is being swallowed (i.e. thin liquid, semi-solid, solid) [5]. This suggests that the ability to modulate swallowing physiology to accommodate variations in bolus characteristics and, thus, maintain swallowing safety is preserved within the early stages of the disease. We have shown that the cortical activation for swallowing changes with varied swallow types (saliva, water, barium) in healthy older adults [14], however, we do not know if similar changes are made in early AD.
Despite the noteworthy overlap of cortical regions that are both active in normal swallowing and degenerative due to AD, no study has examined the neural activity for swallowing in early AD. Investigating the integrity of neurophysiologic parameters of swallowing at this early-stage can mark the initial functional changes in the cortex for processing swallowing and lead to earlier and more effective dysphagia treatments that are specific to the needs of this population. Furthermore, the advanced stages of AD are not feasible for conducting functional imaging studies of swallowing due to the cognitive demand for task compliance and reduced swallowing safety in the supine position.
The primary goal of this investigation was to compare neural activation, as measured with Blood-Oxygen-Level-Dependent (BOLD) signal, during swallowing in early AD and in healthy age-matched controls using functional Magnetic Resonance Imaging (fMRI). We hypothesized that the AD group would elicit decreased responses within the swallowing network, as identified by previous research [14], than the healthy cohort. We hypothesized lower BOLD response in the swallowing cortical network for the AD group because of known cerebral atrophy in these regions associated with volitional swallowing control. Since swallowing kinematics change with varied sensory input (i.e. taste, texture) [15], we examined BOLD signal among different swallow types (sweetened barium, water and saliva) within the AD group. Based on findings from our previous work in healthy older volunteers, we hypothesized that AD participants would elicit more cortical regions in larger clusters for saliva swallows than for water or barium swallows. We expect that saliva swallows will be more difficult to elicit without the added sensory stimulation that a liquid bolus provides, warranting greater neural activation to complete saliva swallows.
The secondary goal of this investigation was to compare swallowing physiology between early AD and healthy age-matched controls using videofluoroscopy. We hypothesized that physiological measures of timing and of range of motion would be significantly different between groups. These differences could be the result of early functional differences in cortical control of swallowing, as discussed in our primary hypothesis. We expected that the early AD group would have swallowing physiology that puts them at greater risk for dysphagia than the healthy controls.
Materials and Methods
Twenty-four participants completed the study (13 AD: mean age 74.3, range 58–88yrs, SD 8.6yrs; 11 controls: mean age 72.3, range 64–83; SD 7.5). Data from the controls were compared with healthy young adults from a previous study [14]. All participants except one healthy control were right-handed as determined by the Edinburgh Handedness Inventory [16]. Mini Mental State Examination scores were obtained for all participants (healthy: mean 28, SD 2.3; and AD: mean 23, SD 2.1). All participants provided written informed consent, which was approved by the Institutional Review Board of the University of Wisconsin.
At entry, all AD participants met the National Institute of Neurological and Communicative Disorders and Stroke/AD and Related Disorders Association (NINCDS/ADRDA) criteria for the diagnosis of probable AD. All had dementia of mild severity, and presented with the characteristic clinical features of AD and were free of any significant medical, neurologic, or psychiatric illness, apart from AD, as detected by detailed clinical evaluation. In particular, no subject had depression, and the Hachinski Ischemia Scale scores did not exceed 4 for any study participant. Extensive laboratory and radiologic tests conducted for each subject failed to detect any other medical disorder that might account for the cognitive symptoms.
fMRI Procedures
Prior to the experimental procedures, a brief training session familiarized each participant with supine swallowing. The experimental tasks involved 30 swallows (10 barium, 10 water, 10 saliva) in a pseudo-random order. 5ml of water and barium (EZEM Varibar®, thin-liquid) were infused directly into the oral cavity via plastic tubing that was dispensed by a MR-safe injector (Spectris Solaris®, Medrad). Participants were instructed to swallow once they felt that the liquid had completely entered their mouths. Saliva swallows were cued visually by the message “swallow saliva once”. Participants viewed cues through a mirror that was mounted atop the head coil using Presentation software (www.neuro-bs.com). Inter-stimulus intervals for barium and water boluses were each 14.5 seconds and saliva swallows and relax intervals (visual cue: “do not swallow”) were each 13 seconds.
Swallow Monitoring
Swallowing events were monitored with a water-filled tube that extended from each participant’s mouth to a pressure transducer in the control room (ADInstruments, Colorado Springs CO). Pressure changes in the oral cavity that occurred during swallowing displaced the water in the tube, providing real-time oral pressure signals synchronized with the scanner transistor-transistor-logic (TTL) pulses.
Functional Imaging
This study used an event-related design. Magnetic resonance images were collected on a 3.0 T GE Signa scanner (Waukesha, WI) using an 8-channel head coil. Functional image data were obtained with a T2* gradient-echo, echo planar imaging (EPI) pulse sequence optimized for blood-oxygen level dependent (BOLD) contrast with the following parameters: echo time (TE) = 30ms; repetition time (TR) = 2000ms; flip angle = 75°; acquisition matrix =64×64; and FOV=240mm. Whole brain coverage was provided with thirty 4.5mm thick inter-leaved axial slices. In each of the two runs, 148 volumes were collected and the first three discarded to allow for magnetization stabilization. Higher order shimming was applied to the static magnetic field (B0) prior to EPI acquisition.
Additionally, a whole-brain high-resolution T1-weighted Inversion-Recovery 3D spoiled gradient echo (3D-IRSPGR) was collected with the following parameters: TR = 9.592ms; TE = 3.00ms; inversion time (TI) = 600ms; flip angle = 10°; acquisition matrix = 256×256; FOV = 230mm; slice thickness = 1.5mm. Inspection by a neuroradiologist confirmed the absence of any structural abnormalities.
fMRI Image Processing
All functional images were processed with Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, University College London, UK) and Analysis of Functional NeuroImages (AFNI, Medical College of Wisconsin, USA) software packages. Images were slice-timing corrected, motion-corrected, normalized to the MNI EPI template using a nonlinear transform, re-sampled to 2mm isotropic voxels, and smoothed with an 8mm FWHM Gaussian kernel. All individuals had less than 3mm of movement in the x, y, and z directions and less than 3 degrees of deflection in pitch, roll, and yaw.
fMRI Statistics
First-level analyses of the time series data were performed for individual participants using a general linear model. Swallow onset times for each condition (barium, water, saliva, spontaneous), obtained pressure transducer (above), were convolved with the canonical hemodynamic response function (HRF) to construct the statistical model. The general linear-model removed the low frequencies with a 128 s high-pass filter. Additionally, vectors were added to the design matrix for the motion parameters and their backward derivatives. Spontaneous, or inadvertent swallows, were not considered further.
Second-level analyses included a repeated measures 2×3 ANOVA that included terms for subject, group, swallow type and group and swallow type interactions to identify regions where there was an interaction between group and swallow type. Significant interactions are reported and then excluded from subsequent analyses of main effects of swallow type and group. Post-hoc T-contrasts were used to identify significant group differences and pair-wise swallow type differences. A 1×3 ANOVA tested for differences among the three swallow types within the AD group. Our analyses were further restricted to the cortical regions that are reported in the literature to be involved in swallowing [14, 17] and areas involved in the cognitive demands of swallowing -- the hippocampus, parahippocampus, amygdala, and orbital frontal gyri (swallowing/AD mask). All analyses were at the α=0.005 uncorrected in at least 20 edge-connected voxels.
We excluded areas of significant difference in grey matter volume as determined with a two-sample t-test (p<.001) controlling for total brain volume using posterior probability derived from VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm/) in SPM5. This exclusion is necessary to ensure that significant differences are based on comparisons of functional activity rather than artifact where the AD group has atrophied cortical regions.
To further characterize the BOLD response during swallowing, we separated active voxels that were shared between groups or exclusive to either group. One sample t-tests were computed for the bias corrected average swallow BOLD responses for each group. The statistical maps were thresholded at alpha<0.005 in at least 20 edge-connected voxels using the “swallowing/AD mask” and converted to binary images. The binary images were combined to identify voxels that were active in only the older adult group or the AD group and voxels that were active in both groups.
Videofluoroscopic Procedures and Image Analysis
Swallowing biomechanics were obtained to determine if physiological differences exist between the two groups in this study. Furthermore, since lingual and pharyngeal kinematics differ in supine compared to upright swallowing [18], biomechanics from supine swallowing were needed to ensure similarity with the fMRI environment where subjects also lay supinely. Videofluoroscopy is a radiographic technique that provides information on swallowing including biomechanics of the upper aerodigestive tract and bolus flow. Full resolution fluoroscopic images were captured in real time (30 frames per second) and digitally recorded for analysis (Siemens Sireskop). The image intensifier was focused on the oral cavity, the posterior pharyngeal wall, and just below the upper esophageal sphincter in the lateral plane. Two speech-language pathologists and a radiologist conducted all videofluoroscopic swallowing studies prior to fMRI procedures.
All participants swallowed three-5ml thin liquid barium boluses (Varibar® Thin, EZ EM, Inc), two-5ml-water boluses and one saliva swallow in random order. The barium and water boluses were manually dispensed onto the mid tongue with a syringe and the same plastic tubing (Medrad® model SSIT_96VLD) as in fMRI procedures.
Videofluoroscopic Image Analysis
For all analyses, the investigators were blinded to group designation.
Durational Measures
Group differences in durational measures of supine swallowing biomechanics (i.e. hyolaryngeal excursion, UES opening) and bolus flow were obtained by using standard criteria and definitions (Table 1) [19, 20].
Table 1.
Standard definitions of durational measures of biomechanics and bolus flow from videofluoroscopic images of swallowing (previously published)
| Duration | Definition |
|---|---|
| Stage transition duration (STD) | time from arrival of bolus head into pharynx until beginning of hyoid excursion |
| Pharyngeal response duration (PRD) |
time from beginning of hyoid excursion until hyoid returns to rest |
| Pharyngeal clearance duration (PCD) |
time from arrival of bolus head at ramus of mandible until bolus tail through UES |
| Duration of hyoid maximum elevation (DOHME) |
time from first maximum hyoid elevation until last maximum hyoid elevation |
| Duration of hyoid maximum anterior excursion (DOHMA) |
time from first maximum hyoid anterior excursion until the last maximum hyoid anterior excursion |
| Duration of laryngeal vestibule closure (LVC) |
time from first contact between arytenoids and base of epiglottis until last contact |
| Duration of UES opening (DOCPO) |
time of UES opening until UES closed |
Measures of Bolus Flow Direction and Clearance
For barium swallows, post-swallow barium contrast residue was judged from the videofluoroscopic image when the hyoid bone returned to rest, operationally defining the end of the swallow. Measurements were taken in the oral cavity, vallecula, posterior pharyngeal wall, pyriform sinus, and upper esophageal sphincter. Ratings were scaled on a 3-point system, in which 0 corresponded to no barium residue, 1 to a coating of barium residue (a line of barium on a structure), and 2 to pooling of barium (an area larger than a line of barium on a structure). An interjudge reliability of 84% and an intrajudge reliability of 90% agreement previously have been reported by using similar datasets [21]. The 8-point Penetration-Aspiration Scale [22, 23] was used to score each swallow observed during the videofluoroscopic swallowing evaluation. Scores on this scale reflect the occurrence, anatomic depth, subject response to, and clearance of material invading the laryngeal vestibule or trachea. All swallowing judgments were blinded to participant group.
Measures of Range of Motion
All videofluoroscopic recordings were digitized using Peak Motus (ViconPeak, Centennial, CO 80112) version 9 for kinematic analysis. The lower anterior corners of the second and fourth cervical vertebrae were marked and a line drawn through these two points served as the y-axis. The x-axis was drawn at a 90-degree angle to y through the point on the fourth cervical vertebra. The following points were located on each frame: the superior/posterior aspect of the subglottal air column (y-axis only - to measure the position of the larynx) and the anterior/inferior most point of the hyoid bone (x- and y- axes). We determined maximum elevation and anterior movement of the hyoid bone and of the larynx for all swallows. Range of motion was derived by subtracting the mean coordinate of the larynx or hyoid bone before swallowing onset (baseline) from the peak coordinate for the respective measure (anterior or superior movement).
Videofluoroscopic Imaging Statistics
The differences between groups for supine swallowing biomechanics (duration and range of motion), residue and swallowing severity were determined using the two-sample t-test (Excel for Mac). Intra-class correlation coefficients (ICC) were computed for all biomechanical measures. The ICC represents the proportion of total variation (between-subject variability and measurement variability) that may be attributed to between-subject variability. Values near 1 suggest nearly all variability is essentially biological variance and not related to measurement, whereas values near 0 indicate that variability is primarily a result of measurement problems [24].
Results
All participants swallowed during the videofluoroscopic and fMRI procedures with no signs of aspiration. Overall, task compliance was 98% for the healthy controls and 96% for AD swallowing during fMRI procedures as determined by the intra-oral pressure device. Among the regions where there was a positive BOLD response during swallowing, 60% was active only in the healthy group, 13% in only AD, and 27% was shared between the two groups. Of the shared regions, the healthy group always had greater BOLD response compared to the AD group.
fMRI Results
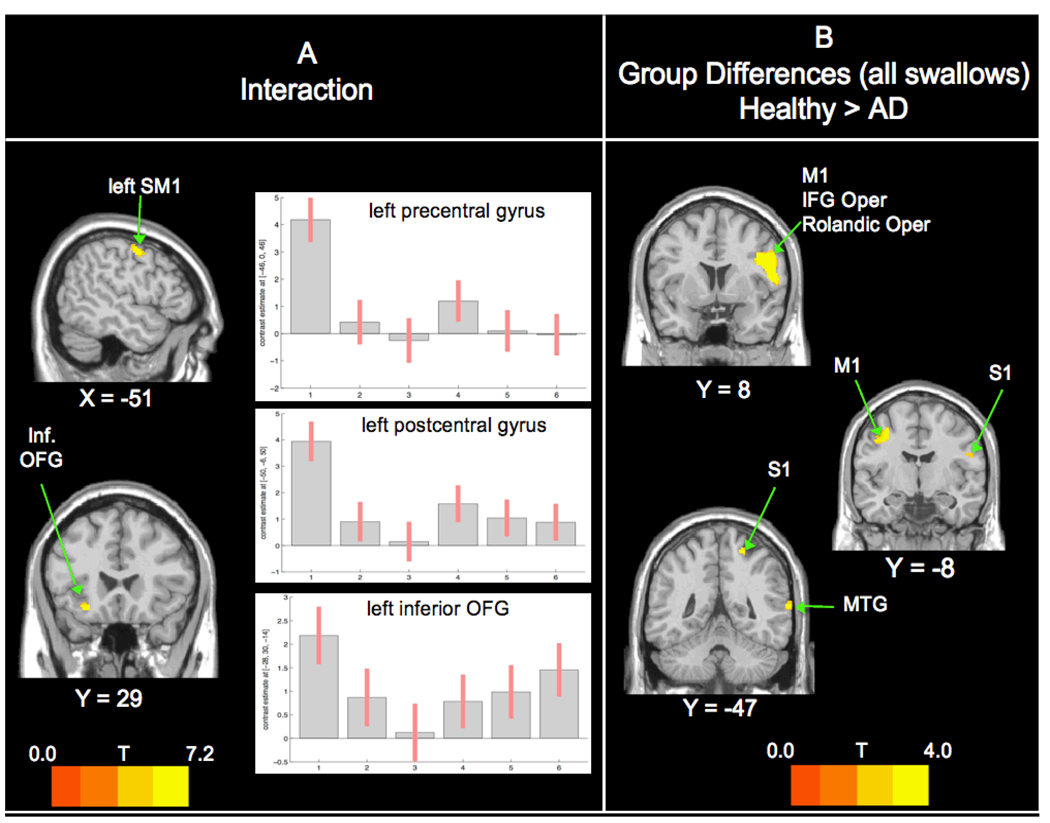
Group by swallow interaction (Figure 1A)
Figure 1. Interaction and Group Comparisons (All Swallows).
A. Significant interaction was found only in the left frontal lobe. In graphs: 1=healthy-saliva; 2=healthy-water; 3=healthy-barium; 4=AD-saliva; 5=AD-water; 6=AD-barium B. Group comparisons show significant BOLD response. Healthy > AD activation bilaterally in the ventro-lateral frontal and parietal lobes and right temporal lobes. Abbreviations: SM1 – precentral and poscentral gyri; Inf OFG – inferior frontal orbital lobe; IFG Oper - inferior frontal gyrus pars opecularis; S1 – Postcentral gyrus; MTG – middle temporal gyrus. Left of image is left side of brain. For all figures, ROI restricted cortical regions that are reported in the literature to be involved in swallowing: precentral gyrus, postcentral gyrus, insular cortex, anterior cingulate cortex, supplementary motor area, supramarginal gyrus, superior frontral gyrus, middle frontal gyrus, inferior frontal gyrus, superior parietal gyrus, middle parietal gyrus, inferior parietal gyrus, frontal inferior opercularis, frontal inferior triangularis, and the Rolandic operculum.
The left precentral and postcentral gyri (MNI −46 0 46 and −50 −6 50; 95 voxel cluster) and in the left orbital frontal gyrus (MNI −28 30 −14; 52 voxel cluster) were significant for an interaction between group and swallow type. These significant results show that the relationship among the 3 swallow types in the healthy group is different from the relationship among the same swallow types in the AD group. More detail can be found in Figure 1, where the precentral and postcentral gyri show a similar pattern of activity, but with greater BOLD response in the healthy group overall. In the orbital frontal gyrus, the 2 participant groups show an inverse relationship for BOLD response relative to swallow types.
Voxel based morphometry
The VBM results show that the AD group had significant difference in grey matter volume in both cerebral hemispheres. The left paracentral lobule, superior temporal pole, and mid frontal gyrus and the right precentral gyrus and cerebellum Crus 2 had significantly less grey matter. The parahippocampus was smaller bilaterally in the AD group.
Group Differences for All Swallows (Figure 1B)
Post-hoc T-tests showed that only the healthy group had greater BOLD response than the AD group (Table 2). These brain regions were primarily right-sided, including the inferior frontal gyrus pars opercularis, pre and post central gyri, Rolandic operculum, and mid temporal gyrus. On the left, the precentral gyrus had greater BOLD response.
Table 2.
Cortical regions with significant BOLD response differences for healthy>AD comparisons.
| Healthy>AD | ||||||
|---|---|---|---|---|---|---|
| cluster | t | z | x | y | z | location |
| 571 | 3.95 | 3.38 | 44 | 8 | 26 | R IFG Oper |
| 3.86 | 3.32 | 50 | 2 | 30 | R Precentral | |
| 3.76 | 3.25 | 54 | 8 | 12 | R Rolandic Oper | |
| 150 | 3.59 | 3.13 | −38 | −8 | 44 | L Precentral |
| 3.12 | 2.79 | −30 | −12 | 52 | ||
| 27 | 3.31 | 2.94 | 22 | −46 | 62 | R Postcentral |
| 32 | 3.27 | 2.9 | 66 | −46 | 10 | R. MTG |
| 26 | 3.05 | 2.75 | 56 | −6 | 24 | R Postcentral |
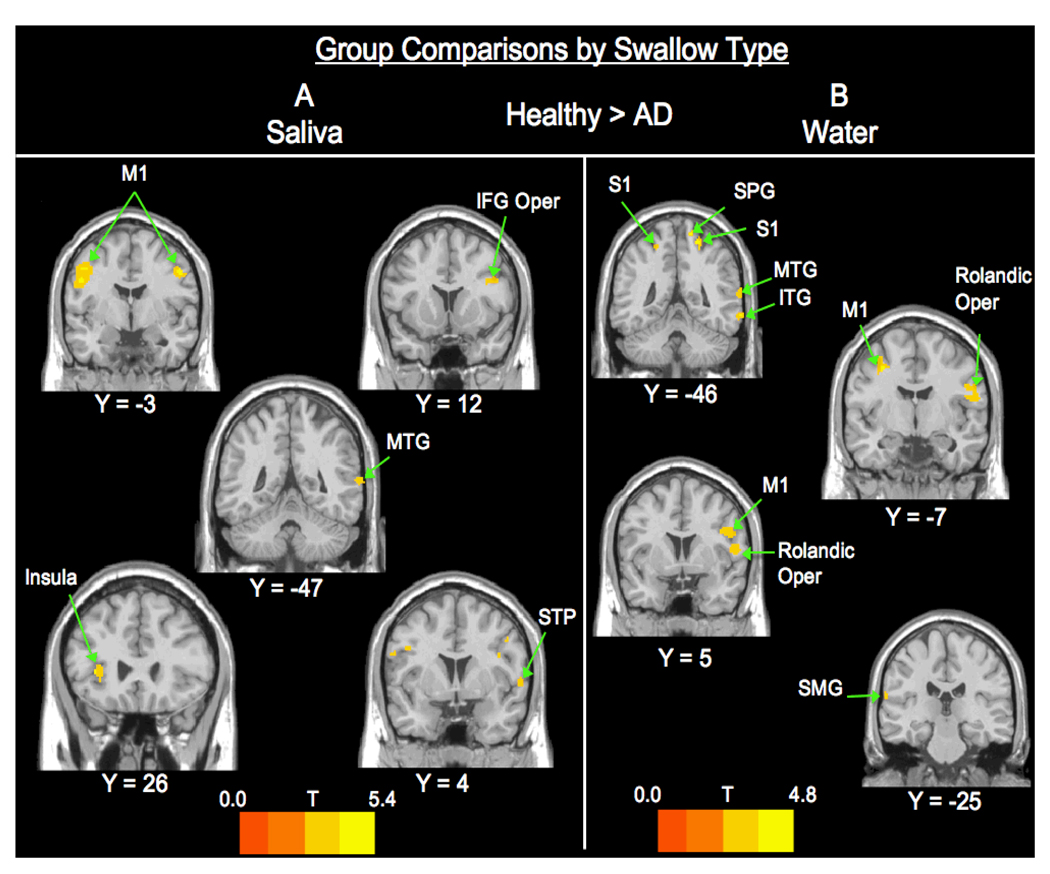
Group Differences by Swallow Type (Figure 2)
Figure 2. Group Comparisons by Swallow Type (Saliva and Water).
A. Saliva swallow healthy > AD contrast shows activation in the frontal, temporal, insular and parietal lobes. B. Water contrasts are significant for BOLD response in frontal, temporal, and parietal lobes. Abbreviations: STP - superior temporal pole; SPG – superior parietal gyrus; ITG – inferor temporal gyrus; SMG – supramarginal gyrus.
Significant BOLD activity differences were found only for the healthy > AD contrast and only for water and saliva swallows, none were found for barium swallows (Table 3). Healthy controls had significantly greater BOLD responses for water swallows in the bilateral pre and post central gyri. Also, there was greater BOLD response in the right inferior and middle temporal gyri, right superior parietal gyrus, right Rolandic Operculum, and left supramarginal gyrus. For saliva swallows, the healthy controls had greater BOLD signal in the bilateral precentral gyrus, left anterior insula, and on the right in the inferior frontal gyrus pars opercularis, superior temporal pole, and middle temporal gyrus.
Table 3.
Cortical regions with significant BOLD response differences by swallow type for healthy > AD comparison.
| Old>AD Saliva | ||||||
|---|---|---|---|---|---|---|
| cluster | t | z | x | y | z | location |
| 475 | 5.36 | 4.21 | −44 | −6 | 42 | L Precentral |
| 4.39 | 3.66 | −50 | −2 | 30 | L Precentral | |
| 96 | 4.42 | 3.68 | 50 | −2 | 40 | R Precentral |
| 59 | 4.16 | 3.51 | −36 | 26 | 6 | L Insula |
| 83 | 3.84 | 3.3 | 38 | 12 | 24 | R IFG Oper |
| 25 | 3.45 | 3.03 | 60 | 4 | 2 | R Sup Temp Pole |
| 38 | 3.36 | 2.97 | 66 | −46 | 10 | R. MTG |
| Old>AD Water | ||||||
| cluster | t | z | x | y | z | location |
| 171 | 4.82 | 3.91 | 16 | −46 | 68 | R. SPG |
| 3.09 | 2.78 | 26 | −40 | 46 | R Postcentral | |
| 315 | 3.57 | 3.12 | 56 | 6 | 12 | R Rolandic Oper |
| 3.33 | 2.95 | 50 | 2 | 30 | R Precentral | |
| 211 | 3.44 | 3.03 | −34 | −6 | 52 | L Precentral |
| 3.38 | 2.99 | −28 | −12 | 62 | L Precentral | |
| 77 | 3.72 | 3.22 | 68 | −48 | −10 | R ITG |
| 3.56 | 3.11 | −20 | −46 | 58 | L Postcentral | |
| 3.51 | 3.08 | −66 | −26 | 20 | L Supramarginal | |
| 3.46 | 3.04 | 66 | −50 | 14 | R MTG | |
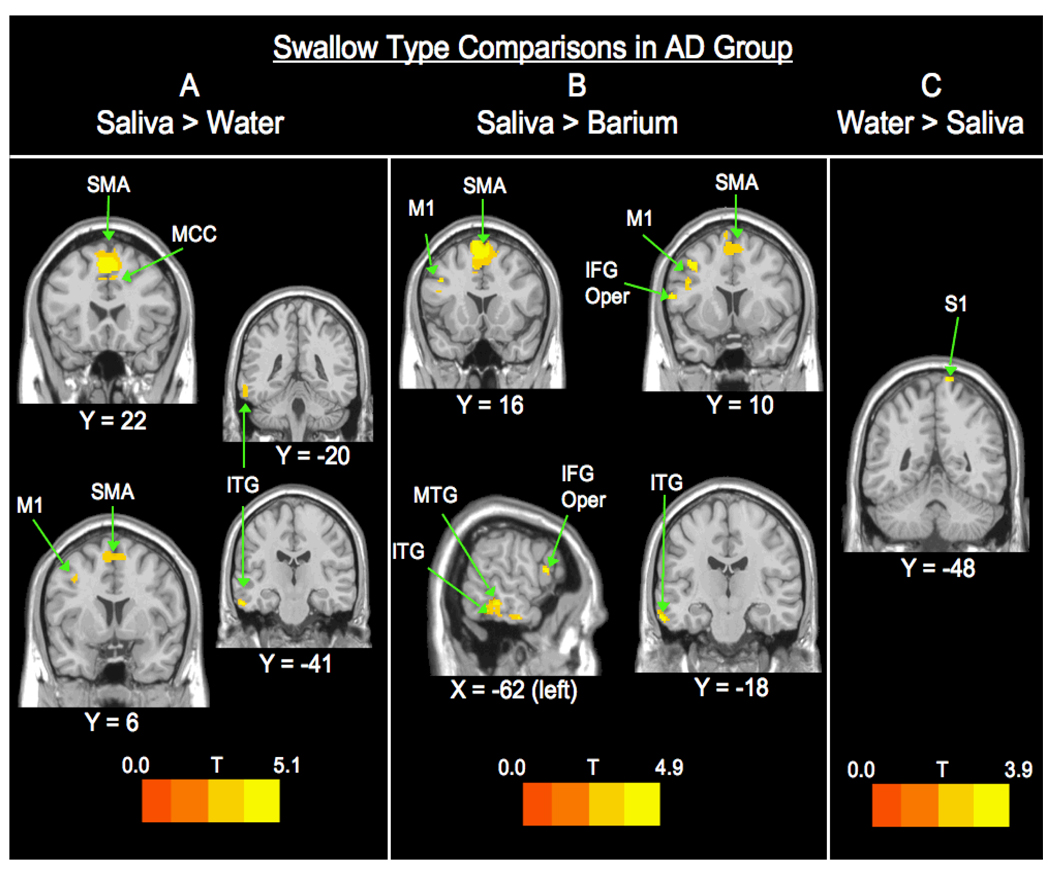
Swallow Type Comparisons in AD group (Figure 3)
Figure 3. Swallow Type Comparisons within the AD Group.
A. Saliva > Water contrasts show activation in the frontal and temporal lobes. B. Saliva > Barium differences are found in more regions within the frontal and temporal lobes. C. Water > Saliva is only significant in the parietal lobe. Abbreviations: SMA – supplemental motor area; MCC – middle cingulate cortex.
Swallow type differences within the AD group were primarily found in the left hemisphere (Table 4). Only 3 contrasts were significant: saliva > barium, saliva > water, and water > saliva. The saliva > barium contrast revealed significant BOLD signal differences bilaterally in the supplementary motor area and in various regions in the left hemisphere including the precentral gyrus, inferior and middle temporal gyri, and inferior frontal gyrus pars opercularis. The saliva > water comparison showed activity in the same regions as the saliva > barium contrast, with the addition of the right mid cingulate gyrus. The water > saliva contrast revealed activation only in the right postcentral gyrus.
Table 4.
Cortical regions with significant BOLD response differences for saliva > barium, saliva > water, and water > saliva within the AD group.
| AD S>W | ||||||
|---|---|---|---|---|---|---|
| cluster | t | z | x | y | z | location |
| 1040 | 5.06 | 4.08 | 2 | 22 | 50 | L SMA |
| 4.72 | 3.88 | −6 | 16 | 56 | ||
| 3.66 | 3.2 | 8 | 2 | 60 | R SMA | |
| 202 | 4.79 | 3.92 | −60 | −20 | −24 | L ITG |
| 3.48 | 3.07 | −64 | −42 | −16 | ||
| 57 | 3.93 | 3.38 | 4 | 30 | 38 | R MCC |
| 3.11 | 2.8 | −6 | 22 | 38 | ||
| 53 | 3.92 | 3.38 | −8 | −8 | 70 | L SMA |
| 26 | 3.21 | 2.88 | −38 | 6 | 40 | L Precentral |
| AD S>B | ||||||
| cluster | t | z | x | y | z | location |
| 967 | 4.85 | 3.96 | −6 | 16 | 70 | L SMA |
| 4.84 | 3.95 | 4 | 24 | 48 | R SMA | |
| 4.74 | 3.89 | −8 | 18 | 60 | L SMA | |
| 51 | 4.3 | 3.62 | −40 | 10 | 44 | L Precentral |
| 65 | 4.28 | 3.61 | −64 | −18 | −22 | L ITG |
| 219 | 4.28 | 3.61 | −64 | −44 | −18 | L ITG |
| 3.89 | 3.35 | −62 | −34 | −10 | LMTG | |
| 3.15 | 2.83 | −62 | −32 | −24 | L ITG | |
| 26 | 4.2 | 3.56 | −62 | 12 | 14 | L ITG |
| 55 | 4.14 | 3.52 | −44 | 12 | 28 | LIFG Oper |
| 37 | 3.47 | 3.06 | −8 | −8 | 70 | L SMA |
| AD W>S | ||||||
| cluster | t | z | x | y | z | location |
| 21 | 3.87 | 3.34 | 12 | −48 | 82 | R Postcentral |
Supine Swallowing Videofluoroscopic Results
Laryngeal vestibule closure (LVC) is visible during swallowing when the epiglottis becomes inverted (due to bolus, lingual and laryngeal forces on it) and contacts the arytenoid cartilages and aryepiglottic folds of the larynx, closing the entrance of the larynx [25]. The control participants had significantly shorter durations of laryngeal vestibule closure than the AD cohort (control .56 msec, AD .67 msec; p<l0.011; ICC > .80). No other swallowing biomechanical comparison survived the corrected alpha threshold level of p=0.016. No statistically significant difference was found between groups for residue or for the penetration-aspiration scale.
Range of motion results
Range of motion data showed that the AD group had significantly less hyoid (p ≤ 0.011; ICC 99.8) and laryngeal elevation (p < 0.0001; ICC 99.5) than the healthy adults. The mean extent of laryngeal elevation for the AD group was 12.7mm (±7.3) and 18.8mm (±7.7) for the healthy group, while mean hyoid ranges were 9.79mm (±7.5) for AD and 13mm (±5.7) for healthy controls. The AD group had greater anterior movement of the hyoid bone and larynx, but neither of these measures survived the corrected alpha threshold level of p=0.0125.
Discussion
Group Differences
This study examined cortical responses to three swallowing tasks to elucidate the early differences in swallowing control in mild AD. This is among the first studies to examine BOLD response for swallowing neurophysiology in individuals with neurological damage. As hypothesized, the AD group had significantly less BOLD response in the swallowing cortical network compared to healthy, age-matched controls. There were no regions where the AD group had greater BOLD response than the healthy cohort for swallowing. This difference in activation likely exists because of disease-related neural atrophy in the patient group.
In a previous investigation, we reported that the BOLD response for swallowing appears to become more lateralized to the right with healthy aging [14], possibly to compensate for increased effort to swallow. In this current study, the healthy group had greater activation in regions that were primarily right-sided (except left precentral gyrus) than the AD group. Thus, it is possible that the early AD group does not have this, apparent, natural shift in activation to the right hemisphere for swallowing as the healthy cohort does. Although the groups are age-matched, the AD group may not be able to involve more global activity for swallowing due to disease-related anatomical or functional neuropathology.
Overall, group differences include regions that are commonly active during normal swallowing across the age span including the pre and post central gyri, and the right Rolandic and frontal operucula [14, 17, 26]. These areas, although not typically atrophied in early AD, receive input from the insula [27], which is involved during preparation to swallow [28]. Many studies have shown that the insula is atrophied in early AD [29–31] and has neuropathological changes at various stages of the disease [32]. The medial temporal gyrus also had greater BOLD response in the healthy group. Neuroimaging studies show activity in the temporal lobe during swallowing [11, 12, 33], likely due to anatomical connections between the temporal lobe and the insula [34] and to motor regions (area 6) [35], as seen in the rhesus monkey. In summary, swallowing-related brain regions that are not directly affected early on in the disease may have less BOLD response in the AD group because of their connections to other affected brain regions.
Swallow types (Group Differences)
The healthy group had greater activation than the AD group for only water and saliva swallows, but not for barium swallows. This same pattern in swallow type differences was found in a healthy old versus young adult comparison that used the same swallowing tasks [14]. It is unknown why BOLD response to barium swallows is not different between groups in the current or previous studies, despite measurable videofluoroscopic differences in swallowing physiology with barium. It is possible that the novelty of our sweetened, thin liquid barium activated a default neural circuit in all participants for whom this material is unfamiliar. Further research is needed to draw more firm conclusions about this result.
For water swallows, the primary active regions include many of the same frontal areas previously discussed, with the addition of parietal and temporal areas. Participants were instructed to hold liquid (water and barium) in the back of the mouth until it was completely infused prior to swallowing. This was likely a novel, salient sensory task for all participants. Also, some judgment or internal planning was necessary to determine when it was time to swallow, especially since no external command was provided to begin swallowing. Others have shown that a salient stimulus (a sensory feature, often novel, of the environment that is attended to) activates many of the same regions as in our group comparison for water (i.e. temporo-parietal junction, supramarginal gyrus) [36, 37]. The AD group may have paid less attention to the oral liquid stimulus for cues to start swallowing than the healthy group.
Saliva swallows had greater BOLD response in larger clusters in the bilateral precentral gyrus and the left anterior insula in the healthy adults. Saliva swallows provide less sensory stimuli than liquid swallows, often requiring an adequate amount of saliva and, occasionally, tongue-pumping to initiate. Therefore, it is no surprise that there was greater BOLD signal within the bilateral primary motor cortex and the left anterior insula for saliva swallows. It is also possible that initiation and execution of the saliva swallow was more difficult in the AD group due to atrophy in the precentral and insular regions. This is supported by evidence that apraxia of speech and dysarthia in Alzheimer’s disease corresponds with significant atrophy in the left anterior insula and left ventrolateral precentral gyrus [38].
Swallow types within AD group
Within the AD group, three swallow type contrasts were significant: saliva > barium, saliva > water, and water > saliva. Comparison results in this analysis were predominantly in the left hemisphere. Overall, in the AD group, saliva swallows had greater activation in regions of interest for swallowing than swallows with a bolus (i.e. SMA, IFG operculum, precentral gyrus, inferior temporal gyrus). Saliva swallows may have elicited greater activation in swallowing areas because they can be more difficult to elicit on command without a bolus present in the oral cavity. On the other hand, water swallows had greater BOLD response only in the right postcentral gyrus, which is important for sensation during swallowing.
Swallowing biomechanics
Biomechanical differences between the two groups centered on hyoid bone and laryngeal function, involving both range and duration of movement. The hyoid bone and larynx (hyolaryngeal complex) is an integral part of functional swallowing, especially during the pharyngeal phase. Normally, they move antero-superiorly as a unit and the larynx closes intrinsically at multiple levels to avoid airway invasion as the bolus passes through the pharynx. Several muscles in the floor of the mouth contract to elevate the hyoid bone (mylohyoid, geniohyoid, anterior belly of the digastrics) and, when combined with thyrohyoid muscle activity, laryngeal elevation is also achieved. Hyolaryngeal elevation and anterior movement also aid in closure of the laryngeal vestibule [25].
Reduced hyolaryngeal elevation can place individuals at risk for aspiration [39]. The AD group had reduced hyolaryngeal movement compared to the healthy group, making swallowing potentially less safe compared to the healthy controls, although no aspiration was observed and swallowing was functional. Motor representation of the larynx in the precentral gyrus [40] for voiced and non-voiced tasks overlap with our findings where our healthy cohort had greater BOLD response (group differences data; MNI coordinates 50,−2,37 and −38,−14,32; Table 1). Thus, these hyolaryngeal biomechanical differences might be explained by functional variances in cortical control of the precentral gyrus where laryngeal control has been found.
Limitations
Some constraints limit our results, including the small number of participants and the inability to image both swallowing biomechanics and the brain simultaneously. Swallowing tasks were completed in the supine position and cannot be directly generalized to swallowing biomechanics in an upright position and only 5ml amounts were studied. Saliva swallows were cued differently (visual) than water and barium swallows (tactile) to ensure that an adequate number of saliva swallows could be analyzed and so that they occurred at predictable intervals (which might account for some differences in activation). Finally, there is a possibility that saliva and water swallows that followed barium swallows may have included trace amounts of barium, possibly affecting BOLD response for those swallow types.
Conclusions
Our results provide evidence that the neurophysiology of swallowing in early AD involved lower BOLD responses in both traditional swallowing cortical areas and in regions commonly affected by AD. Although memory dysfunction is among the first noticeable symptoms of AD, these findings suggest that the brain areas underlying swallowing function also show compromise early on, before clinical dysphagia diagnosis. Furthermore, this, and other investigations with functional neural imaging, shows that swallowing could regularly include cognitive cortical areas (temporal lobe, hippocampus). This is significant because swallowing diagnosis and intervention often involve compensatory maneuvers or require the ability to follow directions and swallow on command. Very little attention has been paid to the importance of cognition on swallowing ability, although aspiration, and often the consequential pneumonia, is prevalent in neurodegenerative populations with cognitive impairment and dysphagia [41–43].
An important clinical implication of this study that can be readily applied to practice is that, in Alzheimer’s disease, changes to swallowing physiology might start long before they are diagnosed clinically. Traditionally, dysphagia, aspiration, and aspiration pneumonia have been viewed as very late stage consequences of the disease. However, our videofluoroscopic findings and those of Priefer and Robbins (1997) show that swallowing and self-feeding changes occur early on in the disease. Therefore, clinical diagnosis and intervention should also be initiated early, before cognitive deficits become too great and reduce the likelihood that patients can alert caregivers or health care providers to their signs or symptoms of dysphagia. For safety, this population might benefit from swallowing rehabilitation that does not challenge their cognitive ability such as bolus modification (i.e. thickened liquids) or even alterations in utensils (i.e. smaller spoon to facilitate smaller bites). Caretakers also should be educated about swallowing impairment in AD alongside memory and cognitive losses once AD is diagnosed. These findings warrant further investigation into differences of both physiology and neurophysiology of swallowing in neurologically-based dysphagias to better understand swallowing impairment in these populations.
Acknowledgements
National Institutes of Health (NIH), NCRR. The Training and Education to Advance Multidisciplinary-Clinical-Research (TEAM) Program. 8K12RR023268-02
Swallowing Physiology and Neurophysiology in Alzheimer’s and Lewy Body Disease. Wisconsin Comprehensive Memory Program (Pilot Funding 2006–2008).
National Institutes of Health and National Institute on Aging grant no. 5T32AG000213-18 and the William S. Middleton Memorial VA Hospital Geriatric Research Education and Clinical Center (GRECC).
This is GRECC manuscript # 2009–12.
References
- 1.van der Steen JT, Ooms ME, Mehr DR, van der Wal G, Ribbe MW. Severe dementia and adverse outcomes of nursing home-acquired pneumonia: evidence for mediation by functional and pathophysiological decline. J Am Geriatr Soc. 2002;50:439–448. doi: 10.1046/j.1532-5415.2002.50108.x. [DOI] [PubMed] [Google Scholar]
- 2.Beard CM, Kokmen E, Sigler C, Smith GE, Petterson T, O'Brien PC. Cause of death in Alzheimer's disease. Ann Epidemiol. 1996;6:195–200. doi: 10.1016/1047-2797(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 3.Burns A, Jacoby R, Luthert P, Levy R. Cause of death in Alzheimer's disease. Age Ageing. 1990;19:341–344. doi: 10.1093/ageing/19.5.341. [DOI] [PubMed] [Google Scholar]
- 4.Wada H, Nakajoh K, Satoh-Nakagawa T, Suzuki T, Ohrui T, Arai H, Sasaki H. Risk factors of aspiration pneumonia in Alzheimer's disease patients. Gerontology. 2001;47:271–276. doi: 10.1159/000052811. [DOI] [PubMed] [Google Scholar]
- 5.Priefer BA, Robbins J. Eating changes in mild-stage Alzheimer's disease: a pilot study. Dysphagia. 1997;12:212–221. doi: 10.1007/PL00009539. [DOI] [PubMed] [Google Scholar]
- 6.Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1994;8:177–189. doi: 10.1097/00002093-199408030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Chu CC, Tranel D, Damasio AR, Van Hoesen GW. The autonomic-related cortex: pathology in Alzheimer's disease. Cereb Cortex. 1997;7:86–95. doi: 10.1093/cercor/7.1.86. [DOI] [PubMed] [Google Scholar]
- 8.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 9.Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer's disease. A clinico-pathological study. Arch Psychiatr Nervenkr. 1976;223:15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- 10.Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G531–G538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- 11.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277:G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 12.Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281–286. [PubMed] [Google Scholar]
- 13.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, Hind J, Robbins J. Neurophysiology of swallowing: Effects of age and bolus type. Neuroimage. 2009;44:982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding R, Logemann JA, Larson CR, Rademaker AW. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: a surface electromyographic study. J Speech Lang Hear Res. 2003;46:977–989. doi: 10.1044/1092-4388(2003/076). [DOI] [PubMed] [Google Scholar]
- 16.Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- 17.Martin R, Barr A, Macintosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V. Cerebral cortical processing of swallowing in older adults. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0592-6. [DOI] [PubMed] [Google Scholar]
- 18.Dejaeger E, Pelemans W, Ponette E, Vantrappen G. Effect of body position on deglutition. Dig Dis Sci. 1994;39:762–765. doi: 10.1007/BF02087420. [DOI] [PubMed] [Google Scholar]
- 19.Lof GL, Robbins J. Test-retest variability in normal swallowing. Dysphagia. 1990;4:236–242. doi: 10.1007/BF02407271. [DOI] [PubMed] [Google Scholar]
- 20.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 21.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82:1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 22.Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetrationaspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 24.Fleiss JL. The design and analysis of clinical experiments. New York: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 25.Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol. 1992;262:G338–G344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- 26.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 28.Dziewas R, Soros P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage. 2003;20:135–144. doi: 10.1016/s1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- 29.Foundas AL, Eure KF, Seltzer B. Conventional MRI volumetric measures of parietal and insular cortex in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1131–1144. doi: 10.1016/s0278-5846(96)00101-7. [DOI] [PubMed] [Google Scholar]
- 30.Bozzali M, Filippi M, Magnani G, Cercignani M, Franceschi M, Schiatti E, Castiglioni S, Mossini R, Falautano M, Scotti G, Comi G, Falini A. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology. 2006;67:453–460. doi: 10.1212/01.wnl.0000228243.56665.c2. [DOI] [PubMed] [Google Scholar]
- 31.Foundas AL, Leonard CM, Mahoney SM, Agee OF, Heilman KM. Atrophy of the hippocampus, parietal cortex, and insula in Alzheimer's disease: a volumetric magnetic resonance imaging study. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:81–89. [PubMed] [Google Scholar]
- 32.Bonthius DJ, Solodkin A, Van Hoesen GW. Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. J Neuropathol Exp Neurol. 2005;64:910–922. doi: 10.1097/01.jnen.0000182983.87106.d1. [DOI] [PubMed] [Google Scholar]
- 33.Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol. 1999;81:1917–1926. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- 34.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 35.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 36.Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- 37.Knight RT, Nakada T. Cortico-limbic circuits and novelty: a review of EEG and blood flow data. Rev Neurosci. 1998;9:57–70. doi: 10.1515/revneuro.1998.9.1.57. [DOI] [PubMed] [Google Scholar]
- 38.Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21:S23–S30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- 39.Lundy DS, Smith C, Colangelo L, Sullivan PA, Logemann JA, Lazarus CL, Newman LA, Murry T, Lombard L, Gaziano J. Aspiration: cause and implications. Otolaryngol Head Neck Surg. 1999;120:474–478. doi: 10.1053/hn.1999.v120.a91765. [DOI] [PubMed] [Google Scholar]
- 40.Brown S, Ngan E, Liotti M. A larynx area in the human motor cortex. Cereb Cortex. 2008;18:837–845. doi: 10.1093/cercor/bhm131. [DOI] [PubMed] [Google Scholar]
- 41.Langmore SE, Olney RK, Lomen-Hoerth C, Miller BL. Dysphagia in patients with frontotemporal lobar dementia. Arch Neurol. 2007;64:58–62. doi: 10.1001/archneur.64.1.58. [DOI] [PubMed] [Google Scholar]
- 42.Logemann JA, Gensler G, Robbins J, Lindblad AS, Brandt D, Hind JA, Kosek S, Dikeman K, Kazandjian M, Gramigna GD, Lundy D, McGarvey-Toler S, Miller Gardner PJ. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson's disease. J Speech Lang Hear Res. 2008;51:173–183. doi: 10.1044/1092-4388(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins J, Gensler G, Hind J, Logemann JA, Lindblad AS, Brandt D, Baum H, Lilienfeld D, Kosek S, Lundy D, Dikeman K, Kazandjian M, Gramigna GD, McGarvey-Toler S, Miller Gardner PJ. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148:509–518. doi: 10.7326/0003-4819-148-7-200804010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]