Abstract
Objectives
We aim to establish the endoscopic pancreatic function test (ePFT) as a method that can safely obtain pancreatic fluid for mass spectrometry analysis from patients during upper endoscopy and to reproducibly identify pancreas-specific proteins.
Methods
We performed SDS-PAGE and mass spectrometry-based proteomic analysis (GeLC-MS/MS) on ePFT-collected pancreatic fluid from three individuals, without evidence of chronic pancreatitis, who were undergoing an upper endoscopy for dyspepsia and chronic abdominal pain.
Results
Pancreatic fluid was safely collected from all subjects. SDS-PAGE analysis of ePFT-collected pancreatic fluid revealed no significant variation (F-statistic 1.33; p-value 0.29) in protein concentration during the 1 hour collection period and a visually reproducible protein banding pattern among the three subjects. GeLC-MS/MS analysis of ePFT-collected fluid identified pancreas-specific proteins previously described from ERCP and surgical collection methods. Gene ontology further revealed that the majority of the proteins identified have molecular function of proteases.
Conclusions
The ePFT is capable of collecting large amounts of pancreatic fluid for proteomic analysis enabling the identification of pancreas-specific proteins. This endoscopic collection method coupled with GeLC-MS/MS is a powerful technique, which can be used in future investigations to elucidate pathways involved in the development and progression of pancreatic disease.
Keywords: proteins, body fluid, biomarker, mass spectrometry, ePFT, pancreatic function test
Introduction
Proteomic techniques can evaluate the protein profile of complex biological samples to understand better the normal physiology and pathogenic mechanisms of human disease. Recent developments in mass spectrometry have revolutionized protein identification enabling the comprehensive analysis of protein mixtures from which hundreds or thousands of proteins may be identified for further investigation [1, 2]. Application of proteomics to the study of pancreatic disease presents a unique opportunity to accelerate the pace of biomarker discovery [1]. Pancreatic juice is a proximal body fluid that is ideal for proteomic analysis, as it is of relatively low complexity, which simplifies the identification of low-abundant proteins [3-5].
Previously published proteomic analysis of pancreatic fluid has been limited to pancreatic disease cases undergoing invasive endoscopic procedures (endoscopic retrograde cholangiopancreatography; ERCP) or pancreatic surgery [4, 6-10]. Retrograde pancreatogram is not justified in healthy subjects to obtain disease-free pancreatic fluid because of the significant risks associated with this procedure [7]. Therefore, previously published pancreatic fluid proteomic analyses have utilized benign, non-malignant patient specimens as “surrogate” controls. To date, the proteome of pancreatic fluid from a disease-free cohort has not been investigated [11].
We have developed an endoscopic pancreatic function test (ePFT) that collects pancreatic fluid without cannulation of the pancreatic duct and without the risk of procedure-related injury [12-14]. Analysis of its electrolyte composition and enzyme activity supports the notion that ePFT-collected fluid reproduces the classic acinar and duct cell secretory profiles following hormonal stimulation [12, 13, 15-17]. Furthermore, the ePFT is now considered an acceptable alternative method for the assessment of pancreas secretory physiology [18, 19]. However, a systematic and comprehensive analysis of the protein composition of ePFT-collected pancreatic fluid has not been performed. As the ePFT collection method is a valuable alternative tool to acquire pancreatic fluid even from subjects without pancreas-related disease, we aim to establish this technique as a viable method to elucidate the proteome of pancreatic fluid.
For proteomic analysis of our ePFT-collected pancreatic fluid, we use GeLC-MS/MS (in-gel tryptic digestion followed by liquid chromatography-tandem mass spectrometry), a powerful approach for proteomic analyses [20, 21]. Proteins are fractionated using one-dimensional SDS-PAGE and entire gel lanes are excised and further subdivided into smaller sections to allow for efficient processing. The proteins in these gel sections are subsequently digested in-gel with trypsin and the generated peptides are subjected to a nanoflow reversed-phase LC-MS/MS experiment to obtain peptide sequence information and hence identify the proteins present in a particular sample of pancreatic fluid.
The primary objectives of our current exploratory investigation are as follows:
collect pancreatic fluid with the ePFT method after secretin stimulation and process it for proteomic analysis,
determine the time course variation in secreted protein concentration during secretin stimulation to optimize sample collection time,
assess the reproducibility and variation of protein banding patterns in ePFT-collected pancreatic fluid specimens using SDS-PAGE,
identify pancreas-specific proteins in ePFT-collected fluid with mass spectrometry (GeLC-MS/MS), and
describe the molecular function of identified pancreas-specific fluid proteins.
The methodology established herein will enable the further investigation of the protein composition of the ePFT-collected pancreatic fluid secretome in healthy and pancreatic disease patients and broaden our knowledge of pancreatic secretory physiology and disease pathogenesis.
Materials and Methods
Study Design and Setting
Proteomic analysis experiment of endoscopically collected fluid in an academic center.
Study Population
This protocol was approved by the Institutional Review Board at Brigham and Women's Hospital and Children's Hospital Boston (IRB # 2007-P-002480/1). The study population included adult patients seen in the Center for Pancreatic Diseases at Brigham and Women's Hospital for abdominal pain and dyspepsia. Subjects were referred to the Center for Pancreatic Disease to eliminate a pancreatic etiology for their gastrointestinal symptoms. All subjects underwent the following: 1) comprehensive review of history and physical examination, 2) review of radiologic and endoscopic data, and 3) upper endoscopy with ePFT followed by a gastric and duodenal mucosal biopsy.
Materials
ChiRhoStim® synthetic human secretin was from ChiRhoClin (Burtonsville MD). SeeBluePlus2 Pre-Stained standard (LC5925), LDS (lithium dodecyl sulfate) sample buffer (NP0008), NuPAGE 4-12% Bis-Tris polyacrylamide gels (NP0335), SimplyBlue Coomassie stain (LC0665), and MES-SDS (2-(N-morpholino)ethanesulfonic acid-sodium dodecyl sulfate) running buffer (NP002) were from Invitrogen (Carlsbad, CA).
Experimental Workflow
Figure 1 illustrates the general workflow for the overall analysis as follows: 1) ePFT sample collection, 2) centrifugation, 3) protein precipitation, 4) SDS-PAGE, 5) in-gel tryptic digest, 6) LC-MS/MS peptide mass determination, and 7) bioinformatic data processing.
Figure 1.
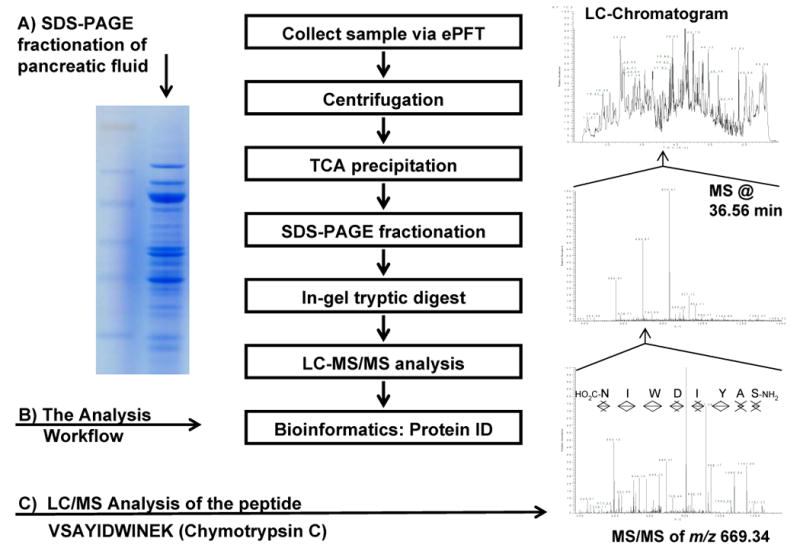
Experimental workflow. A) SDS-PAGE gel image of proteins extracted from pancreatic fluid. B) Workflow of the GeLC-MS/MS experiment for the proteomic profiling of pancreatic fluid. C) Chromatogram of fraction 11 of the proteomic profiling of pancreatic fluid; the survey mass spectrum at 36.56 min; the product ion spectrum of the peptide at m/z 669.34 (marked with an arrowhead in the survey spectrum); database searches identified this MS/MS spectrum as being from VSAYIDWINEK from chymotrypsin C.
Pancreatic Fluid Collection (ePFT method)
The ePFT procedure was as follows: 1) pre-procedural assessment, 2) endoscopic procedure, and 3) post-procedural assessment/recovery.
A. Pre-procedural assessment
Prior to upper endoscopy all study subjects underwent a history and physical examination, including list of allergies, medications, substance use/abuse, vital signs, and physical examination. Pre-procedural sedation review included airway assessment based on Mallampati airway scale and American Society of Anesthesiologists Physical Status Classification (ASA Class). All study subjects in this protocol had a Mallampati score of B, Class 2 and ASA Class II or better.
B. Endoscopic procedure
Endoscopic collection was performed in a stepwise manner as follows:
1) The patient was placed in left lateral decubitus position with slight head elevation. 2) The posterior pharynx was sprayed with topical cetacaine spray. 3) A sedation and analgesia bolus was administered. 4) Further sedation doses were given if necessary for patient comfort. 5) After the sedation bolus, a bite-block was placed. 6) Esophagogastroduodenoscopy (EGD) was performed using a standard (10 mm) gastroscope for visualization of the esophagus, stomach and duodenum (2 to 5 minutes). 7) Gastroduodenal fluid was aspirated (approximately 1 minute) as completely as possible through the gastroscope. 8) A test dose of synthetic human secretin (ChiRhoStim®) was administered and patients were monitored for anaphylaxis or adverse reaction, followed by a standard weight-based intravenous bolus (0.2 μg/kg) given over 1 minute. 9) Pancreatic fluid was aspirated from the descending duodenum at specific timed intervals following hormonal stimulation and kept on ice.
For the purposes of this investigation, duodenal aspirates were collected at 0, 5, 10, 15, 20, 30, 45 and 60 minutes after stimulation. Samples were divided and sent to the Brigham and Women's Hospital Biochemistry Laboratory for measurement of electrolyte profiles and to the Proteomics Center at Children's Hospital Boston for proteomic analysis. Biopsies of the stomach and duodenum were obtained to eliminate microscopic gastrointestinal disease, such as Helicobacter pylori or celiac sprue as a cause of dyspepsia.
C. Post-procedural Assessment / Recovery
Study participants recovered and were discharged from the endoscopy unit based on hospital procedural sedation guidelines assessing level of consciousness, vital signs, oxygen saturation, alertness, gag reflex, degree of nausea, and ability to ambulate.
Pancreatic Fluid Biochemical Analysis
Pancreatic fluid samples were frozen at −80°C and stored until analysis; all measurements were conducted within two weeks of sample collection. A separate in-house validation study has demonstrated no significant difference in pancreatic fluid electrolyte concentrations when stored for two weeks at - 80°C (data not shown). Samples were thawed at room temperature, and an aliquot was passed through a serum filter (ML0550, MarketLab, Caledonia, MI) to remove particulates and fibrin microthrombi prior to analysis. All measurements were conducted in the CLIA-certified Brigham and Women's Hospital Clinical Chemistry Laboratory, under the standard operating procedures on an AU640 (Olympus America, Center Valley, PA) automated chemistry analyzer. Sodium, potassium, and chloride were measured by indirect ion-selective electrodes, and total bicarbonate was measured by the two-step phosphoenolpyruvate carboxylase-malate dehydrogenase enzymatic-photometric method [22]. Samples with results greater than the upper assay limit were diluted into the linear range. The mean peak bicarbonate concentration from previously published studies in secretin-stimulated pancreatic fluid was 103 ± 11 meq/l [16]. A cut-off point of 80 meq/l was two standard deviations below the mean and considered abnormal.
Pancreatic Fluid Proteomic Analysis
A. Pancreatic fluid sample preparation
In brief, pancreatic fluid samples were collected on ice, centrifuged at 4°C at 14,000 rpm to remove cellular debris, and aliquoted (500 μL) prior to storage at -80°C. Protein concentration was determined using the BioRAD protein assay according to the manufacturer's instructions. In preparation for SDS-PAGE analysis, the proteins from pancreatic fluid specimens were isolated by precipitation with the addition of 12.5% trichloroacetic acid (TCA). This process limits protein degradation by instantaneously deactivating enzymes and removing salts that will interfere with the subsequent molecular weight-based fractionation by SDS-PAGE, as described below. Pellets were re-dissolved in 50 μL of reducing Laemmli buffer (with 10 mM dithiothreitol) and alkylated with 1% acrylamide for subsequent GeLC-MS/MS.
B. SDS-PAGE prefractionation and liquid chromatography-tandem mass spectrometry (GeLC-MS/MS) of pancreatic fluid specimens
The proteins were fractionated using 4-12% NuPAGE pre-cast SDS-PAGE gels at 175V for 40 minutes using MES-SDS running buffer. Subsequently, entire gel lanes were divided into 7 sections. Proteins in each gel section were digested in-gel with trypsin [23, 24]. The extracted peptides from each gel section were subjected to peptide fractionation using reversed-phase high performance liquid chromatography (HPLC; Thermo Scientific) and the gradient-eluted peptides were analyzed by an in-line LTQ (linear trap quadrupole) mass spectrometer (Thermo Scientific). The liquid chromatography columns (15 cm × 100 μm ID) were packed in-house (Magic C18, 5 μm, 100°Å, Michrom BioResources, into PicoTips, New Objective, Woburn, MA). Samples were analyzed with a 60 minute linear gradient (0-35% acetonitrile with 0.2% formic acid) and data were acquired in a data dependent manner, in which MS/MS fragmentation is performed on the 6 most intense peaks of every full MS scan.
Bioinformatics and Data Analysis
All data generated from the gel sections were searched against the IPI-human database (v3.36) using the Mascot search engine (v.2.204; Matrix Science). One miscleavage per peptide was allowed and mass tolerances of ± 1 Da (monoisotopic) for precursor and of ± 0.8 Da for fragment ions were used, as was default for LTQ data analysis. Amino acid modifications: fixed: propionamide (Cys); variable: deamidation (Asn/Gln), pyro-glutamate (N-terminal Glu/Gln), and oxidation (Met). Mascot search results were combined using in-house-developed software. In strict compliance with a set of recommendations [25-27] proposed by the major proteomic journals, we present the following protein identification validation method that minimizes false positives and reports only high confidence identifications. Our false discovery rate (FDR) was 1% at the peptide level as determined by searching the same dataset against the target database and a decoy database; the latter featured the reversed amino acid sequences of all the entries in the IPI-human database (v3.36) [28, 29]. We applied the following stringent identification criteria for protein identifications to ensure a false positive rate of ≤ 0.1% at the protein level: 1) a minimum of 2 unique peptides was required for protein identification; 2) each peptide had a score equal or greater than the 1% FDR cut-off (see above); and 3) each matched peptide corresponds to the highest scoring peptide for that MS/MS spectra. Protein lists were imported into Ingenuity Pathways Analysis (Ingenuity Systems, v 7.5) software for additional data comparison and gene ontology analysis. An analysis of variance (ANOVA) for dependent populations was calculated based on the mean protein concentration values of pancreatic fluid at each collection time point over one hour. An F-statistic was calculated and a p-value less than 0.05 was considered statistically significant.
Results
Endoscopic Pancreatic Function Test (ePFT) and Characterization of Study Cohort
Pancreatic fluid was acquired for both biochemical and molecular analysis via secretin-stimulated ePFT from three subjects with chronic abdominal pain (CAP). All subjects underwent a complete history and physical examination at the Center for Pancreatic Disease. All outside procedures and radiology were reviewed and selected radiologic and endoscopic tests were repeated at Brigham and Women's Hospital where necessary. Table 1 summarizes the patients in terms of demographic, radiologic, endoscopic, histologic, and function testing results.
Table 1.
Demographic and clinical data on chronic abdominal pain patients.
| ID | Reason for Referral | Age (yr) | Gender | Serum pancreas enzymes (u/L) | CT Scan | MRI | sMRCP | EUS CP Score (0-9) | ePFT Peak Bicarb. (meq/L) | Endoscopic Biopsy (gastric, duodenum) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| amylase (20-70) | lipase (0-65) | ||||||||||
| CAP1 | Abdominal pain | 28 | Male | 140-159 | 243-352 | Normal | Normal | Normal | 3 | 84 | Normal |
| CAP2 | Abdominal pain | 54 | Male | 82-153 | 31-32 | Normal | Normal | Abnormal side branches | - | 114 | Normal |
| CAP3 | Abdominal pain | 53 | Female | 44-53 | 33-94 | Normal | Normal | Normal | 1 | 92 | Gastropathy |
The study cohort had a mean age of 45 years and 2 out of 3 were male. There was no history of alcohol abuse, acute recurrent pancreatitis or therapeutic endoscopic pancreaticobiliary procedures / instrumentation. All imaging studies including computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) were without evidence of acute or chronic pancreatic disease. The mean peak bicarbonate concentration in secretin-stimulated pancreatic fluid was 96 meq/L. Endoscopic biopsies of the stomach and duodenum were normal in all patients and without evidence of Helicobacter pylori infection or celiac sprue. Thus, careful review of all clinical, laboratory, and radiologic data eliminated pancreatic disease as a cause of their gastrointestinal dyspeptic symptoms.
SDS-PAGE Analysis of Pancreas Fluid Samples
ePFT time course revealed no significant variation in protein content
In the analysis of ePFT-collected pancreatic fluid, it was crucial to maximize the amount of protein obtained via ePFT, as larger protein amounts allowed for an increased analytical depth. To avoid the risk of collecting fluid at a sub-optimal time point, we investigated the temporal variation of the proteins secreted in secretin-stimulated pancreatic fluid. Pancreatic fluid was collected at a series of time points (t = 0, 5, 10, 15, 20, 30, 45, and 60 minutes) following secretin stimulation. Samples were TCA-precipitated and separated by SDS-PAGE. As shown in Figure 2, there was little deviation from the overall visual protein band pattern after secretin stimulation among and within the time courses of different patients.
Figure 2.
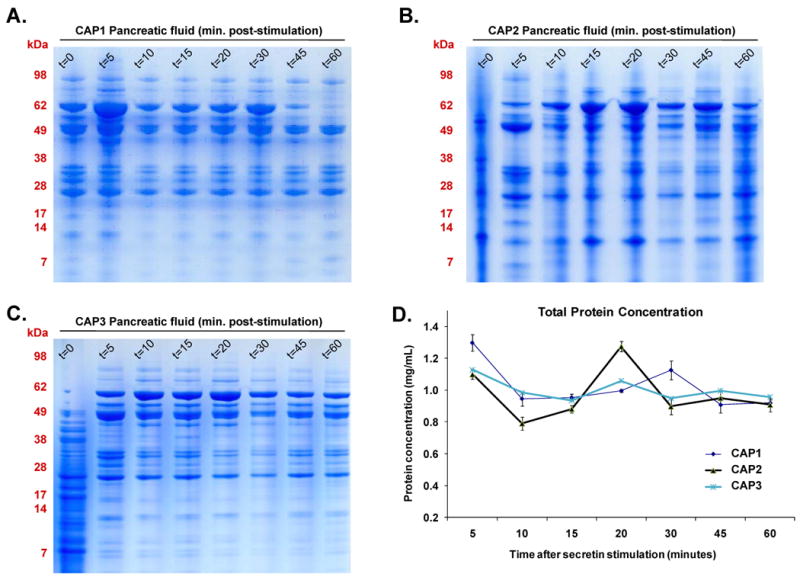
A-C) SDS-PAGE gels of time courses of secretin-stimulated ePFT-collected pancreatic fluid proteins of individuals with chronic abdominal pain (A: CAP1, B: CAP2, C: CAP3). D) Temporal variation of pancreatic protein concentration secretion following secretin stimulation for the three samples (CAP1, CAP2 and CAP3). Error bars are standard deviations of technical replicates.
The variability in protein content of the t=0 samples may be attributed to the nature of the collection. Prior to the collection of the t=0 sample, the gastroduodenal fluid was aspirated; thus, incomplete aspiration may result in prominent contamination of this sample with gastroduodenal fluid, although much less likely in subsequent collections. In addition, there was little fluid secreted during the initial time point and such fluid was more viscous than later samples, which can adversely affect protein extraction and distort the SDS-PAGE protein band patterning, as was evident by the t=0 lanes in Figure 2 A-C. We suggest that this sample (t=0) not be used for proteomic analysis aimed at identifying pancreatic fluid proteins.
Protein concentration, as determined by the BioRAD (Bradford-based) protein assay, ranged between 0.8 to 1.4 mg/mL for all samples (Figure 2 D). Although the 5 minute time point (t=5) indicated a slightly increased protein concentration, there was no statistically significant difference (F-statistic 1.33; p-value 0.29) among the three curves, indicating stable protein secretion over 60 minutes after secretin stimulation. The 30 and 45 minute time points, which are routinely processed for the previously-described biochemical electrolyte analysis, are both convenient and practical for subsequent mass spectrometric analysis.
Protein profiling of ePFT-collected pancreatic fluid is reproducible
Previous studies have illustrated significant patient-to-patient variation of pancreatic fluid protein profiles on SDS-PAGE gels [4]. We have observed that protein degradation of samples following a 30-minute incubation at 23°C and 37°C was apparent via SDS-PAGE gels (manuscript in preparation). We questioned the potential effect of specimen collection/processing methodology and analyzed biological replicates from our cohort, which showed visually consistent protein patterns among and within the time courses of different patients (Figure 2). We attribute the improvement in the reproducibility of our gels when comparing our data with those of previous reports to our systematic and careful collection, handling, and processing of the pancreatic fluid samples.
Proteomic Analysis of Pancreas Fluid Samples
Pancreas-specific proteins were identified in ePFT-collected pancreatic fluid
Pancreas-specific proteins that were common among the three samples analyzed by GeLC-MS/MS are listed in Table 2. These proteins included carboxypeptidase A1, carboxypeptidase A2, chymotrypsinogen B1, chymotrypsinogen B2, chymotrypsin C, elastase, lipase, and trypsin. These proteins were likely produced and secreted by the pancreas, and have also been identified in fluid collected via ERCP or surgery [4, 6-10]. The right-most column of Table 2 lists studies in which the corresponding proteins have also been identified.
Table 2.
Pancreas-specific proteins identified by our GeLC-MS/MS analysis that are present in ePFT collected samples. References in the right-most column are of published pancreatic fluid studies in which the specified protein was identified.
| Enterez GeneID | Protein name | Subcellular location | Molecular function | No. of peptides | Mascot score | Previously identified |
|---|---|---|---|---|---|---|
| AMY2B | amylase, alpha 2B | extracellular space | enzyme | 41 | 101 | [4, 7, 10] |
| ANPEP | aminopeptidase N | extracellular space | peptidase | 14 | 80 | [4] |
| CEL | carboxyl ester lipase | extracellular space | enzyme | 24 | 107 | [7, 10] |
| CLPS | colipase, pancreatic | extracellular space | enzyme | 6 | 73 | [7, 9] |
| CPA1 | carboxypeptidase A1 | extracellular space | peptidase | 19 | 108 | [4, 7] |
| CPA2 | carboxypeptidase A2 | extracellular space | peptidase | 7 | 95 | [4, 7, 10] |
| CTRB1 | chymotrypsinogen B1 | extracellular space | peptidase | 1 | 28 | [4, 7, 9, 10] |
| CTRC | chymotrypsin C (caldecrin) | extracellular space | peptidase | 11 | 107 | [4, 7] |
| CTRL | chymotrypsin-like | extracellular space | peptidase | 8 | 61 | [7] |
| ELA2A | elastase 2A | extracellular space | peptidase | 16 | 93 | [4, 7, 10] |
| ELA3B | elastase 3B, pancreatic | extracellular space | peptidase | 13 | 92 | [4, 7, 9, 10] |
| KLK1 | kallikrein 1 | unknown | peptidase | 3 | 68 | [10] |
| PLA2G1B | phospholipase A2 | extracellular space | enzyme | 5 | 69 | [4, 7, 9] |
| PNLIP | pancreatic lipase | extracellular space | enzyme | 22 | 111 | [10] |
| PNLIPRP1 | pancreatic lipase-related protein 1 | extracellular space | enzyme | 1 | 21 | [7, 9] |
| PRSS1 | protease, serine, 1 (trypsin 1) | extracellular space | peptidase | 12 | 90 | [4, 7, 9, 10] |
| PRSS2 | protease, serine, 2 (trypsin 2) | extracellular space | peptidase | 6 | 101 | [4, 7, 10] |
| PRSS3 | protease, serine, 3 | extracellular space | peptidase | 6 | 90 | [7, 10] |
| SERPINA1 | alpha-1 antitrypsin | extracellular space | inhibitor | 20 | 129 | [7, 10] |
| SERPINA3 | alpha-1-antichymotrypsin | extracellular space | inhibitor | 9 | 96 | [7, 10] |
Gene ontology reveals identified proteins mainly have molecular function of proteases
To characterize further the 20 pancreas-specific proteins that we identified in all three samples, we performed a gene ontology analysis using the Ingenuity Pathways Analysis program (Ingenuity Systems, v 7.5). As expected, the majority of the pancreas-specific proteins identified by our mass spectrometry experiment have molecular functions of proteases (Table 2).
Discussion
In this study, using a robust fluid analysis strategy, we have described the first proteomic analysis of ePFT-collected pancreatic fluid and identified pancreas-specific proteins. The pancreas is relatively inaccessible and difficult to study due to its retroperitoneal location. A histologic biopsy is not routinely obtained due to the concern for bleeding, pancreatitis, and fistulae formation. Traditionally, highly-invasive procedures (ERCP and surgery) have been used to collect pancreatic fluid for proteomic analysis [4, 6-10]. In contrast, ePFT causes significantly less morbidity for the patients, lowers cost and allows for the collection of larger volumes of fluid. These advantages of our collection methodology combined with GeLC-MS/MS enables a more detailed analysis of the pancreatic fluid.
The development of advanced molecular biology technology has led to an explosion in information about the human genome and the proteins it encodes. Whereas technologies have been developed to quantitate messenger RNA (mRNA) levels to obtain quantitative data regarding transcription, the mRNA abundance levels do not always correlate with the protein concentration of a cell or of the cellular secretome. Proteomics can deal with problems that cannot be approached by genomic analysis, namely, relative abundance of the protein products, post-translational modifications, compartmentalization, turnover, and protein interactions. Clinical proteomics evaluates the protein profile of complex biological samples and can enhance our understanding of normal physiology and pathogenic mechanisms of human disease. Many biological fluids with clinical applications (e.g., plasma, serum, urine, cerebrospinal fluid, saliva) contain proteins of physiological and diagnostic significance that require direct analysis using proteomic technologies. Similarly, pancreatic fluid is a proximal biological fluid specimen that is well-suited for proteomic analysis.
Many previously reported proteomic analysis studies of pancreatic fluid, such as those aiming to investigate or identify biomarker proteins of pancreatic cancer obtained pancreatic fluid during ERCP or at surgery [4, 6-10]. None of the previously-published investigations utilize our endoscopic collection method (ePFT), which significantly enhances fluid collection volume (>10-fold), thereby facilitating a more comprehensive proteome analysis that is not limited by a small sample. More importantly, however, our ePFT method is much less invasive than the aforementioned techniques (ERCP and surgery) and allows for the safe-collection of pancreatic fluid. We believe our collection method combined with high-throughput proteomics will help to clearly differentiate the pancreatic fluid proteome of patients with pancreatic disease and those without pancreatic disease.
There are some potential aspects of our methodology that should be addressed by future investigations. For example, the fluid collected from the duodenum is a mixture of biliary, gastric, duodenal, and pancreas secretions. However, duodenal protein secretion is minimal, and potential gastric fluid efflux is decreased by placing the patient in the left lateral decubitus position. Moreover, both fluids (collectively known as gastroduodenal fluid) are evacuated prior to ePFT and any remnants are subsequently diluted by the protein-rich secretin-stimulated pancreatic secretions. Nevertheless, common gastric secretion proteins, pepsinogen and mucins, have also been identified in pancreatic fluid collected by both ERCP [4, 7] and our own ePFT method. Also, for both ePFT and ERCP, bile contamination is virtually inevitable, due to basal secretions emanating from the common bile duct. For example, in a recent proteomic analysis of ERCP-collected human bile almost 25% of the proteins identified were pancreatic enzymes, such as chymotrypsin, trypsin, elastase, carboxypeptidase, and lipase [30]. Pancreas-specific proteins were also identified in more recent bile proteomics publications investigating malignant biliary stenosis [31, 32]. Because we ultimately aim to compare the differences in protein expression between diseased and healthy states, the presence of bile and gastroduodenal proteins in our samples will not be detrimental to our conclusions.
In summary, we have performed an exploratory proteomic analysis of ePFT-collected, secretin-stimulated pancreatic fluid from non-pancreatic disease controls. Application of a robust and stringent analysis has allowed us to identify common pancreatic fluid proteins which were also found in previous studies where pancreatic fluid was collected by ERCP or surgery. With our initial investigation, we have established ePFT as a reproducible and reliable endoscopic technique to collect pancreatic fluid for further testing and molecular analysis. The ePFT technique combined with GeLC-MS/MS provides a valuable foundation upon which future proteomic investigations of pancreatic fluid can be developed. Application of our technique will be valuable in the characterization of the pathogenesis of pancreatic disease at the molecular level and for the comparison of the pancreatic fluid proteome of healthy individuals and those with pancreatic disease with the intention to elucidate disease-specific biomarkers.
Acknowledgments
The authors would like to thank the Harvard Digestive Disease Center (NIH NIDDK 5 P30 DK034854-24) and the Burrill Family for generous grant support. We would also like to thank members of the Steen Lab, in particular Robert Everley, Yin Yin Lin, Wiebke Timm, and Zachary Waldon for their assistance in data collection and analysis. We would also like to thank members of the therapeutic endoscopy group at Brigham and Women's Hospital and Harvard Medical School, in particular David Carr-Locke, Neil I. Lindeman, John Saltzman, and Christopher Thompson, for their assistance in specimen collection techniques.
NIH financial support: The Harvard Digestive Disease Center (NIH NIDDK 5 P30 DK034854-24)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joao A. Paulo, Department of Pathology, Children's Hospital Boston and Harvard Medical School, Boston, MA, Proteomics Center at Children's Hospital Boston, Boston, MA, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
Linda S. Lee, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
Bechien Wu, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
Kathryn Repas, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
Koenraad J. Mortele, Department of Radiology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
Peter A. Banks, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
Hanno Steen, Department of Pathology, Children's Hospital Boston and Harvard Medical School, Boston, Proteomics Center at Children's Hospital Boston, Boston.
Darwin L. Conwell, Center for Pancreatic Disease, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital and Department of Medicine, Harvard Medical School, Boston.
References
- 1.Lee RS, Monigatti F, Briscoe AC, et al. Optimizing sample handling for urinary proteomics. J Proteome Res. 2008;7(9):4022–30. doi: 10.1021/pr800301h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thongboonkerd V. Proteomics of human body fluids : principles, methods, and applications. Totowa, N.J.: Humana Press; 2007. p. xiii.p. 533. [Google Scholar]
- 3.Anderson NL, Polanski M, Pieper R, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004;3(4):311–26. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Gronborg M, Bunkenborg J, Kristiansen TZ, et al. Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res. 2004;3(5):1042–55. doi: 10.1021/pr0499085. [DOI] [PubMed] [Google Scholar]
- 5.Muthusamy B, Hanumanthu G, Suresh S, et al. Plasma Proteome Database as a resource for proteomics research. Proteomics. 2005;5(13):3531–6. doi: 10.1002/pmic.200401335. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Brentnall TA, Pan S, et al. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteomics. 2007;6(8):1331–42. doi: 10.1074/mcp.M700072-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Pan S, Cooke K, et al. Comparison of pancreas juice proteins from cancer versus pancreatitis using quantitative proteomic analysis. Pancreas. 2007;34(1):70–9. doi: 10.1097/01.mpa.0000240615.20474.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronborg M, Kristiansen TZ, Iwahori A, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5(1):157–71. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Chen R, Pan S, Yi EC, et al. Quantitative proteomic profiling of pancreatic cancer juice. Proteomics. 2006;6(13):3871–9. doi: 10.1002/pmic.200500702. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Lu Z, Yang A, et al. Comparative proteomic analysis of human pancreatic juice: methodological study. Proteomics. 2007;7(8):1345–55. doi: 10.1002/pmic.200600086. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Pan S, Brentnall TA, et al. Proteomic profiling of pancreatic cancer for biomarker discovery. Mol Cell Proteomics. 2005;4(4):523–33. doi: 10.1074/mcp.R500004-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Conwell DL, Zuccaro G, Jr, Vargo JJ, et al. An endoscopic pancreatic function test with cholecystokinin-octapeptide for the diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2003;1(3):189–94. doi: 10.1053/cgh.2003.50028. [DOI] [PubMed] [Google Scholar]
- 13.Conwell DL, Zuccaro G, Jr, Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57(1):37–40. doi: 10.1067/mge.2003.14. [DOI] [PubMed] [Google Scholar]
- 14.Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol. 2009;104(10):2381–3. doi: 10.1038/ajg.2008.181. [DOI] [PubMed] [Google Scholar]
- 15.Stevens T, Conwell DL, Zuccaro G, et al. Electrolyte composition of endoscopically collected duodenal drainage fluid after synthetic porcine secretin stimulation in healthy subjects. Gastrointest Endosc. 2004;60(3):351–5. doi: 10.1016/s0016-5107(04)01809-7. [DOI] [PubMed] [Google Scholar]
- 16.Stevens T, Conwell D, Zuccaro G, et al. Analysis of pancreatic elastase-1 concentrations in duodenal aspirates from healthy subjects and patients with chronic pancreatitis. Dig Dis Sci. 2004;49(9):1405–11. doi: 10.1023/b:ddas.0000042238.80040.cc. [DOI] [PubMed] [Google Scholar]
- 17.Conwell DL, Zuccaro G, Morrow JB, et al. Analysis of duodenal drainage fluid after cholecystokinin (CCK) stimulation in healthy volunteers. Pancreas. 2002;25(4):350–4. doi: 10.1097/00006676-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Pollack BJ, Grendell JH. Where have all the dreiling tubes gone? Am J Gastroenterol. 2006;101(2):356–9. doi: 10.1111/j.1572-0241.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 19.Forsmark CE. The early diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6(12):1291–3. doi: 10.1016/j.cgh.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Shevchenko A, Tomas H, Havlis J, et al. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1(6):2856–60. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 21.Shevchenko A, Wilm M, Vorm O, et al. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 22.Forrester RL, Wataji LJ, Silverman DA, et al. Enzymatic method for determination of CO2 in serum. Clin Chem. 1976;22(2):243–5. [PubMed] [Google Scholar]
- 23.Neubauer G, Mann M. Mapping of phosphorylation sites of gel-isolated proteins by nanoelectrospray tandem mass spectrometry: potentials and limitations. Anal Chem. 1999;71(1):235–42. doi: 10.1021/ac9804902. [DOI] [PubMed] [Google Scholar]
- 24.Steen H, Kuster B, Fernandez M, et al. Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Anal Chem. 2001;73(7):1440–8. doi: 10.1021/ac001318c. [DOI] [PubMed] [Google Scholar]
- 25.Carr S, Aebersold R, Baldwin M, et al. The need for guidelines in publication of peptide and protein identification data: Working Group on Publication Guidelines for Peptide and Protein Identification Data. Mol Cell Proteomics. 2004;3(6):531–3. doi: 10.1074/mcp.T400006-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Taylor GK, Goodlett DR. Rules governing protein identification by mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(23):3420. doi: 10.1002/rcm.2225. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins MR, Appel RD, Van Eyk JE, et al. Guidelines for the next 10 years of proteomics. Proteomics. 2006;6(1):4–8. doi: 10.1002/pmic.200500856. [DOI] [PubMed] [Google Scholar]
- 28.Elias JE, Gibbons FD, King OD, et al. Intensity-based protein identification by machine learning from a library of tandem mass spectra. Nat Biotechnol. 2004;22(2):214–9. doi: 10.1038/nbt930. [DOI] [PubMed] [Google Scholar]
- 29.Moore RE, Young MK, Lee TD. Method for screening peptide fragment ion mass spectra prior to database searching. J Am Soc Mass Spectrom. 2000;11(5):422–6. doi: 10.1016/S1044-0305(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 30.Kristiansen TZ, Bunkenborg J, Gronborg M, et al. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3(7):715–28. doi: 10.1074/mcp.M400015-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Farina A, Dumonceau JM, Lescuyer P. Proteomic analysis of human bile and potential applications for cancer diagnosis. Expert Rev Proteomics. 2009;6(3):285–301. doi: 10.1586/epr.09.12. [DOI] [PubMed] [Google Scholar]
- 32.Farina A, Dumonceau JM, Frossard JL, et al. Proteomic analysis of human bile from malignant biliary stenosis induced by pancreatic cancer. J Proteome Res. 2009;8(1):159–69. doi: 10.1021/pr8004925. [DOI] [PubMed] [Google Scholar]


