Abstract
Objective
Previous research has shown important developmental shifts in genetic and environmental influences for disordered eating. However, little research has examined age differences for weight/shape concerns, two key components of eating disorders. The goal of the present study was to investigate these age differences in pre-adolescent, adolescent, young adult, and mid-adult twins.
Method
Participants included 2,618 female twins (ages of 10-41 years) from three large twin registries. Shape and weight concerns were assessed with the Eating Disorders Examination Questionnaire.
Results
Genetic influences were modest in pre-adolescent twins, but significant from early-adolescence through middle adulthood. Shared environmental factors showed the opposite pattern, with the largest shared environmental contributions occurring in the youngest age group. Nonshared environmental effects remained relatively constant across age.
Discussion
Findings highlight the importance of age differences in genetic and environmental influences. Possible mechanisms include gene × environment interactions and biological changes associated with key developmental stages.
Twin studies have suggested that anorexia nervosa (AN), bulimia nervosa (BN), and eating disorders not otherwise specified (e.g., binge eating disorder) [1-9] are moderately heritable in adulthood. However, heritability for disordered eating symptoms (e.g., weight preoccupation, binge eating, compensatory behaviors) is unlikely to be stable over the lifetime. Disordered eating shows important developmental shifts in etiologic influences across adolescence, with increasing genetic and decreasing shared environmental influences. For example, cross-sectional and longitudinal studies have shown lower heritability of overall levels of disordered eating symptoms (i.e., levels of binge eating, compensatory behaviors, weight preoccupation) in pre- and early adolescent twins as compared to twins in middle and late adolescence [4, 10, 11]. Moreover, longitudinal data suggest that there are no new genetic factors influencing general disordered eating symptoms from age 14 through age 18 [10]. Explanations for these developmental shifts have frequently focused on puberty, where analyses from two twin registries (MTFS) [12] (Michigan State University Twin Registry; MSUTR) [13] suggest that puberty moderates genetic effects on disordered eating, with no genetic influence in early puberty but significant genetic effects in middle- to post-puberty [14-16].
These findings have been important for identifying developmental stages in which genetic effects may become more pronounced. This information has, in turn, narrowed hypotheses regarding regulators of genetic effects (e.g., ovarian hormones) [17] and potential candidate genes [18]. Nonetheless, much more information is needed to fully characterize the developmental trajectories of genetic and environmental influences on disordered eating. For example, we currently have no data beyond the adolescent period. Thus, it is unclear if the magnitude of genetic and environmental influences remains constant from late adolescence (a peak time of eating disorder risk) through young and middle adulthood.
We also have little information about developmental changes in different types of disordered eating. One critical type of disordered eating is weight and shape concerns (i.e., concern about the size or shape of one's body, preoccupation with body weight, etc.). Weight and shape concerns contribute to cognitive symptoms of eating disorders (e.g., fear of fatness, misperceptions of body shape, importance of weight and shape on self-evaluation) [19] and have been shown to be strong prospective risk factors for eating disorders [20]. This strong prospective relationship likely explains the emphasis on weight/shape concerns in etiological [21] and treatment [22] conceptualizations of the disorders.
Despite this emphasis, few studies have examined age differences in genetic and environmental influences for these symptoms. Initial research comparing genetic and environmental effects across age suggested low heritability of weight concerns in pre-adolescent twins [4] but significant heritability (>50%) in adolescent twins [4]. This general pattern has been replicated in studies examining one age group only, which tend to show moderate heritability of weight and/or shape concerns in late adolescence and young adulthood [23-25]. However, others have found lower heritability of weight and shape concerns and related symptoms (i.e., the undue influence of weight and shape on self-evaluation) [9, 26, 27], particularly during middle adulthood [21, 26]. These discrepant results cannot be due entirely to differences in assessment instrument across studies (e.g., weight/shape concerns measured with the Minnesota Eating Behavior Survey versus the Eating Disorders Examination Questionnaire (EDE-Q)), as different results have been obtained when using the same family of assessment instruments (i.e., the EDE and EDE-Q) (see Wade and Bulik, 2007 and Spanos, Burt and Klump, in press) [21, 23]. This has led some to speculate that discrepant findings are actually due to age/developmental differences in etiologic effects rather than measurement error per se [23].
The purpose of our study was to directly examine this possibility by investigating age differences in etiologic effects on weight and shape concerns. We combined data across three twin registries (i.e., the MTFS, the MSUTR, and the Australian Twin Registry (ATR) [28]) to investigate cross-sectional age differences in genetic and environmental effects in 2,618 same-sex female twins who were pre-adolescence, adolescence, young adulthood, or middle adulthood. Our study was the largest to date to examine age differences in etiologic effects and was the first to examine middle adulthood.
Methods
Participants
Participants included 2,618 female twins (1,624 MZ, 994 DZ) from the MSUTR, the MTFS, and the ATR. Data from all three registries were collected during a similar time period (i.e., 1996-2007), particularly the MSUTR and ATR data (2000-2007). Although recruitment procedures vary somewhat by registry, all are population-based with the majority of twins being Caucasian (>82%) and of middle socioeconomic class [12, 28, 29]. The MSUTR and MTFS twins are broadly representative of their respective regions in terms of ethnicity and socioeconomic class [12, 29]. Likewise, only limited sampling bias has been noted in the ATR for self-report questionnaires [28]. Although the MSUTR and MTFS have been described in detail elsewhere [12, 13] the cohort of ATR [28] twins included in this study have not been reported upon previously. Participation rates (77%) and demographic characteristics were excellent for this new cohort.
Twins were between the ages of 10 and 41 years (M = 21.99 years; SD = 7.76 years). Given the wide range of ages, we divided the sample into 6 age groups for analysis (see Table 1). We divided pre-adolescent and adolescent twins into three groups comprised of 10-12 year-old, 13-15 year-old, and 16-19 year-old twins that were identical to twin groups examined in previous developmental studies of pre-adolescent and adolescent twins [4, 10]. This similarity allowed us to directly examine whether weight/shape concerns show similar developmental differences to those observed for overall disordered eating. We then divided the remaining twins into three additional groups comprised of 20-25 year-old, 26-30 year-old, and 31-41 year-old twins. These groupings were based on previous research examining peak periods for eating disorder symptoms [30-32] and sample size considerations. The peak period of onset for eating disorder symptoms extends to age 25 and then levels off [30-32]; thus, we used 25 years as the cut-off for twins in their early 20s. We grouped ages 26-30 and 31-41 years separately to capture theoretically different developmental stages (e.g., late 20s versus mid-30s). Moreover, sample sizes were largest for twins in their early-to-mid 30s and relatively small for twins over 35 years (n = 55 twins). Thus, we used 30 years as the cut-off for the oldest group. Notably, results remained unchanged when twins over the age of 35 were excluded from analyses (data not shown), suggesting that our inclusion of twins in their early and late 30s in the same group did not unduly influence results.
Table 1. Sample Sizes by Twin Registry and Age Group (Total N = 2,618 twins from 1,100 complete and 209 incomplete pairs).
| MSUTR | MTFS | ATR | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | Total | MZ | DZ | Total | MZ | DZ | Total | MZ | DZ | Total | MZ | DZ |
| 10-12 yrs | 172 | 84 | 88 | 0 | 0 | 0 | 0 | 0 | 0 | 172 | 84 | 88 |
| 13-15 yrs | 146 | 78 | 68 | 532 | 342 | 190 | 0 | 0 | 0 | 678 | 420 | 258 |
| 16-19 yrs | 152 | 94 | 58 | 24 | 26 | 22 | 0 | 0 | 0 | 200 | 120 | 80 |
| 20-25 yrs | 164 | 92 | 72 | 516 | 346 | 170 | 0 | 0 | 0 | 680 | 438 | 242 |
| 26-30 yrs | 16 | 8 | 8 | 0 | 0 | 0 | 334 | 206 | 128 | 350 | 214 | 136 |
| 31-41 yrs | 0 | 0 | 0 | 0 | 0 | 0 | 538 | 348 | 190 | 538 | 348 | 190 |
| Total | 650 | 356 | 294 | 1,072 | 714 | 382 | 872 | 554 | 318 | 2,618 | 1,624 | 994 |
Note. MSUTR = Michigan State University Twin Registry; MTFS = Minnesota Twin Family Study; ATR = Australian Twin Registry; MZ = monozygotic; DZ = dizygotic. Values are the number of twins per age category and twin registry.
Measures
Demographic Questionnaire
Twin age was assessed via self-report using standard demographic questionnaires.
Zygosity Determination
In all three registries, twin zygosity was established using standard physical similarity questionnaires that show accuracies of 95% or better (see Lykken, Bouchard, McGue and Telegen, 1990 and Peeter, Van Gestel, Vlietinck, Derom and Derom, 1998) [33, 34]. These questionnaires were completed by either the twins and/or the mother, depending upon the age of the twin. In the MTFS and MSUTR, research assistants also frequently completed the questionnaire, and responses were compared for accuracy. Disagreements were resolved through either: 1) a review of questionnaire data and twin photographs (when available) by one of the principal investigators (MSUTR); or 2) by DNA markers (MTFS).
Weight and Shape Concerns
Weight and shape concerns were assessed with the Eating Disorders Examination Questionnaire (EDE-Q) [35]. The EDE-Q is a 36-item self-report questionnaire derived from the Eating Disorders Examination (EDE) [36] that assesses eating pathology over the past 28 days. The EDE-Q was the only measure of disordered eating that was shared among all three registries.
The EDE-Q includes a 7-item shape concerns subscale (i.e., assessing dissatisfaction and discomfort with body shape) and a 5-item weight concerns subscale (i.e., assessing preoccupation with weight and a desire to lose weight) whose items are rated on a 6-point scale (0 = not at all, 2 = slightly, 4 = moderately, 6 = markedly) and then averaged to create subscale scores. In adult populations, these EDE-Q scales correlate highly with scores from the EDE (r=.77-.80) [37, 38] and exhibit good internal consistency [39] and long-term (∼315 days) test-retest reliability [40]. Although the EDE-Q weight and shape concerns subscales have been studied less extensively in children and adolescents, initial data suggest that correlations with the EDE are high [37, 41-44]. Moreover, in the MSUTR adolescent sample (ages 10-17 years) included in this report, the scales showed excellent internal consistency (alphas > .85) and exhibited high correlations (r = .66 - .69) with the weight preoccupation and body dissatisfaction subscales from the Minnesota Eating Behavior Survey (MEBS) [45]. The MEBS was designed for use in girls ages 9 and older, and thus provides good evidence of convergent validity for the EDE-Q weight/shape concerns scales in our pre-adolescent and young adolescent twins.
Despite the strong psychometric properties of the EDE-Q, recent factor analyses in non-clinical samples have failed to identify two separate EDE/EDE-Q shape and weight concerns subcales [39, 46-48]. Indeed, factor analytic work tends to identify one factor that includes most all of the items from these two subscalesa. Not surprisingly then, the intercorrelation between the two subscales was very high (r = .87 - .95) across all of our age groups. Given these data, we opted to combine items on the two subscales into a single weight/shape concerns scale for all analyses. This combined scale was scored the same way as the individual subscales, i.e., items were added then averaged to create the final score. Similar to findings for the separate weight/shape concerns scales, the combined scale showed excellent internal consistency across all twin registries and ages in our sample (see Table 2).
Table 2. Internal Consistency and Descriptive Statistics for Weight and Shape Concerns by Twin Registry and Age Group.
| Sample | Alphas | Means (SD) | Score Range |
|---|---|---|---|
| Registry: | |||
| MSUTR | .94 | 1.70 (1.50) | .00-5.80 |
| MTFS | .95 | 1.42 (1.43) | .00-6.00 |
| ATR | .94 | 1.89 (1.52) | .00-6.00 |
| Age Group: | |||
| 10-12 years | .92 | 1.19 (1.31)a | .00-4.60 |
| 13-15 years | .95 | 1.22 (1.39)a | .00-6.00 |
| 16-19 years | .95 | 1.78 (1.63)b | .00-5.75 |
| 20-25 years | .95 | 1.89 (1.51)b | .00-6.00 |
| 26-30 years | .94 | 1.95 (1.45)b | .00-5.80 |
| 31-41 years | .94 | 1.99 (1.51)b | .00-6.00 |
Note. MSUTR = Michigan State University Twin Registry; MTFS = Minnesota Twin Family Study; ATR = Australian Twin Registry. Means with different superscripts are significantly different from each other at p < .05.
p < .001
Statistical Analyses
Intraclass twin correlations were used to provide initial indications of genetic and environmental influences on the weight/shape concerns subscale both within and across age. Cross-sectional, univariate twin models were then fit to the raw twin sum scores using the maximum likelihood option in Mx [49]. The raw data option in Mx treats missing data as missing at random. This treatment of missing data is expected to produce less biased and more consistent estimates than other techniques (e.g., pairwise or listwise deletion).
We used the cross-sectional models to estimate the relative contribution of additive genetic (A; genetic influences that add across genes), shared environmental (C; environmental influences that are shared by reared-together twins and are a source of behavioral similarity), and nonshared environmental (E; environmental influences that are not shared by reared together twins and are a source of behavioral dissimilarity) factors to EDE-Q scales across age. Because of the potentially large number of models that could be fit across the six age groups, we initially fit the fully unconstrained model that allows A, C, and E to vary across age. We then examined parameter estimates from this model to identify appropriate submodels that differentially constrained parameters across groups. This approach allowed us to test theoretically relevant submodels without unduly increasing the number of statistical tests. In addition to these submodels, we also fit the fully constrained model that constrains parameters to be equal across all ages, as this model is the most parsimonious and directly tests the null hypothesis (i.e., that there are no differences in A, C, and E across age). We present standardized (or proportional) parameter estimates for all models in the data tables. Following previous recommendations [50], however, we also report the unstandardized parameter estimates for the best-fitting model. These estimates more accurately depict absolute differences in genetic and environmental influences than standardized estimates which represent these differences as proportions of the total variance.
Model fit was determined by examining the difference in the minimized value of minus twice the log-likelihood (-2lnL) between nested models. This difference yields a likelihood-ratio χ2 test that is used to test the significance of the more restrictive model. A nonsignificant χ2 indicates that the more restrictive model provides an appropriate fit to the data. In addition, models that minimize Akaike's information criterion (AIC; AIC=χ2-2*Δdf) [51] are preferred, as the AIC balances model fit with parsimony.
Importantly, before conducting the analyses described above, we first tested for twin registry differences in mean levels of disordered eating and genetic and environmental effects to ensure that findings reflect differences in age rather than twin registry. We used age regressed, standardized EDE-Q scores in these analyses to account for the fact that twin registry was somewhat confounded with age (i.e., the 10-12 group was from the MSUTR; the 13-25 group was from the MSUTR and MTFS; and the oldest two age groups were from the ATR; see Table 1). Mean differences in weight and shape concerns across registry were uniformly of small effect (d = .03-.12; see Table 1 for raw means and SD), suggesting minimal differences in weight/shape concerns after controlling for age. Likewise, biometric model-fitting indicated no significant differences in additive genetic (.55; 95% CI = .35-.60), shared environmental (.00; 95% CI = .00-.18), or nonshared environmental (.45; 95% CI = .40-.50) influences on age-regressed weight and shape concern scores across the three twin registries (Unconstrained model AIC = 1617.35; Constrained model AIC = 1612.85). These findings strongly support our inclusion of data from all three registries and confirm that any observed differences in etiologic factors across age are not due to other differences across twin registries.
Results
Descriptive Statistics
Descriptive statistics are presented in Table 2. Although variability in weight/shape concerns were present for all age groups, a one-way analysis of variance (ANOVAs) with Tukey's B post hoc t-tests revealed lower mean scores in the two youngest age groups (10-12; 13-15) as compared to the older groups (i.e., 16-19, 20-25, 26-30, and 31-41). These findings corroborate those previously showing the largest increase in eating disorder symptoms during adolescence [30-32] and relative stability in symptoms from adolescence on [30-32]. Notably, these mean differences do not affect our ability to examine age differences in genetic and environmental influences, as the later uses the covariation between co-twins (i.e., similarity between twin 1 and twin 2) to estimate etiologic effects rather than individual twin means.
Twin Intraclass Correlations
Twin intraclass correlations are presented in Table 3. The pattern of correlations suggests developmental differences in genetic and environmental effects. In the youngest (i.e., ages 10-12) age group, the MZ twin correlation was not significantly different from the DZ correlation, indicating little genetic influence but significant shared and nonshared environmental effects. By contrast, in ages 13-30, the MZ/DZ twin correlation differences tended to be larger and reach or almost reach significance. These results suggested greater heritability of weight and shape concerns from mid-adolescence through young adulthood. Finally, in the oldest age group, MZ twin correlations were larger than DZ correlations, but differences were more modest, suggesting that heritability may be somewhat lower in this age group.
Table 3. Intraclass Twin Correlations for the Weight and Shape Concerns Subscale.
| Test of Equality | |||
|---|---|---|---|
| Age Group | Twin Correlation | z | One-tailed p |
| 10-12 years | |||
| MZ (n = 31 pairs) | .54*** | .21 | .42 |
| DZ (n = 30 pairs) | .51** | -- | -- |
| 13-15 years | |||
| MZ (n = 181 pairs) | .52*** | 3.44 | .0003 |
| DZ (n = 115 pairs) | .16* | -- | -- |
| 16-19 years | |||
| MZ (n = 56 pairs) | .59*** | 1.52 | .06 |
| DZ (n = 28 pairs) | .30† | -- | -- |
| 20-25 years | |||
| MZ (n = 199 pairs) | .51*** | 3.34 | .0004 |
| DZ (n = 110 pairs) | .16* | -- | -- |
| 26-30 years | |||
| MZ (n = 60 pairs) | .59*** | 1.16 | .054 |
| DZ (n = 38 pairs) | .32* | -- | -- |
| 31-41 years | |||
| MZ (n = 117 pairs) | .59*** | 1.41 | .079 |
| DZ (n = 45 pairs) | .40** | -- | -- |
Note. MZ = monozygotic; DZ = dizygotic. The “Test of Equality z” tests for differences between the MZ and DZ twin correlations. Only pairs with complete data for both twins were included in the analysis.
p < .10,
p < .05,
p < .01,
p < .001.
The twin correlation is greater than zero.
Biometric Model-Fitting
Standardized parameter estimates from the fully unconstrained model suggested substantial changes in genetic and shared environmental effects across age (see Table 4), with modest genetic influence at ages 10-12 years, but significant genetic effects from ages 13-30 years. Genetic effects were somewhat lower again at ages 31-41, although the decrease was not as dramatic as that observed in the 10-12 year-old age group. Overall, shared environmental factors tended to show the opposite pattern of effects (highest at the youngest age), while nonshared environment changed much less across age.
Table 4. Parameter Estimates.
| Models | |||||
|---|---|---|---|---|---|
| Estimates/Model Fit Statistics | Fully Unconstrained | Constrain ACE Ages 13-30a | Constrain ACE Ages 13-41b | Fully Constrainedc | |
| Parameter Est: | |||||
| 10-12 years | |||||
| A | .00 (.00-.44) |
.00 (.00-.44) |
.00 (.00-.44) |
.54 (.38-.59) |
|
| C | .58 (.12-.73) |
.58 (.12-.73) |
.58 (.12-.73) |
.00 (.00-.15) |
|
| E | .42 (.27-.64) |
.42 (.27-.64) |
.42 (.27-.64) |
.46 (.41-.51) |
|
| 13-15 years | |||||
| A | .52 (.30-.61) |
.49 (.30-.48) |
.54 (.40-.59) |
-- | |
| C | .00 (.00-.18) |
.03 (.00-.20) |
.00 (.00-.13) |
-- | |
| E | .48 (.39-.59) |
.48 (.42-.55) |
.46 (.41-.51) |
-- | |
| 16-19 years | |||||
| A | .62 (.09-.75) |
-- | -- | -- | |
| C | .00 (.00-.44) |
-- | -- | -- | |
| E | .38 (.25-.57) |
-- | -- | -- | |
| 20-25 years | |||||
| A | .51 (.28-.60) |
-- | -- | -- | |
| C | .00 (.00-.20) |
-- | -- | -- | |
| E | .49 (.40-.60) |
-- | -- | -- | |
| 26-30 years | |||||
| A | .50 (.00-.70) |
-- | -- | -- | |
| C | .07 (.00-.55) |
-- | -- | -- | |
| E | .43 (.30-.61) |
-- | -- | -- | |
| 31-41 years | |||||
| A | .28 (.00-.65) |
.28 (.00-.65) |
-- | -- | |
| C | .27 (.00-.60) |
.27 (.00-.60) |
-- | -- | |
| E | .45 (.35-.57) |
.45 (.35-.57) |
-- | -- | |
| Model Fit: | |||||
| -2lnL (df) | 7732.09 (2217) | 7756.79 (2225) | 7749.98 (2229) | 7762.35 (2232) | |
| Δχ2 (df) | -- | 33.70 (8) | 17.89 (12) | 30.26 (15) | |
| p | -- | <.001 | .12 | .01 | |
| AIC | 3298.09 | 3306.79 | 3291.98 | 3298.35 | |
Note. A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects; -2lnL = -2 times the log likelihood; Δχ2 = the difference in -2lnL values between the fully unconstrained and the nested constraint models; AIC = Akaike's Information Criteria. Twins from complete and incomplete pairs were included in the analysis. The best-fitting model is noted in bolded text.
Parameter estimates are constrained to be identical from ages 13-31; thus, these estimates are only listed for the age 13-15 group.
Parameter estimates are constrained to be identical from ages 13-41; thus, these estimates are only listed for the age 13-15 group.
Parameter estimates are constrained to be identical across all ages; thus, these estimates are only listed for the age 10-12 group.
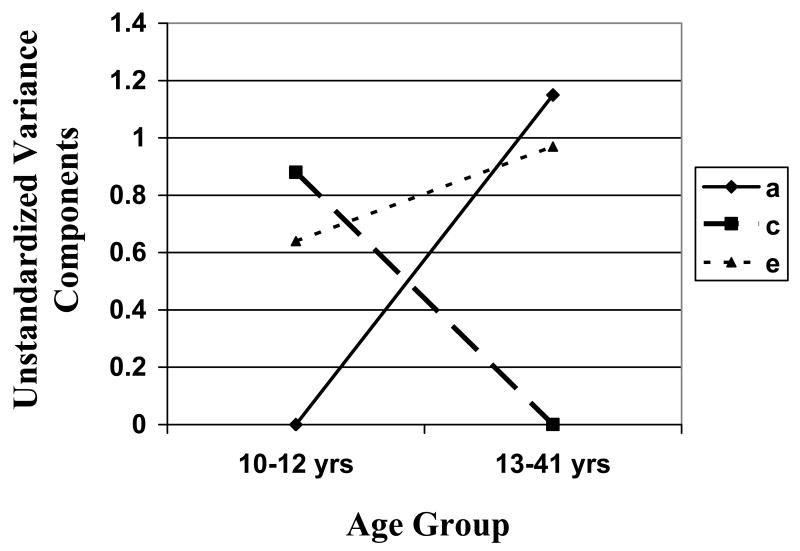
Based on these results, we fit three submodels to the data: 1) a model that constrained A, C, and E across ages 13-30; 2) a model that constrained A, C, and E across ages 13-41; and 3) the fully constrained model that constrained A, C, and E across all ages. Model fit comparisons indicated that the best fitting model was one in which A, C, and E were constrained to be equal across ages 13-41, but were allowed to vary in the 10-12 year old group. This model had the lowest AIC value, and the chi-square difference tests comparing the model to the fully unconstrained model was non-significant (see Table 4). Standardized (or proportional; see Table 4) and unstandardized (or absolute; see Figure 1) parameter estimates from this model indicated that shifts in additive genetic and shared environmental influences primarily account for these age differences. In the 10-12 year old age group, genetic influences were nominal, while shared and nonshared environmental factors accounted for substantial proportions of variance. By contrast, at ages 13-41, additive genetic and nonshared environmental influences predominated with little-to-no shared environmental influence.
Figure 1. Unstandardized Estimates of Additive Genetic, Shared Environmental, and Nonshared Environmental Factors from the Best-Fitting Model for the Weight and Shape Concerns Scale.
Abbreviations include: a = additive genetic effects; c = shared environment; e = nonshared environment.
Discussion
Our study is the first to examine age differences in genetic and environmental effects on weight and shape concerns from pre-adolescence through middle adulthood. We found evidence for modest genetic influences in pre-adolescence and significant genetic effects from early adolescence through middle adulthood. Shared environmental factors tended to show the opposite pattern, with the largest shared environmental contributions occurring in the youngest age groups. Finally, nonshared environmental factors remained relatively constant across age groups. Overall, our findings underscore the likely importance of developmental changes in etiologic effects for weight and shape concerns and their potential role in the development of eating disorders.
Our data are significant in showing that the pre-adolescent period is marked by low genetic risk for core weight and shape concerns of eating disorders. These findings extend previous work by showing that in addition to developmental changes in general disordered eating (i.e., sum scores that include binge eating, compensatory behavior, body dissatisfaction, and weight concerns) (see Klump, McGue and Iacono, 2000 and Klump, Burt, McGue and Iacono, 2007) [4, 10], key symptoms of eating disorders also show nominal genetic effects in pre-adolescence. Previous explanations for these effects have focused on puberty as an activating event for genetic risk for eating pathology [15-18]. This focus partially stems from extant data showing that ovarian hormones regulate gene transcription within several neurotransmitter systems known to be disrupted in eating disorders (e.g., serotonin) [18, 52]. Thus, the activation of ovarian hormones at puberty may activate genetic risk for eating disorders through differential transcription of risk genes. New data indirectly implicate this process, as estrogen levels moderate genetic effects on disordered eating during puberty, with little-to-no genetic effects in girls with low estrogen levels, and significant genetic effects (>50%) in girls with moderate-to-high estrogen levels [53]. Unfortunately, pubertal stage information was not available for many of our 10-12 year-old twins and thus, we were unable to directly examine the effect of puberty on age differences. Nonetheless, it is likely that puberty may partially account for observed differences in genetic effects, and that puberty and ovarian hormones will be critically important mechanisms to examine in future twin and genetic research.
The lack of genetic effects for weight and shape concerns in pre-adolescent twins was countered by the effects of shared environment during this time period. This finding replicates those previously for overall levels of disordered eating [4, 10, 15, 16] and highlights shared environment as a risk factor for disordered eating during pre-adolescence. Shared environment has generally been ignored since the late 1980s when nonshared environmental factors were found to be most important for behavioral phenotypes in adulthood [54]. However, our results add to a growing literature suggesting that shared environment is critically important for the development of psychological symptoms in childhood [55], where a recent meta-analysis found shared environment to significantly contribute to the variance in externalizing and internalizing disorders in childhood [55]. These findings point to a need for studies identifying the factors that underlie these main effects of shared environment. Important shared environmental factors to examine in future research include (but are not limited to) cultural messages about thinness [56, 57] and modeling of disordered eating behavior by family members [58]. The examination of these factors in genetically informative, pre-adolescent samples will significantly advance understanding of the development of eating disorders during this understudied developmental period.
We found moderate heritability for weight/shape concerns during adolescence and young adulthood. This period is one in which females experience the highest level of eating disorder risk [30-32]. Our data suggest that at least part of this increased risk is due to genetic factors that may “tip the scales” toward eating disorders, particularly in the presence of other risk factors (e.g., stressful life events, involvement in weight-focused peer groups). Importantly, although shared environmental influences were non-significant during this time, they may contribute to disordered eating through gene-environment interactions [55]. For example, exposure to cultural messages regarding beauty/thinness ideals may interact with genetic risk to increase susceptibility to eating disorders in some females relative to others. These types of gene × shared environment interactions are partitioned into the additive genetic estimates within twin models, leading to increased heritability and decreased shared environmental variance, a pattern that is similar to that observed in our study (see Figure 1). Adolescence and young adulthood are therefore prime developmental periods for investigating genetic risk and gene × shared environment interactions in the development of eating disorders.
Our study was the first to examine age differences in middle adulthood. Findings from the fully unconstrained model suggested that genetic factors decrease somewhat during this time period, while shared and nonshared environmental factors again become important. However, model fit comparisons suggested that these changes were not statistically significant, as the best fitting model was one which genetic and environmental factors could be constrained across late adolescent, young adult, and mid-adult groups (see Table 4). Given parameter estimates in Table 4, it is possible that a larger sample would have detected significant differences in etiologic effects. Future research should directly examine this possibility.
In the meantime, our data indicate few differences in the broad categories of etiologic factors (i.e., genetic and nonshared environment) contributing to weight/shape concerns in middle adulthood, adolescence, and young adulthood. Nonetheless, it is important to note that while the broad categories of risk may be the same across these developmental periods, the types of genetic and nonshared environmental factors that are important probably vary. For example, it is likely that nonshared environmental risk factors experienced by middle aged women are very different from those experienced by women during adolescence and young adulthood. Factors such as marital separations/divorces [59], stressors emanating from work (e.g., job loss or change of job) and/or family life (e.g., parent-child conflict) [59], the number of pregnancies [60] and physiological changes (e.g., weight changes) [59] are likely to be critical. Although some of these factors (e.g., physical changes) are partially under genetic control [61], their differential experience and/or timing could serve as nonshared environmental risk factors that contribute to weight concerns in one twin relative to another.
The results of this study should be interpreted within the context of its limitations. First, although our overall sample size was quite large, the size of some age groups was relatively small (e.g., the 10-12 age group, and twins over the age of 35). This resulted in broad confidence intervals that, at times, included 0 (e.g., see Table 4). Moreover, and as noted above, sample sizes likely impacted our ability to detect significant differences between some models. Additional studies with larger samples are needed to investigate this possibility. These studies should include a higher proportion of twins over the age of 35 in order to directly examine genetic and environmental factors that may be specific to the peri-menopausal and menopausal period.
Second, age was confounded with twin registry in the youngest and oldest age groups. However, initial analyses confirmed that there were no significant differences in mean levels of weight/shape concerns or genetic and environmental influences across registry after controlling for age. Moreover, data from all three registries were collected during a narrow and similar time period (i.e., 1996-2007); cohort effects would be unlikely to significantly impact the etiology of weight/shape concerns during such a relatively small window of time. Taken together then, these data suggest that our findings likely reflect true age differences in etiologic influences rather than simply registry or cohort effects.
Third, we were limited to the EDE-Q weight/shape concerns scale, which may have posed problems for our phenotypic measurement. For example, it would have been preferable to access interview-based measures as well as questionnaires to ensure that we captured the full range of weight/shape concerns. In addition, it would have been ideal to use latent scores in addition to EDE-Q sum scores in the model-fitting analyses, as sum scores can sometimes produce biased estimates of genetic and environmental effects [see Neale, Lubke, Aggen and Dolan, 2005 62]. Nonetheless, questionnaire measures [4, 23-25] of eating disorder symptoms provide similar estimates of genetic influences as interviews [1, 6, 63, 64], and sum scores tend to produce more biased estimates when binary items are used, scores are based on parental reports, and/or the phenotypes are directly observable by others (e.g., twin activity level) [62]. Given that our sum scores do not fit these criteria, it is unlikely that our findings were unduly influenced by assessment or scoring method.
A final limitation is that our data were cross-sectional rather than longitudinal in nature. Additional studies examining the same cohort of twins across time is needed to ensure that cross-sectional findings are robust and extend to within-twin changes in genetic and environmental influences.
In summary, our results underscore the importance of age differences in etiologic influences on weight and shape concerns. Future studies should improve upon our work by examining more extensive measures of shape/weight concerns and using cross-national, longitudinal samples that span childhood through middle and older adulthood. Such analyses will allow us to develop more comprehensive models of eating disorder risk that take into account the dynamic interplay of genes and environment across development.
Acknowledgments
The study design and data collection for the research presented herein were supported by grants from the National Institute of Mental Health awarded to Dr. Klump (MH 070542), by grants from the Intramural Research Grants Program at Michigan State University awarded to Drs. Burt (04-232) and Klump (71-4831), by grants from the National Institute on Drug Abuse (DA 05147, DA 13240) and the National Institute of Alcohol Abuse and Alcoholism (AA 09367) awarded to Drs. McGue and Iacono, and by grants from the National Health and Medical Research Council (160009 and 310667) awarded to Dr. Wade.
The authors would like to thank the twins for their participation in this research, the MSUTR, MTFS, and ATR staff, and Ms Jacqueline Bergin for coordinating subject recruitment and data collections.
Footnotes
Notably, in the current sample, we conducted an exploratory factor analysis and also obtained a one-factor solution that contained nearly all of the weight and shape concerns items (data not shown). These findings supported our decision to use a single combined scale and partially guided the focus of the current work on weight and shape concerns rather than other disordered eating constructs assessed by the EDE-Q (e.g., restraint, eating concerns, binge eating, purging, etc.). These other constructs appear to be less well-defined by the EDE-Q for our age groups, limiting their usefulness for the twin model-fitting analyses.
None of the authors have financial conflicts of interest.
References
- 1.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychological Medicine. 2001;31:737–40. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 2.Kortegaard LS, Hoerder K, Joergensen J, Gillberg C, Kyvik KO. A preliminary population-based twin study of self-reported eating disorder. Psychological Medicine. 2001;31:361–4. doi: 10.1017/s0033291701003087. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS, MacLean C, Neale M, Kessler R, Heath A, Eaves L. The genetic epidemiology of bulimia nervosa. American Journal of Psychiatry. 1991;148:1627–35. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- 4.Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in female adolescent twins. Journal of Abnormal Psychology. 2000;109(2):239–51. [PubMed] [Google Scholar]
- 5.Javaras KN, Laird NM, Reichborn-Kjennerud T, Bulik CM, Pope HG, Hudson JI. Familiality and heritability of binge eating disorder: Results of a case-controlled family study and a twin stufy. International Journal of Eating Disorders. 2008;41:174–9. doi: 10.1002/eat.20484. [DOI] [PubMed] [Google Scholar]
- 6.Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly-defined bulimia nervosa. Biological Psychiatry. 1998;44:1210–8. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- 7.Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Archives of General Psychiatry. 2006;63(3):305–12. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: A review. International Journal of Eating Disorders. 2000;27:1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Wade TD, Martin NG, Tiggemann M. Genetic and environmental risk factors for the weight and shape concerns characteristic of bulimia nervosa. Psychological Medicine. 1998;28:761–71. doi: 10.1017/s0033291798006989. [DOI] [PubMed] [Google Scholar]
- 10.Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: A longitudinal twin study. Archives of General Psychiatry. 2007;64:1409–15. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- 11.Silberg JL, Bulik CM. The developmental association between eating disorders symptoms and symptoms of depression and anxiety in juvenile twin girls. Journal of Child Psychology and Psychiatry. 2005;46:1317–26. doi: 10.1111/j.1469-7610.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- 12.Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- 13.Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics Special Issue: Twin registries: An ongoing success story. 2006;9:971–7. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- 14.Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating: a replication study. doi: 10.1037/a0017207. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klump KL, McGue M, Iacono WG. Differential heritability of eating pathology in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33:287–92. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- 16.Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–34. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- 17.Klump KL, Culbert KM, Edler C, Keel PK. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. doi: 10.1017/S0033291708002997. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klump KL, Culbert KM. Molecular genetic studies of eating disorders: current status and future directions. Current Directions in Psychological Science. 2007;16:37–41. doi: 10.1111/j.1467-8721.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association AP. Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition -Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 20.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Stewart A. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Wade TD, Bulik CM. Shared genetic and environmental risk factors between undue influence of body shape and weight on self-evaluation and dimensions of perfectionism. Psychological Medicine. 2007;37:635–44. doi: 10.1017/S0033291706009603. [DOI] [PubMed] [Google Scholar]
- 22.Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. New York: The Guilford Press; 2008. [Google Scholar]
- 23.Spanos A, Burt SA, Klump KL. Do weight and shape concerns exhibit genetic effects? Investigating discrepant findings International. Journal of Eating Disorders. doi: 10.1002/eat.20666. in press. [DOI] [PubMed] [Google Scholar]
- 24.Keski-Rahkonen A, Bulik CM, Neale BM, Rose RJ, Rissanen A, Kaprio J. Body dissatisfaction and drive for thinness in young adult twins. Int J Eat Disord. 2005;37:188–99. doi: 10.1002/eat.20138. [DOI] [PubMed] [Google Scholar]
- 25.Rutherford J, McGuffin P, Katz RJ, Murray RM. Genetic influences on eating attitudes in a normal female twin population. Psychological Medicine. 1993;23:425–36. doi: 10.1017/s003329170002852x. [DOI] [PubMed] [Google Scholar]
- 26.Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Tambs K, Torgersen S, et al. Undue influence of weight on self-evaluation: a population-based twin study of gender differences. International Journal of Eating Disorders. 2004;35:123–32. doi: 10.1002/eat.10252. [DOI] [PubMed] [Google Scholar]
- 27.Wilksch SM, Wade TD. An investigation of temperament endophenotype candidates for early emergence of the core cognitive component of eating disorders. Psychological Medicine. doi: 10.1017/S0033291708004261. in press. [DOI] [PubMed] [Google Scholar]
- 28.Heath AC, Howells W, Kirk KM, Madden PAF, Bucholz KK, Nelson EC, et al. Predictors of non-response to a questionnaire survey of a volunteer twin panel: Findings from the Australian 1989 twin cohort. Twin Research. 2001;4:73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- 29.Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite-sex and same-sex twins. Archives of General Psychiatry. 2008;65:329–36. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychological Medicine. 1998;28:109–26. doi: 10.1017/s0033291797005928. [DOI] [PubMed] [Google Scholar]
- 31.Woodside DB, Garfinkel P. Age of onset in eating disorders. International Journal of Eating Disorders. 1992;12:31–6. [Google Scholar]
- 32.Stice E, Killen JD, Hayward C, Taylor CB. Age of onset for binge eating and purging during adolescence: A four-year survival analysis. Journal of Abnormal Psychology. 1998;107:671–5. doi: 10.1037//0021-843x.107.4.671. [DOI] [PubMed] [Google Scholar]
- 33.Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Gemellogicae et Medicae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- 34.Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28:159–61. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- 35.Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–70. [PubMed] [Google Scholar]
- 36.Cooper Z, Fairburn CG. The Eating Disorder Examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. International Journal of Eating Disorders. 1987;6:1–8. [Google Scholar]
- 37.Carter JC, Aime AA, Mills JS. Assessment of bulimia nervosa: A comparison of interview and self-report questionnaire methods. International Journal of Eating Disorders. 2001;30:187–92. doi: 10.1002/eat.1071. [DOI] [PubMed] [Google Scholar]
- 38.Mond JM, Hay PJ, Rodgers B, Owen C, Beamont PJV. Validity of the eating disorder examination questionnaire (EDE-Q) in screening for eating disorders in community samples. Behaviour Research and Therapy. 2004;42:551–67. doi: 10.1016/S0005-7967(03)00161-X. [DOI] [PubMed] [Google Scholar]
- 39.Peterson CB, Crosby RD, Wonderlich SA, Joiner T, Crow SJ, Mitchell JE, et al. Psychometric properties of the eating disorder examination-questionnaire: Factor structure and internal consistency. International Journal of Eating Disorders. 2007;40:386–9. doi: 10.1002/eat.20373. [DOI] [PubMed] [Google Scholar]
- 40.Mond JM, Hay PJ, Rodgers B, Owen C, Beamont PJV. Temporal stability of the eating disorder examination questionnaire. International Journal of Eating Disorders. 2004;36:195–203. doi: 10.1002/eat.20017. [DOI] [PubMed] [Google Scholar]
- 41.Binford RB, Le Grange D, Jellar CC. Eating Disorders Examination versus the Eating Disorders Examination-Questionnaire in adolescents with full and partial-syndrome bulimia nervosa and anorexia nervosa. International Journal of Eating Disorders. 2005;37:44–9. doi: 10.1002/eat.20062. [DOI] [PubMed] [Google Scholar]
- 42.Grilo CM, Masheb RM, Wilson TG. A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. Journal of Consulting and Clinical Psychology. 2001;69:317–22. doi: 10.1037//0022-006x.69.2.317. [DOI] [PubMed] [Google Scholar]
- 43.Passi VA, Bryson SW, Lock J. Assessment of eating disorders in adolescents with anorexia nervosa: Self-report questionnaire versus interview. International Journal of Eating Disorders. 2003;33:45–54. doi: 10.1002/eat.10113. [DOI] [PubMed] [Google Scholar]
- 44.Wolk SL, Loeb KL, Walsh BT. Assessment of patients with anorexia nervosa: Interview versus self-report. International Journal of Eating Disorders. 2005;37:92–9. doi: 10.1002/eat.20076. [DOI] [PubMed] [Google Scholar]
- 45.von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: A brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;6:373–92. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Pennings C, Wojciechowski FL. The Eating Disorder Questionnaire (EDE-Q): Netherlands normative scores for anorexic patients and a non-eating disorder control group. Gedragstherapie. 2004;37:293–301. [Google Scholar]
- 47.Wade TD, Byrne S, Bryant-Waugh R. The eating disorder examination: Norms and construct validity with young and middle school girls. International Journal of Eating Disorders. 2008;41:551–8. doi: 10.1002/eat.20526. [DOI] [PubMed] [Google Scholar]
- 48.Waller G, Hamill M, van Gerko K, Hughes ML. Psychometric properties and discriminant validity of the Eating Disorder Examination-Questionnaire form. submitted. [Google Scholar]
- 49.Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical Modeling. 5th. Department of Psychiatry; Richmond, VA: 1999. [Google Scholar]
- 50.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5(6):554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 51.Akaike H. Factor analysis and aic. Psychometrika. 1987;52:317–32. [Google Scholar]
- 52.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 53.Klump KL, Sisk CL, Keel PK, Burt SA. Estrogen moderates genetic risk on disordered eating during puberty. in preparation. [Google Scholar]
- 54.Plomin R, Daniels Why are children in the same family so different from one another? Behavioral and Brain Sciences. 1987;10:1–60. [Google Scholar]
- 55.Burt SA. Environmental contributions to sibling similarity: Meta-analyses of shared environmental influences on child and adolescent psychopathology. Psychological Bulletin. doi: 10.1037/a0015702. in press. [DOI] [PubMed] [Google Scholar]
- 56.Cafri G, Yamamiya Y, Brannick M, Thompson JK. The influence of sociocultural factors on body image: A meta-analysis. Clinical Psychology: Science and Practice. 2005;12:421–33. [Google Scholar]
- 57.Thompson JK, Stice E. Thin-ideal internalization: Mounting evidence for a new risk factor for body-image disturbance and eating pathology. Current Directions in Psychological Science. 2001;10:181–3. [Google Scholar]
- 58.Stice E. Modeling of eating pathology and social reinforcement of the thin-ideal predict onset of bulimic symptoms. Behaviour Research and Therapy. 1998;36:931–44. doi: 10.1016/s0005-7967(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 59.Keel PK, Baxter MG, Heatherton TF, Joiner T. A 20-year longitudinal study of body weight, dieting, and eating disorder. Journal of Abnormal Psychology. 2007;116:422–32. doi: 10.1037/0021-843X.116.2.422. [DOI] [PubMed] [Google Scholar]
- 60.Bulik CM, Von Holle A, Hamer R, Berg CK, Torgersen L, Magnus P, et al. Pattern of remission, continuation and incidence of boradly dfined eating disorder during early pregnancy in the Norwegian mother and child cohort study (MoBa) Psychological Medicine. 2007;37:1109–18. doi: 10.1017/S0033291707000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox CS, Heard-Costa NL, Vasan RS, Murabito JM, D'Agostino RB, Atwood LD. Genomewide linkage analysis of weight change in the Framington heart study. The Journal of Clinical Endocrinology and Metabolism. 2005;90:3197–201. doi: 10.1210/jc.2004-1752. [DOI] [PubMed] [Google Scholar]
- 62.Neale MC, Lubke G, Aggen SH, Dolan CV. Problems with using sum scores for estimating variance components: Contamination and measurement nonvariance. Twin Research and Human Genetics. 2005;8:553–68. doi: 10.1375/183242705774860231. [DOI] [PubMed] [Google Scholar]
- 63.Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: An examination of shared genetic and environmental risk factors. American Journal of Psychiatry. 2000;157:469–71. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 64.Kendler KS, MacLean C, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of bulimia nervosa. American Journal of Psychiatry. 1991;148:1627–35. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]