Abstract
Background
Despite improvements in outcomes after completion of the Fontan circulation, long-term functional state varies. We sought to identify pre- and postoperative characteristics associated with overall function.
Methods and Results
We analyzed data from 476 survivors with the Fontan circulation enrolled in the Pediatric Heart Network Fontan Cross-sectional Study. Mean age at creation of the Fontan circulation was 3.4 plus or minus 2.1 years, with a range from 0.7 to 17.5 years, and time since completion was 8.7 plus or minus 3.4 years, the range being from 1.1 to 17.3 years. We calculated a functional score for the survivors by averaging the percentile ranks of ventricular ejection fraction, maximal consumption of oxygen, the physical summary score for the Child Health Questionnaire, and a function of brain natriuretic peptide. The mean calculated score was 49.5 plus or minus 17.3, with a range from 3 to 87. After adjustment for time since completion of the circulation, we found that a lower score, and hence worse functional state, was associated with: right ventricular morphology (p less than 0.001), higher ventricular end-diastolic pressure (p equals 0.003) and lower saturations of oxygen (p equals 0.047) prior to completion of the Fontan circulation, lower income for the caregiver (p equals 0.003), and, in subjects without a prior superior cavopulmonary anastomosis, arrhythmias after completion of the circulation (p equals 0.003). The model explained almost one-fifth (18%) of the variation in the calculated scores. The score was not associated with surgical centre, sex, age, weight, fenestration, or the period of stay in hospital after completion of the Fontan circuit. A validation model, using 71 subjects randomly excluded from initial analysis, weakly correlated (R equals 0.17, p equals 0.16) with the score calculated from the dataset.
Conclusions
Right ventricular morphology, higher ventricular end-diastolic pressure and lower saturations of oxygen prior to completion of the Fontan circuit, lower income for the provider of care, and arrhythmias after creation of the circuit, are all associated with a worse functional state. Unmeasured factors also influence outcomes.
Keywords: Clinical outcomes, single ventricle, univentricular heart
Advances in medical and surgical techniques have increased the survival of patients with functionally univentricular heart into adolescence and young adulthood following the final stage of palliation, namely creation of the Fontan circulation. While patients with this circulation may lead relatively normal lives, their exercise tolerance and functional state often decline over time. Patients with a failing circuit may manifest signs and symptoms of exercise intolerance, protein-losing enteropathy, intractable arrhythmias, poor growth, cyanosis, or recurrent effusions. Although ventricular dysfunction may underlie these symptoms, patients with normal ventricular function may also be affected. As the population with the functionally univentricular circulation ages, an increasing number are being referred for cardiac transplantation due to their poor functional state.1 Unfortunately, poor nutritional state, or end-organ dysfunction, increases the risk of death while waiting for, or following, transplantation. Identification of factors associated with a worse functional state following creation of the Fontan circuit may allow the early recognition of patients at risk for poor outcome, permitting appropriate intervention and earlier referral for evaluation for transplantation. The aim of our analysis, therefore, was to identify characteristics of the patients before and after conversion to the functionally univentricular circulation associated with a prospectively collected measure of overall functional state, analysing a well characterized cohort of patients who have undergone the Fontan operation.
Methods
Study Design
The National Heart, Lung, and Blood Institute Pediatric Heart Network Fontan Cross-sectional Study, a multi-centric assessment completed in 2004 by the 7 network centres, enrolled 546 survivors with the Fontain circulation, aged from 6 to 18 years. Details of the overall study have been published previously.2–4 In brief, 4 clinically useful and independent measures of functional state, specifically ventricular function, exercise performance, health-related measures for quality of life, and evidence of neurohormonal activation, were measured in subjects at a mean of 8.7 plus or minus 3.4 years after completion of the circuit, with the goal of determining the relationship among the variables collected. At the time of enrollment, we recorded the medical history related to the underlying cardiac anatomy, clinical, and echocardiographic state prior to completion of the circuit, the surgical outcomes, and the course after completion of the functionally univentricular circulation. Each centre obtained approval from the local ethics review committee, and informed consent was obtained for each subject.
Assessment of ventricular function
Ventricular ejection fraction as assessed by echocardiography provided a quantitative measure of ventricular function. In order to justify the use of echocardiographic calculated ejection fractions in the population, we compared the ejection fractions in a 125 subjects who underwent both magnetic resonance imaging and echocardiography, and found the absolute mean difference between modalities to be less than 2%. Imaging was performed at each participating centre using a standard protocol, and all measurements were conducted in a standardized fashion by the Core Laboratory of the Fontan Cross-Sectional Study. Cross-sectional echocardiograms and Doppler evaluations of standard short- and long-axis views of the ventricle or ventricles were interpreted by one of two readers. Ejection fraction was calculated using end-diastolic and end-systolic volumes obtained using the biplane-modified Simpson’s method.
Assessment of exercise capacity
A maximal bicycle ergometry exercise test was performed using a standard ramp protocol across all centres. We recorderd the percentage predicted maximal consumption of oxygen normalized for age and sex.5,6
Assessment of perceived functional state of health
The Child Health Questionnaire Parent Report Form7 is a validated self-administered instrument for parents to report on the general health and functioning of their child. It yields two summary scores, one physical and the other psychosocial. We used the physical score, which includes questions about functional limitations at school and at home, physical discomfort, and overall health, in our analysis.
Assessment of neurohormonal activation
We measured centrally the resting concentration of brain natriuretic peptide in the plasma using the Shionogi brain natriuretic peptide-32 human assay.8
Statistical methods
Functional Score
In an effort to characterize the overall functional state at the time of participation in the study, we created a functional score. This score is novel, and was developed over the course of multiple discussions by 15 representatives, including both physicians and statisticians, of the 7 participating centres, the New England Research Institute, and the National Heart, Lung, and Blood Institute. The components were determined by consensus from first principles prior to analysis of study data, and are considered to represent clinically useful and independent measures of the functional state at any given time in the period of follow-up. The composite score was calculated for each subject by averaging the percentile rank, determined relative to the other participants in the study, of the four main assessments collected during the study, namely ventricular ejection fraction measured by echocardiography, percentage predicted maximal consumption of oxygen on exercise testing, the physical summary score on the Child Health Questionnaire, and the negative of the residual of age- and gender-adjusted concentration of brain natriuretic peptide in the serum. Equal weight was attributed to each component of the score. The scale ranged from 0 to 100, with a higher score indicative of better functional state.
As an example, the severity score for an individual subject would be calculated as follows:
| Percentage predicted maximal consumption of oxygen | 57th percentile |
| Physical score for the Child Health Questionnaire | 49th percentile |
| Negative brain natriuretic peptide | 66th percentile |
| Total ejection fraction | 33th percentile |
The overall score equals 57 plus 49 plus 66 plus 33 divided by 4, in other words, 51.3.
Due to known effects of gender on the levels of brain natriuretic peptide, we adjusted the raw values prior to calculating the score. Since an adequate external reference does not exist for children, we normalized the values to take account of age and sex, and we used the percentile rank of the residuals to calculate the score. The equation used for adjustment is:
Normalized brain natriuretic peptide equals
where x1 is age at enrollment, 11.86 is mean age, x2 is 0 if female and 1 if male, and 0.603 is the prevalence of males in the study group.
Candidate Predictors of the Functional Score
A list of potential correlates of the functional score was generated by discussion after review of available medical history data. In Table 1, we show the complete list of potential variables. Due to their low prevalence in our sample, we did not use an anatomic diagnosis of totally anomalous pulmonary venous connection, found in only 7 subjects, procedures performed on the pulmonary arteries after completion of the circuit, undertaken in only 4 subjects, and repair of the aortic arch during completion of the circuit, undertaken in only 1 subject. Medications were not considered as correlates due to wide variation in institutional practices.
Table 1.
Demographic, anatomic, and surgical characteristics of the cohorts. All variables listed were considered in modelling as potential correlates of Fontan Functional Score, unless otherwise noted in Methods.
| Study Cohort |
Modelling Cohort |
Validation Cohort |
|||
|---|---|---|---|---|---|
| Variables | Number missing | Mean ± SD, Median (Interquartile Range) or % | Mean ± SD, Median (Interquartile Range) or % | Mean ± SD, Median (Interquartile Range) or % | p value* |
| Number of subjects | 476 | 405 | 71 | ||
| Fontan Functional Score | 49.5 ± 17.3 | 49.4 ± 17.7 | 50.0 ± 14.9 | 0.803 | |
| Age at enrollment, year | 12.0 ± 3.4 | 12.0 ± 3.4 | 12.1 ± 3.3 | 0.825 | |
| Race | 1 | 0.133 | |||
| White | 382 (80.4%) | 319 (79.0%) | 63 (88.7%) | ||
| Black | 47 (9.9%) | 44 (10.9%) | 3 (4.2%) | ||
| Other | 46 (9.7%) | 41 (10.1%) | 5 (7.0%) | ||
| Male | 287 (60.3%) | 244 (60.2%) | 43 (60.6%) | 1.000 | |
| Parental highest grade of school | 6 | 0.391 | |||
| Some high school or less | 29 (6.2%) | 22 (5.5%) | 7 (10.3%) | ||
| High school graduate or GED | 117 (24.9%) | 105 (26.1%) | 12 (17.6%) | ||
| Vocational school, some college | 159 (33.8%) | 136 (33.8%) | 23 (33.8%) | ||
| 4 year college graduate | 110 (23.4%) | 93 (23.1%) | 17 (25.0%) | ||
| Graduate degree | 55 (11.7%) | 46 (11.4%) | 9 (13.2%) | ||
| Household income | 61 | 0.174 | |||
| <$20 000 | 52 (12.5%) | 46 (12.9%) | 6 (10.2%) | ||
| $20 000–39 000 | 76 (18.3%) | 70 (19.7%) | 6 (10.2%) | ||
| $40 000–59 000 | 69 (16.6%) | 53 (14.9%) | 16 (27.1%) | ||
| $60 000–79 000 | 63 (15.2%) | 54 (15.2%) | 9 (15.3%) | ||
| $80 000–99 000 | 58 (14.0%) | 51 (14.3%) | 7 (11.9%) | ||
| >$100 000 | 97 (23.4%) | 82 (23.0%) | 15 (25.4%) | ||
| Pulmonary atresia | 29 (6.1%) | 25 (6.2%) | 4 (5.6%) | 1.000 | |
| Hypoplastic left heart syndrome | 100 (21.0%) | 83 (20.5%) | 17 (23.9%) | 0.529 | |
| Common atrioventricular valve/Heterotaxy/Unbalanced atrioventricular septal defect | 52 (10.9%) | 42 (10.4%) | 10 (14.1%) | 0.355 | |
| Tricuspid atresia | 114 (24.0%) | 97 (24.0%) | 17 (23.9%) | 1.000 | |
| Totally anomalous pulmonary venous return | 8 (1.7%) | 5 (1.2%) | 3 (4.2%) | 0.103 | |
| Ventricular type | 0.671 | ||||
| Left | 244 (51.3%) | 211 (52.1%) | 33 (46.5%) | ||
| Right | 162 (34.0%) | 135 (33.3%) | 27 (38.0%) | ||
| Mixed | 70 (14.7%) | 59 (14.6%) | 11 (15.5%) | ||
| Pre-Fontan mean pulmonary arterial pressure, mmHg | 43 | 11.4 ± 3.3 | 11.4 ± 3.3 | 11.9 ± 3.3 | 0.200 |
| Pre-Fontan end-diastolic pressure, mmHg | 22 | 7.8 ± 3.3 | 7.8 ± 3.4 | 8.0 ± 2.6 | 0.562 |
| Pre-Fontan oxygen saturation, percent | 13 | 84 ± 5.2 | 84.0 ± 5.0 | 84.3 ± 6.4 | 0.694 |
| Age at volume unloading surgery (either superior cavopulmonary anatomosis or Fontan), year | 6 | 1.0 (0.6, 2.3) | 1.0 (0.6, 2.3) | 1.1 (0.6, 2.2) | 0.949 |
| Superior cavopulmonary anatomosis | 351 (73.7%) | 297 (73.3%) | 54 (76.1%) | 0.770 | |
| Number of pulmonary arterial surgeries | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.5 ± 0.7 | 0.819 | |
| Moderate/Severe atrioventricular valvar regurgitation | 48 | 20 (4.7%) | 17 (4.7%) | 3 (4.4%) | 1.000 |
| Moderate/Severe ventricular dysfunction | 31 | 6 (1.4%) | 5 (1.4%) | 1 (1.5%) | 1.000 |
| Age at Fontan, year | 2.9 (2.1, 4.0) | 2.9 (2.1, 3.9) | 3.0 (1.9, 5.0) | 0.657 | |
| Weight for age z score at Fontan | 8 | −1.0 ± 1.2 | −1.0 ± 1.2 | −0.9 ± 1.2 | 0.457 |
| Fenestration | 319 (67.0%) | 262 (64.7%) | 57 (80.3%) | 0.009 | |
| Cardpulmonary bypass time, minutes | 37 | 113.9 ± 47.4 | 114.0 ± 48.1 | 113.0 ± 43.4 | 0.865 |
| Type of Fontan | 0.346 | ||||
| Atriopulmonary connection | 67 (14.1%) | 61 (15.1%) | 6 (8.5%) | ||
| Total cavopulmonary connection intracardiac lateral tunnel | 287 (60.3%) | 244 (60.2%) | 43 (60.6%) | ||
| Total cavopulmonary connection extracardiac lateral tunnel | 53 (11.1%) | 41 (10.1%) | 12 (16.9%) | ||
| Total cavopulmonary connection extracardiac conduit | 60 (12.6%) | 51 (12.6%) | 9 (12.7%) | ||
| Other | 9 (1.9%) | 8 (2.0%) | 1 (1.4%) | ||
| Post-operation complication: Prolonged pleural/pericardial effusions/chylothorax | 177 (37.6%) | 149 (37.3%) | 28 (39.4%) | 0.791 | |
| Length of hospital stay, days | 9 | 11 (9, 18) | 12 (9, 18) | 11 (8, 18) | 0.454 |
| Post-Fontan arrhythmia | 101 (21.2%) | 86 (21.2%) | 15 (21.1%) | 1.000 | |
| Post-Fontan surgeries ≥2 | 13 (2.7%) | 12 (3.0%) | 1 (1.4%) | 0.702 | |
| Number of post-Fontan catheterization interventions | 0.8 ± 1.1 | 0.7 ± 1.1 | 1.0 ± 1.2 | 0.107 | |
| Aortic arch repair | 1 (0.2%) | 0 (0.0%) | 1 (1.4%) | 0.149 | |
| Pulmonary arterial surgeries | 4 (0.8%) | 4 (0.9%) | 0 (0.0%) | 1.000 | |
| Number of years since Fontan | 8.7 ± 3.4 | 8.8 ± 3.4 | 8.4 ± 3.7 | 0.398 | |
| Currently on pacemaker | 64 (13.5%) | 51 (12.6%) | 13 (18.3%) | 0.191 | |
Test of significance for differences between model sample (405 subjects) and validation sample (71 subjects).
Variable reduction
Cluster analysis was used to explore collinearity among variables, and to limit the number of variables considered for building our model.
Imputation of potential predictors
Some variables had missing values that were replaced prior to building the model using mean imputation from the modeling cohort. These were ventricular end-diastolic pressure, in 21 subjects, saturations of oxygen measured at preoperative catheterization, in 8 subjects, the income of the caregiver, in 49 subjects, and age at unloading surgery, in 5 subjects. Missing values in length of hospital stay, discovered in 12 subjects, were replaced by the median number of hospital days. Of note, although data relating to income was missing for 12% of the subjects, when a complete-case analysis was conducted, excluding the cases with unknown income, the multivariate model contained the same variables.
Development of the model
Linear regression was used to explore the relationship between the calculated functional score and potential correlates, and generalized additive modeling and graphical displays were used to determine whether any associations were non-linear. The number of years since completion of the circulation demonstrated a non-linear relationship with the score and was therefore categorized as less than 6 years, from 6 to 11 years, and greater than 11 years since completion, based on lower and upper quartiles. The number of operative procedures performed after completion of the circuit also demonstrated a non-linear relationship, and was categorized as up to 2, or more than 2, procedures.
Multivariate linear regression was used to identify associations between the calculated score and the potential correlates. The criterion for entry to the model was set at p equals 0.2. The criterion to remain in the model was p equals 0.05. Stepwise regression was first used to select a multivariate model that contained main effects only. Time since completion of the Fontan circuit was retained in all multivariate modelling, as the value of some potential correlates depended on follow-up time. Bootstrap bagging was used to indicate reliability of variable selection for inclusion in the final multivariable model. In order to assess whether the predictors identified are important across clinical subgroups, we explored the effects of interactions. We examined 3 different interactions with each candidate predictor in separate models. One interaction term was for the presence of a prior superior cavopulmonary anastomosis, noting yes or no, one interaction term was for the age at Fontan completion of less than 3 years as opposed to equal to or more than 3 years, and the final interaction term was for left versus right versus mixed morphology for the dominant ventricle.
Validation of the model
We selected randomly 71 subjects, accounting for 15% of the overall study sample, to serve as a validation cohort. The data of these 71 subjects were not used in the construction of the primary model. The objective was to compare the predicted score from the regression equation with the score that was calculated from the database.
Results
Calculation of the Functional Score
Data for all components of the score were available for 285 subjects. Analyses indicated that the calculated score was robust when using an average of 3 variables if 4 were not available. Using 3 variables for definition resulted in scores that were within one standard deviation of the score based on complete data for 98% of subjects. On this basis, we included 476 subjects, in whom we had at least 3 components of the score. Similar results were achieved using a score restricted to those with all four components.
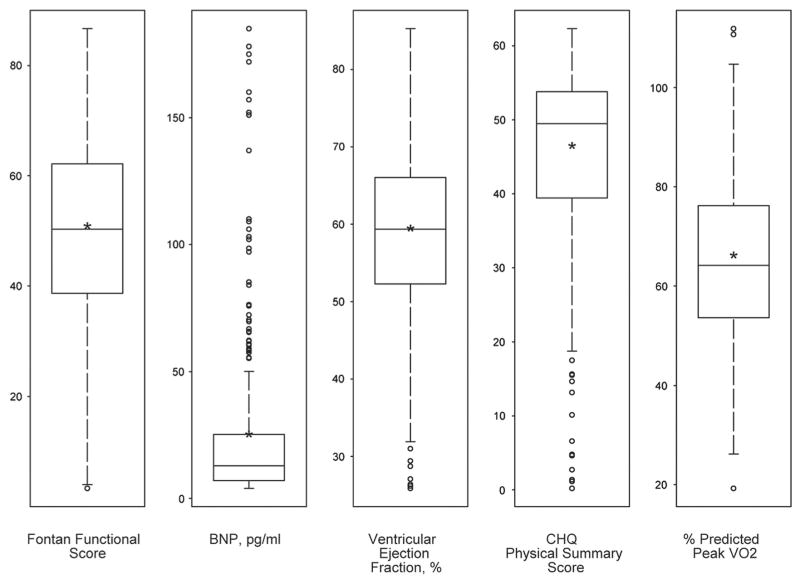
Among the 476 subjects, 396 (83%) had an ejection fraction available for analysis, 392 (82%) had percentage predicted maximal consumption of oxygen, 463 (97%) had a physical summary score, and all had a measured level of brain natriuretic peptide available. In Figure 1, we show the distribution of each component of the score, and the overall calculated functional score.
Figure 1.
Distribution of the functional score and its components. The middle line in each box indicates median, asterisk indicates the mean, and lower and upper edges of boxes represent the 25th and 75th percentiles, respectively. Three extreme values of Brain Natriuretic Peptide (377, 500, 652) are not shown.
Spearman correlation analysis demonstrated that the four components of the score correlated weakly with each other, confirming that their contributions to a composite measure of functioning are largely independent. Only the physical summary score and the percentage predicted maximal consumption of oxygen had a correlation coefficient exceeding 0.15 (R equals 0.24). The calculated functional score itself had a symmetric distribution, with a mean of 49.5 plus or minus 17.3, and a range from 3 to 87.
Results of the Functional Score
In Table 1, we show the characteristics for the entire cohort of 476 subjects, the model cohort of 405 subjects, and the validation cohort of 71 subjects. For the modelling cohort, mean plus or minus standard deviation age at the Fontan procedure was 3.4 plus or minus 2.0 years and, at enrollment, the time since the procedure was 8.8 plus or minus 3.4 years. Of the subjects, 60% were male, and 79% were white. The dominant ventricle was morphologically left in 52% of the subjects, morphologically right in 33%, and was deemed to be of mixed morphology in the remaining 15%. The cohorts used for modeling and validation are similar with respect to all but one variable. The percentage of subjects with a surgical fenestration is lower in the cohort used for analysis than in the one used for validation, at 64.7% versus 80.3% (p equals 0.009).
Results of modelling
Reduction of variables
Cluster analysis revealed expected correlations such as right ventricular morphology and an anatomic diagnosis of hypoplastic left heart syndrome, cardiac anatomic diagnosis and age at Fontan, and pulmonary arterial pressure and end diastolic pressure. Of these, we retained ventricular morphology, age at Fontan, and end-diastolic pressure. Postoperative complications were not used in modeling, as this variable was highly collinear with length of hospital stay. Similarly, since annual household income and highest level of education for the caregiver were collinear, we excluded education.
The results of univariate analysis of the candidate predictors using the modelling subset of 405 subjects are listed in Table 2. We identified 6 independent predictors of the functional score as determined by multivariate regression modelling (Table 3). We regarded the time since the procedure as an adjustment variable. The functional score was associated with ventricular morphology (p less than 0.001), with a lower score for those with a dominant right as compared to dominant left (p less than 0.001) or mixed ventricular types (p equals 0.002). The score did not differ between those having dominant left or mixed ventricular morphology (p equals 0.55). A lower score was also associated with higher ventricular end-diastolic pressure (p equals 0.003), and lower saturations of oxygen (p equals 0.047) at the time of the preoperative catheterization, as well as with lower annual family income (p equals 0.003). The multivariate model explained 18 percent of the variation in the calculated score. The multivariate model also included an interaction between performance of a prior superior cavopulmonary anastomosis and the presence of out of hospital arrhythmias after the procedure (p equals 0.003). Among subjects without a superior cavopulmonary anastomosis, the score was significantly lower, with a mean difference of 16.7 points, for those with compared to those without arrhythmias (p less than 0.001). Among subjects with a superior cavopulmonary anastomosis, the mean scores were similar, with a difference of 3.4 points, when those with were compared to those without arrhythmias (p equals 0.153; see Table 3). Thus, the presence of a postoperative arrhythmia was associated with a lower score if a prior superior cavopulmonary anastomosis had not been performed.
Table 2.
Unadjusted linear regression of Fontan Functional Score on each independent variable (405 subjects).
| Variables | R2 | Estimate | Mean | p |
|---|---|---|---|---|
| Demographics | ||||
| Race | 0.008 | 0.216 | ||
| White | 1.04 | 50.1 | ||
| Black | −3.93 | 45.2 | ||
| Other | – | 49.1 | ||
| Male | 0.001 | 1.03 | 0.566 | |
| Parental highest grade of school | 0.019 | 0.109 | ||
| Some high school or less | −4.25 | 47.1 | ||
| High school graduate/GED | 1.04 | 52.4 | ||
| Vocational school, some college | −4.77 | 46.6 | ||
| 4 year college graduate | −1.08 | 50.3 | ||
| Graduate degree | – | 51.4 | ||
| Annual household income, $20K increments | 0.027 | 1.76 | 0.001 | |
| Ventricular type | 0.022 | 0.011 | ||
| Right | – | 45.9 | ||
| Left | 4.79 | 50.7 | ||
| Mixed | 7.19 | 53.1 | ||
| Pre-Fontan end-diastolic pressure, mmHg | 0.041 | −1.08 | < 0.001 | |
| Pre-Fontan oxygen saturation, % | 0.019 | 0.50 | 0.005 | |
| Superior cavopulmonary anastomisis: | ||||
| Yes | 0.020 | 5.68 | 50.9 | 0.004 |
| No | 45.2 | |||
| Age at unloading surgery | 0.022 | −1.68 | 0.003 | |
| Moderate/Severe atrioventricular valvar regurgitation: | ||||
| Yes | 0.003 | −1.85 | 45.8 | 0.502 |
| No | 2.22 | 49.8 | ||
| Unknown | – | 47.6 | ||
| Number of pulmonary arterial surgeries | 0.001 | −0.74 | 0.546 | |
| Age at Fontan, year | 0.005 | −0.62 | 0.157 | |
| Weight for age z score at Fontan | 0.001 | 0.55 | 0.446 | |
| Surgical fenestration: | ||||
| Yes | 0.004 | 2.35 | 50.2 | 0.202 |
| No | – | 47.9 | ||
| Type of Fontan | 0.032 | 0.011 | ||
| Atriopulmonary connection | – | 46.6 | ||
| Total cavopulmonary connection intracardiac lateral tunnel | 2.25 | 48.9 | ||
| Total cavopulmonary connection extracardiac lateral tunnel | 6.59 | 53.2 | ||
| Total cavopulmonary connection extracardiac conduit | 7.86 | 54.5 | ||
| Other | −11.3 | 35.3 | ||
| Cardpulmonary bypass time, minutes | 0.002 | −0.02 | 0.347 | |
| Length of hospital stay, days | 0.009 | −0.09 | 0.062 | |
| Log transformed length of stay | 0.018 | −3.62 | 0.007 | |
| Arrhythmias: | ||||
| Yes | 0.034 | −7.93 | 43.2 | < 0.001 |
| No | – | 51.1 | ||
| Currently on pacemaker: | ||||
| Yes | 0.021 | −7.73 | 42.7 | 0.004 |
| No | – | 50.4 | ||
| Post-Fontan surgeries ≥2 | 0.004 | |||
| Yes | 0.020 | −14.9 | 34.9 | |
| No | – | 49.9 | ||
| Number of post-Fontan catheterization interventions | 0.011 | −1.68 | 0.033 | |
| Number of years since Fontan | 0.013 | −0.59 | 0.023 | |
| Number of years since Fontan categorized | 0.038 | < 0.001 | ||
| Less than 6 years | 4.91 | 49.3 | ||
| 6 to 11 years | 8.16 | 52.5 | ||
| Greater than or equal to 11 years | – | 44.4 | ||
Table 3.
Independent predictors of Fontan Functional Score (Model R2 = 0.18).
| Variable | Estimate ± Standard Error | Least-Squares Mean | p-value |
|---|---|---|---|
| Years since Fontan | 0.042 | ||
| Less than 6 | 2.15 ± 2.54 | 45.3 | |
| 6 to 11 | 5.19 ± 2.19 | 48.3 | |
| Greater than or equal to 11 | – | 43.1 | |
| Ventricular type | <0.001 | ||
| Left | −1.45 ± 2.42 | 47.2 | |
| Right | −7.88 ± 2.57 | 40.8 | |
| Mixed | – | 48.7 | |
| Annual household income, $20 K increments | 1.53 ± 0.50 | 0.003 | |
| Pre-Fontan end diastolic pressure, mmHg | −0.75 ± 0.25 | 0.003 | |
| Pre-Fontan oxygen saturation | 0.34 ± 0.17 | 0.047 | |
| Post Fontan arrhythmia* | <0.001 | ||
| Superior Cavopulmonary anastomosis | 0.004 | ||
| Superior cavopulmonary anastomosis × post-Fontan arrhythmia interaction | 0.003 | ||
| No superior cavopulmonary anastomosis | |||
| No arrhythmia | 50.3 | <0.001 | |
| Arrhythmia | 33.6 | ||
| Superior cavopulmonary anastomosis performed | |||
| No arrhythmia | 50.9 | 0.153 | |
| Arrhythmia | 47.5 | ||
Main effects estimates for post-Fontan arrhythmia and superior cavopulmonary anastomosis are not interpretable and not shown due to the presence of a significant interaction.
No association with the score was found for the centre where the Fontan circulation was created, gender, race, age at the time of the Fontan procedure, weight for age z-score at the time of the procedure, atrioventricular valvar regurgitation observed prior to the procedure, fenestration of the Fontan baffle, time of cardiopulmonary bypass, length of stay in hospital at the time of the Fontan procedure, and the number of operative surgeries or interventions undertaken subsequent to creation of the functionally univentricular circulation.
Validation of the model
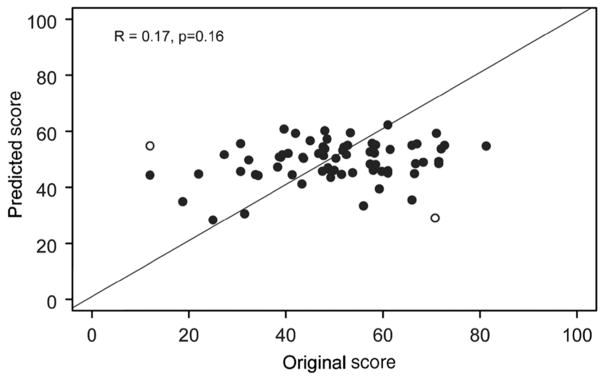
The mean score for the cohort of 71 subjects used for validation was 50.0 plus or minus 14.9, with an interquartile range of 40.5 to 59.8, and a range from 12.0 to 81.3. There was no significant difference between the score predicted from the regression equation and the calculated score, the mean difference being 0.9 plus or minus 15.4 (p equals 0.625). In Figure 2, we show a scatter plot for the predicted score versus the original calculated score, revealing a weak correlation (R equals 0.17, p equals 0.16). The scatter plot demonstrates that the derived model predicts the outcome of ‘middle’ patients reasonably well, but for those with a higher functional state in particular, the model provides an underestimate. There are two extreme observations where the absolute difference between the predicted and the original calculated scores is greater than 40. After removing the two outliers (open circles), the correlation between the predicted and the original score in the remaining 69 subjects increases to 0.29 (p equals 0.017).
Figure 2.
Validation cohort predicted versus empirical functional score. Solid line is line of identity. Pearson correlation is 0.17, p equals 0.16. Outliers are depicted by open circles.
Discussion
Despite improvements in surgical outcomes following completion of the Fontan circulation, functional state varies considerably among survivors. Through creation of a functional score, averaging the percentile ranks of four main evaluations collected during our Fontan Cross-Sectional Study, we aimed to identify pre- and postoperative characteristics associated with overall function for patients in the years following their Fontan operation. We chose our 4 specific components because each was felt to represent a unique and important measure of overall functional state of the individual with Fontan physiology. After adjustment for time since the Fontan procedure, multivariate linear regression demonstrated that a lower functional score, indicating a worse functional state, was associated with: right ventricular morphology, higher ventricular end-diastolic pressure, lower saturations of oxygen, lower annual family income, and, in subjects without a superior cavopulmonary anastomosis, occurrence of arrhythmias after the Fontan procedure. The model explained almost one-fifth of the variation in the calculated score.
Previous series have evaluated risk factors for poor outcomes following palliation by creation of the Fontan circulation. In a recent report of 406 patients at a mean follow up of 6.1 plus or minus 5.7 years, factors associated with poor late outcome included qualitatively decreased ventricular function and elevated pulmonary arterial pressures prior to completion of the Fontan circuit.9 Cluster analysis of our data demonstrated a strong correlation between pulmonary arterial and ventricular end-diastolic pressures prior to the Fontan procedure, and therefore we removed pulmonary arterial pressure as a variable prior to building our model. In a cohort of 220 patients studied 10.2 plus or minus 0.6 years after a Fontan procedure, early failure of the circulation was associated with right ventricular morphology, preoperative elevated pulmonary vascular resistance and pulmonary arterial distortion, and postoperative elevated right atrial pressures.10 Late death was associated with a history of repair of coarctation and right ventricular morphology. Our data also suggests that worse functional state is associated with right ventricular morphology. Patients with a dominant right ventricle may fare worse due to an increased likelihood of poor coronary arterial perfusion during the initial stage of surgery, as in patients with hypoplastic left heart syndrome and aortic atresia. Differences intrinsic to the molecular biology of right ventricular myocardium or to anatomic geometry may also contribute to this finding. Another group reported that atrioventricular valvar regurgitation and heterotaxy were associated with late failure among 121 patients at a mean follow up time of 10.9 plus or minus 5.2 years, albeit that only heterotaxy remained significant in a multivariate equation.11 In our study, the preoperative echocardiographic finding of moderate to severe atrioventricular valvar regurgitation in 20 subjects was not significantly associated using univariate analysis with the functional score (p equals 0.360). We had 33 subjects with heterotaxy syndrome in our cohort, albeit that cluster analysis revealed high collinearity with age at the time of the Fontan procedure and the presence of heterotaxy syndrome. Because of this we eliminated heterotaxy syndrome from the list of potential predictors.
Increased ventricular end-diastolic pressure is presumed to be detrimental to patients with the Fontan circulation due to the impeding of pulmonary venous inflow, the increased pulmonary pressures, and hence compromise of the passive flow dynamics of the Fontan circuit. In addition, elevated ventricular end-diastolic pressure can lead to atrial enlargement, which increases the risk for tachyarrhythmias, another reported risk factor for failure of the Fontan circuit. Reasons for the worse functional state of those with lower saturations of oxygen prior to creating the circuit are unclear. This was the weakest of the independent predictors identified. Chronic hypoxaemia may be a marker for decreased pulmonary arterial flow, and poorer pulmonary arterial growth, leading to less favourable outcomes following completion of the Fontan circuit.
The negative impact of postoperative arrhythmias on the patient with a Fontan circuit, both from a functional and a psychosocial perspective, has been well described. The decreased ventricular filling time associated with tachyarrhythmias rapidly leads to impaired ventricular relaxation, increased ventricular end-diastolic pressures, and potentially decreased flow through the Fontan circuit. Interventions such as cardioversion, and medications to control the arrhythmias, may negatively impact on the quality of life, and increase the risk of adverse effects. Our finding that arrhythmias were predictive of the functional score only in the absence of a superior cavopulmonary anastomosis is curious. The effect could not be explained by any single component of the score. Of note, most patients who did not undergo a prior superior cavopulmonary anastomosis had a history of tachyarrhythmia rather than bradyarrhythmia. Within the group of those with a superior cavopulmonary anastomosis, the prevalences of tachy-versus bradyarrhythmia were similar, consistent with the reported association between staged surgery and sinus nodal dysfunction.12 Although highly statistically significant, we cannot exclude the possibility that the effect of this interaction is spurious. Independent replication of this interaction is of interest, as earlier studies may not have examined interactions by clinical subgroups.
Family income, another independent predictor of the functional score, was associated with the level of education of the parents. This association between the level of education and scores for quality of life has been reported previously.13
Limitations
Requirements for entry into the cross sectional study, including survival to the time of the study, ability to attempt an exercise test, and surgery performed at one of the 7 participating centres, necessarily resulted in bias of selection towards higher functioning patients. Brain natriuretic peptide was normalized using least-square residuals derived from the study sample, limiting the ability to apply this equation to all patients with the Fontan circuit. In general, as the score is derived from the percentile ranks of the population studied, caution should be used if seeking to extrapolate the score to other patients with the Fontan circuit. Assessment of cardiac function using calculations of ejection fraction derived from those with normal left ventricles may also introduce error. Despite utilization of all available data in this well characterized cohort, we were able to explain 18% of the variation noted in functional state following the completion of the Fontan circulation. Hence, over four-fifths of the variation remains unexplained. Unmeasured factors, such as subclinical myocarditis or subtle intraoperative events, may contribute significantly to outcomes. Alternatively, this cohort may be too highly functioning and homogeneous with respect to clinical state to allow us to identify predictors of good and poor function following completion of the Fontan circuit. Future studies including patients with failed Fontan physiology, such as those who have died or have undergone cardiac transplantation, are needed to investigate predictors of long-term outcomes following completion of the Fontan circulation.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288). Dr. Williams reports support from Grant Number KL2 RR024157 from the National Centre for Research Resources, a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Centre for Research Resources or National Institutes of Health. Information on National Centre for Research Resources is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Appendix
National Heart, Lung, and Blood Institute: Gail Pearson, Mario Stylianou, Judith Massicot-Fisher, Marsha Mathis, Victoria Pemberton
Data Coordinating Centre: New England Research Institutes, Lynn Sleeper, Steven Colan, Paul Mitchell, Dianne Gallagher, Patti Nash, Gloria Klein, Minmin Lu
Network Chair: Lynn Mahony, University of Texas Southwestern Medical Centre
Clinical Site Investigators: Children’s Hospital Boston, Jane Newburger (PI), Stephen Roth, Roger Breitbart, Jonathan Rhodes, Jodi Elder, Ellen McGrath; Children’s Hospital of New York, Welton M. Gersony (PI), Seema Mital, Beth Printz, Ashwin Prakash, Darlene Servedio; Children’s Hospital of Philadelphia, Victoria Vetter (PI), Bernard J. Clark, Mark Fogel, Steven Paridon, Jack Rychik, Margaret Harkins, Jamie Koh; Duke University, the late Page A. W. Anderson (PI), Rene Herlong, Lynne Hurwitz, Jennifer S. Li, Ann Marie Nawrocki; Medical University of South Carolina, J. Philip Saul (PI), Andrew M. Atz, Andrew D. Blaufox, Girish Shirali, Jon Lucas, Amy Blevins; Primary Children’s Medical Centre, Salt Lake City, Utah, LuAnn Minich (PI), Richard Williams, Linda Lambert, Michael Puchalski; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Timothy Bradley, Kevin Roman, Jennifer Russell, Shi-Joon Yoo, Elizabeth Radojewski, Nancy Slater
Core Laboratories:
Cardiac MRI, Children’s Hospital Boston: Tal Geva (Director); Andrew J. Powell
Echocardiography, Children’s Hospital Boston: Steven Colan (Director), Marcy Schwartz, Renee Margossian
Protocol Review Committee: Michael Artman, Chair; Dana Connolly, Timothy Feltes, Julie Johnson, Jeffrey Krischer, G. Paul Matherne
Data and Safety Monitoring Board: John Kugler, Chair; Kathryn Davis, David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Catherine L. Webb, Lawrence Wissow
References
- 1.Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, et al. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol. 2004;44:2065–2072. doi: 10.1016/j.jacc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 2.McCrindle BW, Williams RV, Mitchell PD, et al. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113:1123–1129. doi: 10.1161/CIRCULATIONAHA.105.576660. [DOI] [PubMed] [Google Scholar]
- 3.Sleeper LA, Anderson P, Hsu DT, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson PA, Sleeper LA, Mahony L, et al. Contemporary Outcomes after the Fontan Procedure: A Pediatric Heart Network Multicentre Study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 6.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 7.Landgraf JM. Measuring health-related quality of life in pediatric oncology patients: a brief commentary on the state of the art of measurement and application. Int J Cancer Suppl. 1999;12:147–150. doi: 10.1002/(sici)1097-0215(1999)83:12+<147::aid-ijc26>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu H, Aono K, Masuta K, Asada H, Misaki A, Teraoka H. Stability of brain natriuretic peptide (BNP) in human blood samples. Clin Chim Acta. 1999;285:169–172. doi: 10.1016/s0009-8981(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 9.Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments’? Eur J Cardiothorac Surg. 2007;31:344–352. doi: 10.1016/j.ejcts.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Stamm C, Friehs I, Mayer JE, Jr, et al. Long-term results of the lateral tunnel Fontan operation. J Thorac Cardiovasc Surg. 2001;121:28–41. doi: 10.1067/mtc.2001.111422. [DOI] [PubMed] [Google Scholar]
- 11.Ono M, Boethig D, Goerler H, Lange M, Westhoff-Bleck M, Breymann T. Clinical outcome of patients 20 years after Fontan operation – effect of fenestration on late morbidity. Eur J Cardiothorac Surg. 2006;30:923–929. doi: 10.1016/j.ejcts.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Manning PB, Mayer JE, Jr, Wernovsky G, Fishberger SB, Walsh EP. Staged operation to Fontan increases the incidence of sinoatrial node dysfunction. J Thorac Cardiovasc Surg. 1996;111:833–839. doi: 10.1016/s0022-5223(96)70344-6. [DOI] [PubMed] [Google Scholar]
- 13.Saliba Z, Butera G, Bonnet D, et al. Quality of life and perceived health status in surviving adults with univentricular heart. Heart. 2001;86:69–73. doi: 10.1136/heart.86.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]