Abstract
Objective
Burn injury is frequently complicated by bacterial infection. Following burn injury, exposure to endotoxin produces a measurable decrease in cardiomyocyte sarcomere contractile function. Lipopolysaccharide-binding protein (LBP) is an acute phase protein that potentiates the recognition of lipopolysaccharide (LPS) by binding to the lipid A moiety of LPS. In this study we sought to determine the effect of recombinant rat LBP (rLBP) on cardiomyocyte sarcomere function after burn or sham injury in the presence or absence of bacterial endotoxin.
Methods
Rats underwent a full-thickness 30% total body surface area scald or sham burn. At 24 hours post injury, cardiomyocytes were isolated, plated at 50,000 cells/well and incubated with 50 μg/mL LPS and rLBP) or chloramphenicol acetyltransferase (BVCat, an irrelevant control protein produced using the same expression system as rLBP) at concentrations by volume of 1, 5, 10, and 30%. Subsets of cardiomyocytes were incubated with 5 % rat serum or 30% rLBP and blocking experiments were conducted using an LBP-like synthetic peptide (LBPK95A). In-vitro sarcomere function was measured using a variable rate video camera system with length detection software.
Results
Co-culture of burn and sham injury derived cardiomyocytes with high-dose rLBP in the presence of LPS resulted in a significant reduction to the functional impairment observed in peak sarcomere shortening following exposure to LPS alone. LBP-like peptide LBPK95A at a concentration of 20 μg/mL, in the presence of LPS, abolished the ability of 30 % rLBP and 5% rat serum to restore peak sarcomere shortening of cardiomyocytes isolated following burn injury to levels of function exhibited in the absence of endotoxin exposure.
Conclusions
In the setting of LPS challenge following burn injury, rLBP at high concentrations restores cardiomyocyte sarcomere contractile function in vitro. Rather than potentiating the recognition of LPS by the cellular LPS receptor complex, rLBP at high concentrations likely results in an inhibitory binding effect that minimizes the impact of endotoxin exposure on cardiomyocyte function following thermal injury.
Keywords: Burn trauma, Burn injury, Cardiac function, Endotoxin, Langendorff preparation, Sarcomere contraction and relaxation, Lipopolysaccharide-binding protein (LBP)
Introduction
Lipopolysaccharide-binding protein (LBP) is a 60kDa acute-phase glycoprotein that is expressed by various tissue cell types following burn injury (1, 2). In stress states such as burn injury and infection, LBP interacts with membrane-bound or soluble CD14, LPS, and toll-like receptor 4 to initiate cellular production of proinflammatory cytokines, immune cell recruitment, and endotoxin clearance. The complete spectrum of LBP function and interactions in the setting of a burn wound and local or systemic infection is not yet fully understood. Recent experimental data has shown that absence of the LBP gene renders mice incapable of combating and surviving Gram-negative bacterial infections (3). Lamping and colleagues have demonstrated that high concentrations of LBP can have a beneficial effect on survival and clearance of bacteria in a murine sepsis model (4). In humans, high serum LBP levels are known to be inhibitory to monocyte production of TNF-α in the setting of sepsis (5).
Bacterial LPS is the principal outer leaflet lipid component of the membrane in Gram-negative bacteria. LPS itself consists of a sugar moiety including a core oligosaccharide and an O-antigen portion, which is covalently bound to the lipid A component. It has been shown in animal models that exposure to bacterial LPS (E.coli, 011:B4) can induce cardiomyocyte dysfunction in vitro (6, 7). LBP has been demonstrated to modulate the effects of LPS on macrophages and monocytes and other immunocompetent cells; however, its impact on cardiomyocyte function in burn trauma has not been investigated. Recognition of LPS-LBP interaction is usually associated with the presence of either membrane-bound (mCD14) or soluble (sCD14) CD14 receptor (Figure 1). The expression of the CD14 receptor on rat cardiomyocytes has been demonstrated by Comstock and colleagues (8). Both CD14-dependent and CD14-independent LPS signaling have been demonstrated in mouse models (9, 10). Situations such as sepsis and infection are known to increase serum LBP by up to 200μg/mL as part of the acute phase response (11).
Figure 1.
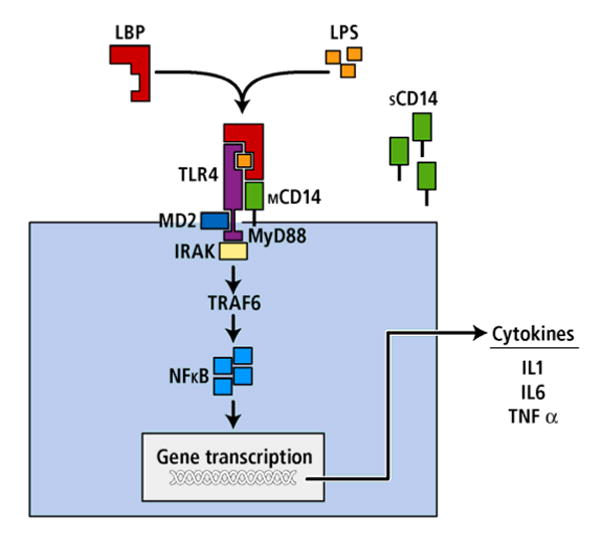
Endotoxin binding: Schematic overview of the LPS, LBP, CD14 and TLR-4 signal transduction pathway. LBP conveys LPS to membrane-bound or soluble CD14, which is surface-anchored by GPI (Glycosylphosphatidylinositol) of host cells. LPS then comes into contact both with TLR4 and with MD-2, a small protein associated with the TLR-4 ectodomain. MyD88 (myeloid differentiation primary response gene) and the IL-1-receptor-associated kinase (IRAK) are recruited and induce activation of the transcription factor nuclear factor kappa B (NFκB), which leads to downstream gene transcription and production of proinflammatory cytokines such as TNF-α, IL-1β and IL-6.
These facts regarding the role of LBP in host defense mechanisms, and our own experience with LBP in the setting of pneumonia prompted us to evaluate the functional impact of LBP in burn injury (12-14). We hypothesized that LBP might have a modulating role on cardiomyocyte function in the setting of burn injury and sepsis. Specifically, we sought to evaluate the effect of recombinant rat LBP (rLBP) on cardiomyocyte sarcomere contraction following thermal injury and exposure to bacterial endotoxin. We also wished to investigate if in-vitro blockade of LPS-LBP interactions with a synthetic peptide could be used to therapeutic advantage.
Materials and Methods
Experimental animals
Adult male Sprague-Dawley rats (Harlan, Inc., Indianapolis, IN) weighing 300 to 350 grams were used in all experiments. Prior to use, the animals were allowed to acclimate to their surroundings for one week. All experiments were performed in accordance with the guidelines set forth by the National Institutes of Health for care and use of animals. The experimental protocol was approved by the University Committee on Use and Care of Animals at the University of Michigan.
Burn procedure
Rats were anesthetized by intraperitoneal injection of ketamine hydrochloride 100 mg/kg (Ketaset®, Fort Dodge Inc., Fort Dodge, IA) and xylazine hydrochloride 5 mg/kg (AnaSed®, Lloyd Lab., Shenandoah, IA). A full-thickness scald burn injury was produced using our previously described technique (7). The exposed skin surface was immersed in 60° C water for 40 seconds. This technique produces a full-thickness dermal burn over 30% of the total body surface area (TBSA). Sham burn animals underwent an identical procedure, except that they were immersed in room temperature water (21° C). The animals received buprenorphine hydrochloride 0.1 mg (Buprenex® Injectable, Reckitt Benckiser Pharmaceuticals, Richmond, VA) by subcutaneous injection every 8 hours for the first 24 hours following burn injury. Sham burn animals received the same resuscitation and analgesia treatment.
Cardiomyocyte isolation
To prepare cardiac myocyte suspensions, animals were anti-coagulated with 1000 units of heparin sodium (Abbott Laboratories, North Chicago, IL) and anesthetized with pentobarbital sodium 65 mg/kg (Nembutal®, Abbott Laboratories, North Chicago, IL) injected intraperitoneally at time 24 hours post-burn or sham injury. A bilateral thoracotomy flap was created and the heart was rapidly excised. Immediate perfusion of the heart using a Langendorff perfusion technique and single cell cardiomyocyte isolation was performed as described previously (7). Cell suspensions with a viability of > 75 % were used for all subsequent experiments.
Fluorescent terminal-deoxyuridine-nick-end-labeling (TUNEL) assay
Apoptosis was detected in situ with fluorescein based labeling of DNA strand breaks using the terminal deoxyuridine nick-end labeling (TUNEL) assay (ApopTag™, CHEMICON International, Inc. Temecula, CA). Cardiac tissue samples were fixed in 10% buffered formalin, embedded in paraffin, cut in 8 μm thick sections and fixed to slides for indirect immunofluorescence according to supplier's instructions. Sections were washed once with water and cover slips were applied using ProLong Gold Antifade™ (Molecular Probes, Inc., Eugene, OR) which included 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) counterstaining. Rat mammary gland tissue obtained four days post-weaning were used as positive controls (provided with the kit), mammary gland sections analyzed omitting terminal transferase served as negative controls.
Image analysis
Images of fluorescent-labeled TUNEL slides were captured digitally at identical time post labeling to control fading of fluorescence using an Olympus BX-51 fluorescence microscope (OLYMPUS America Inc., Melville, NY) at fixed image capture settings and 40× magnification. For each slide three randomly chosen regions of interest of standardized size were digitally defined and digitally captured. Fluorescence was captured using an excitation/filter channel visualizing fluorescein-labeled TUNEL positive cells and images were digitally recorded.
Production of recombinant rat LBP (rLBP)
Recombinant rat LBP was produced using a baculovirus expression system (15). The full-length rat LBP complementary DNA was cloned in-frame into a pBluebacHis2c vector (Invitrogen, Carlsbad, CA). This construct was used with the linearized defective baculovirus DNA, Bac-N-Blue (Invitrogen Corp., Carlsbad, CA) to co-transfect Sf9 insect cells. PCR technique and plaque assays were used to select recombinant viral clones. The production of protein by virally transfected Sf9 cells was optimized using Western Blotting of anti-Xpress-treated cell pellets and supernatants. Chloramphenicol acetyltransferase (BVCat) was used as an irrelevant control protein and was produced using the same baculovirus-based cell expression system. Infection of High Five insect cells (Invitrogen Corp., Carlsbad, CA), which were cultured under serum-free conditions in Excell 400 media (JRH Biosciences, Lenexa, KS), was used to produce high volumes of both recombinant proteins. Biological activity of the rLBP was documented previously (15).
Production of synthetic, LBP-blocking peptide LBPK95A
With the assistance of The University of Michigan Protein Structure Facility, we synthesized a LBP-like peptide (LBPK95A). The peptide synthesized consisted of the 86-99 amino acid sequence of human LBP, which is RVQGRWKVRKSFFK, with substitution of the lysine 95 (Lys95) amino acid with alanine (RVQGRWKVRASFFK). Substitution of the Lys95 amino acid with alanine has been shown to display the strongest blocking effect on LPS-LBP interaction in-vitro (16).
To ascertain that our synthesized LBPK95A peptide has biologic activity we stimulated RAW 267.6 cells with LPS (1 ng/mL) in the presence of 3% rLBP or 1% rat serum and 0, 5, 10, and 20 μM of the LBPK95A inhibitory peptide. TNF-α production was determined after 6 hours in culture using ELISA.
Lipopolysaccharide stimulation of cardiomyocytes
Bacterial endotoxin purified by gel filtration chromatography (LPS, lipopolysaccharide, Lot#013K4148, derived from E.coli 0111:B4, Sigma Corp., St. Louis, MO) was prepared by dissolving 25 mg of the lyophilized powder in 25mL M199 medium. This suspension was sonicated and boiled for 10 minutes. The solution was separated into 1mL aliquots and stored frozen at -20° C.
Cardiomyocytes were plated onto sterile 22×22 mm glass coverslips pre-coated with 40μg/mL natural mouse laminin (Invitrogen Corp., Carlsbad, CA) at a density of 5 × 104 cells/coverslip/well. The coverslips were incubated in 6-well tissue culture plates (37° C, 5 % CO2) for 3 hours to allow for cell attachment and adhesion. The initial culture medium was then carefully pipetted off and replaced with 2 mL/well serum-free M199 medium (Sigma Corp., St. Louis, MO) supplemented with 10mM Glutathione (Sigma Corp., St. Louis, MO), 0.2 mg/mL BSA (GIBCO, Grand Island, NY), 15mM HEPES (Sigma Corp., St. Louis, MO), 26 mM NaHCO3 (Sigma Corp., St. Louis, MO) and the desired experimental concentration of LPS. The plates containing single cell suspensions of cardiomyocytes and reagents were placed in an incubator (37° C, 5% CO2) for 18 hours. All media and other reagents used for the cardiomyocyte isolation were certified endotoxin-free by the manufacturers. At the end of the incubation period supernatants were collected from the wells or the cells underwent single-cell sarcomere contraction analysis.
Determination of rLBP effect on cardiomyocyte function – experimental conditions
At time 0 of an 18-hour cell culture interval, LPS was added to the appropriate wells at a concentration of 50 μg/mL media. This concentration elicited significant suppression of cardiac function in prior experiments (7). In addition, we added either 0, 1, 5, 10 or 30 % by volume of rLBP or BVCat to the cell culture media for a total volume of 2mL in all experiments. To evaluate intrinsic effects of rLBP or BVCat we analyzed the sarcomere contraction of myocytes that were exposed to rLBP or BVCat alone (without LPS). We have shown previously, that in Kupffer cells, peak TNF-α levels were achieved with the addition of LPS and 3% to 10% rLBP (15, 17). Thus, our experimental design included the addition of a low-dose (1%) and a high-dose (30%) rLBP to the cell culture. To evaluate the potential of LBPK95A to block naturally occurring LBP, we also conducted a subset of experiments using LPS, 5% rat serum, and 20 μg/mL LBPK95A peptide exposure.
Single-cell sarcomere contraction and relaxation analysis
Plated cardiomyocytes incubated for 18 hours under experimental conditions underwent single-cell sarcomere contraction and relaxation analysis using a variable rate CCD video camera system (MyoCam®, IonOptix Corp., Milton, MA) equipped with sarcomere length detection software (IonWizard®, IonOptix Corp., Milton, MA). A cover slip with the plated cardiomyocytes was placed in a prefabricated chamber, which was filled with warm (37° C) M199 cell culture media and mounted on the microscope system. The chamber system was connected to a Grass stimulator system (Grass Inc., West Warwick, RI). Electrical pacing stimulation was performed using a 100 mV stimulus of 4 ms duration and a frequency of 1 Hz. The measurement of sarcomere contraction at the different experimental conditions (LPS dose) and the selection of the cardiomyocytes from each coverslip were performed in a randomized fashion. For each measured cardiomyocyte, a rectangle-shaped region of interest was defined and sarcomeres within the focused region were selected for analysis. Typically, this region included approximately 15 – 20 sarcomeres. Sarcomere contractions were recorded for 75 seconds. Raw data for sarcomere shortening and relaxation was collected and stored using the IonWizard® software. Analysis of values at each experimental condition was performed by averaging normalized values for 4-8 cardiomyocytes per coverslip, times 3 wells, times 3 animals for each of the experimental conditions.
Limulus amebocyte assay
A chromogeneic Limulus amebocyte assay (QCL-1000®, Cambrex Corp., Baltimore, MD) was performed to confirm endotoxin-free conditions in the cardiomyocyte suspensions which received no LPS stimulation.
Statistical analysis
All statistical analysis was performed using STATA Statistics/Data Analysis 8.0 software (Stata Corporation, College Station, TX). Results are expressed as the mean value ± standard error of the mean (SEM) unless otherwise noted. Analysis of variance (ANOVA) followed by Tukey's post-hoc tests was used to test for differences among the experimental groups for each of the grouping variables (burn injury vs. sham injury, LPS stimulation vs. no LPS stimulation, and LPS dose). Student's t-test was used to analyze the cytokine data. Statistical significance was defined as a p-value ≤ 0.05.
Results
TUNEL labeling shows increased apoptosis in burn-derived vs. sham animal derived cardiomyocytes
Burn injury markedly increased the rate of cardiomyocytes undergoing apoptosis. Figure 2 shows fluorescent TUNEL stains of cardiomyocytes derived from either sham animals (Figure 2A), or burn animals (Figure 2B). Samples derived from sham animals demonstrate only minimal amounts of TUNEL positive cardiomyocytes, whereas qualitative analysis of cardiomyocytes derived from burn injured animals shows evidence of a marked increase in TUNEL positive cells.
Figure 2. Burn injury increases cardiomyocyte apoptosis.
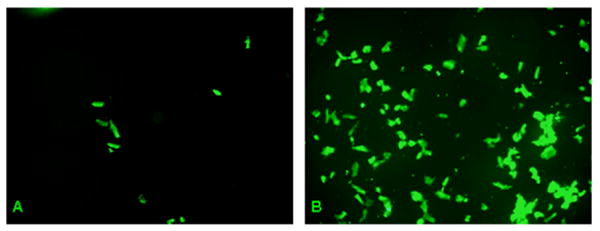
Fluorescent TUNEL stains of cardiomyocytes from either sham animals (A) or burn injured animals (B). Qualitative analysis shows a marked increase in TUNEL positive cardiomyocytes derived from burn injured animals, suggesting that burn trauma has a strong pro-apoptotic effect on cardiac tissue.
rLBP addition to cardiomyocytes in-vitro has no effect on peak sarcomere shortening in the absence of LPS
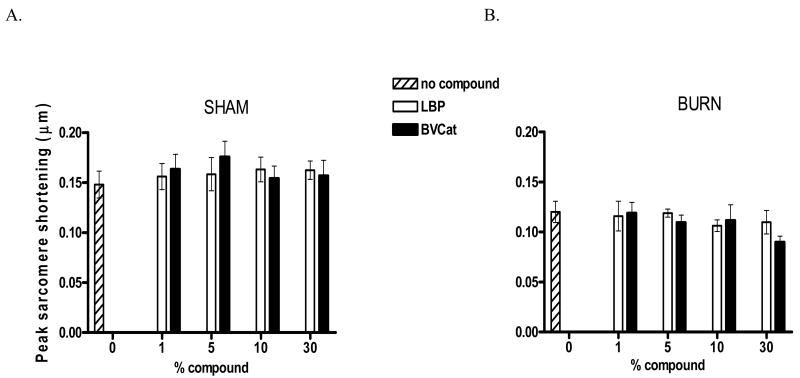
Initially, we sought to determine whether in vitro addition of rLBP to cardiomyocyte culture suspensions isolated 24 hours after burn or sham injury has an effect on peak sarcomere shortening. As depicted in Figure 3, we tested five subgroups: 0 % rLBP and 1, 5, 10 and 30 % (of the total culture media volume, 2mL) rLBP or the equivalent amount of BVCat as a negative control. The y axis represents peak sarcomere shortening in μm. All experiments were performed in triplicate with an average number of 40 cells analyzed per experiment (n=120 cardiomyocyte cells). Our results show that there was no effect of rLBP addition to cardiomyocytes after burn injury regardless of rLBP concentration. Peak sarcomere shortening in the presence of rLBP or presence of the control protein BVCat was the same as observed in the absence of rLBP. Cardiomyocytes derived from sham animals and harvested 24 hours after sham injury revealed the same trend, however, the baseline peak sarcomere shortening levels were higher (sham, no compound, vs. burn, no compound, 0.148 ± 0.013 vs. 0.120 ± 0.011 μm, p≤0.01, Figure 3A, sham injury) indicating that there is cardiomyocyte contractility suppression after burn injury (Figure 3B, burn injury).
Figure 3.
A, sham injury: Peak sarcomere shortening is independent of rLBP or control protein (BVCat) concentrations in sham animals.
This chart depicts peak sarcomere shortening levels of myocytes isolated from sham-injured animals at t = 24 hours post sham injury. No influence of increasing rLBP or BVCat doses was seen (white bars (rLBP), black bars (BVCat) and statistical analysis revealed non-significance (p=n.s., ANOVA). Also, peak sarcomere shortening levels of myocytes from sham animals without rLBP or BVCat exposure were not significantly different from those measured in cardiomyocytes with protein exposure.
B, burn injury: Peak sarcomere shortening is independent of rLBP or control protein (BVCat) concentrations in burn animals.
Measured peak sarcomere shortening levels (μm) of cardiomyocytes isolated from burn animals at t = 24 hours post injury. No significant differences of peak sarcomere shortening were seen with increasing concentrations (Vol.-%, depicted on x axis as % compound) of rLBP (white bars) and the irrelevant control protein BVCat (black bars). Also, there were no significant differences in peak sarcomere shortening between burn cardiomyocytes without rLBP or BVCat exposure (striped bars) and any of the rLBP or BVCat groups (p=n.s., ANOVA).
Addition of rLBP to endotoxin-challenged cardiomyocyte cultures harvested from sham-injured animals improves peak sarcomere shortening in a dose-dependent fashion
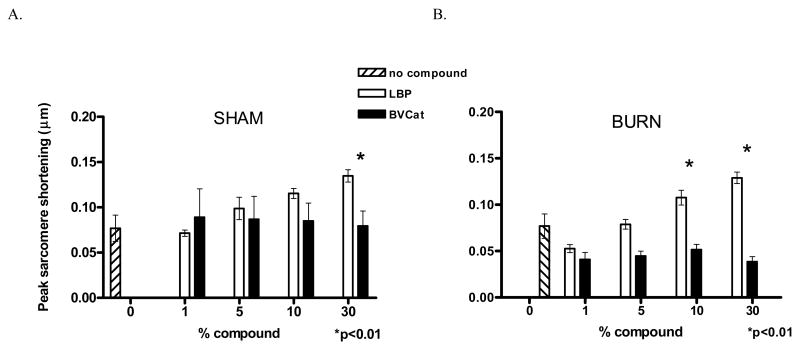
These experiments investigated the addition of rLBP in doses of either 0 (no compound group), 1, 5, 10 or 30 % by volume to sham cardiomyocytes exposed to 50 μg/mL LPS (E. coli 0111:B4). This experiment was designed to evaluate the effect of endotoxin stimulation of cardiomyocytes in the presence of rLBP within the culture medium. Our results showed that at higher rLBP concentrations there was less suppression of peak sarcomere shortening by exposure to LPS (10 and 30 % LBP, Figure 4A, sham injury) with a statistically significant improvement observed for the 30 % rLBP group compared to the 0 % rLBP group (30 % rLBP, 0.135 ± 0.006 μm vs. 0 % LBP, 0.077 ± 0.015 μm, p<0.01). This effect was present, but did not reach statistical significance in groups exposed to LPS at lower concentrations of rLBP.
Figure 4.
A, sham injury: Increased rLBP concentrations reduce LPS-induced suppression of peak sarcomere shortening.
In this chart, results from experiments using sham-animal derived cardiomyocytes that had been cultured with 50 μg/mL LPS are depicted: The peak sarcomere shortening was recorded (μm) and compared to levels obtained from cells with additive rLBP or BVCat exposure (1 to 30%, black and white bars). (30 % rLBP, 0.135±0.006μm vs. 0 % rLBP, 0.077±0.015μm, p<0.01).
B, burn injury: Reductions in peak sarcomere shortening following burn injury and subsequent LPS exposure in rLBP-exposed, burn-injured cardiomyocytes.
LPS-induced peak sarcomere shortening suppression was completely abolished in cells exposed to 10 and 30 % rLBP. At doses of 1% rLBP, suppression of peak sarcomere shortening was observed, there were no significant differences compared to the 0 % rLBP group (1% rLBP 0.053±0.004 μm vs 0 % rLBP 0.077±0.013μm, p≥0.05, n.s.)
Burn injury and LPS + rLBP stimulation improves cardiomyocyte peak sarcomere shortening at lower levels of rLBP than in experiments with sham-injured cardiomyocytes
This set of experiments was designed to evaluate the potential of rLBP to abolish reductions in peak sarcomere shortening following burn injury and subsequent LPS exposure. At 1% rLBP, suppression of peak sarcomere shortening was observed, however, there was not a significant difference compared to the 0 % rLBP group. (Figure 4B, burn injury, 1% rLBP 0.053 ± 0.004 μm vs 0 % LBP 0.077 ± 0.013 μm, p≥0.05). Similar to the experiments using cardiomyocytes derived from sham animals, the reductions in peak sarcomere shortening levels were significantly less at higher rLBP concentrations (10 and 30% rLBP), whereas cells that were exposed to the control protein BVCat experienced a reduction in cardiomyocyte function as evidenced by reduced levels of peak sarcomere contraction. Culture of burn cardiomyocytes with LPS in the presence of 30% rLBP abolished the negative effect of the endotoxin and resulted in peak sarcomere shortening that was equivalent to burn cardiomyocyte cells that were not exposed to LPS (Figure 3B, burn injury vs. Figure 4B, burn injury, Burn + 50 μg/mL LPS + 30% LBP 0.129 ± 0.006 μm vs. Burn + no LPS exposure + 0 % LBP 0.12 ± 0.011 μm, p≥0.05).
Biologic activity of LBP like peptide LBPK95A
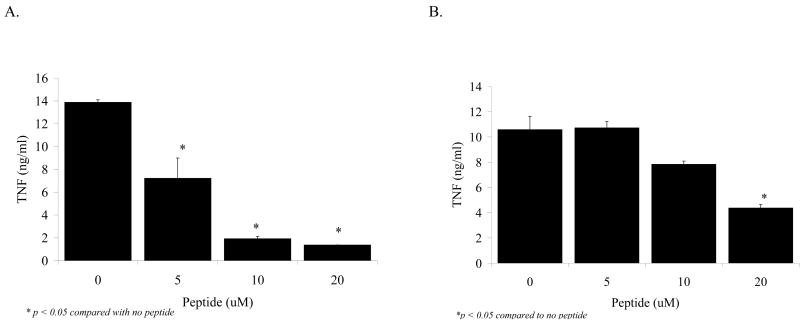
In our next experiment we synthesized a LBP-like peptide, LBPK95A, which has been demonstrated to block recognition of LPS and improve survival in a murine sepsis model (16). When RAW cells were exposed to LPS (1 ng/mL) and 3 % rLBP in the presence of LBP-like peptide LBPK95A there was a dose-dependent decrease in the production of TNFα by the stimulated macrophages (Figure 5A). This dose-dependent LBPK95A blocking effect on the production of TNFα was not as pronounced in the presence of co-culture of the RAW cells with 1 % rat serum instead of rLBP (Figure 5B).
Figure 5.
A: Dose-dependent decrease in the production of TNFα by the stimulated macrophages with rising LBPK95A concentrations.
RAW cells were exposed to LPS (1 ng/mL) and 3 % rLBP in the presence of LBP-like peptide LBPK95A. Note the dose-dependent decrease in the production of TNFα by the stimulated macrophages with rising LBPK95A concentrations (x-axis: 5, 10 and 20 μM of peptide LBPK95A, black bars, *p<0.05 compared with no peptide).
B: 1 % rat serum abolishes the dose-dependent LBPK95A blocking effect on TNFα production of RAW cells.
The dose-dependent decrease in the production of TNFα was not as pronounced in the presence 1 % rat serum instead of rLBP (*p<0.05 compared with no peptide).
Addition of LBP-like peptide LBPK95A abolishes LPS-induced inhibition of cardiomyocyte peak sarcomere shortening
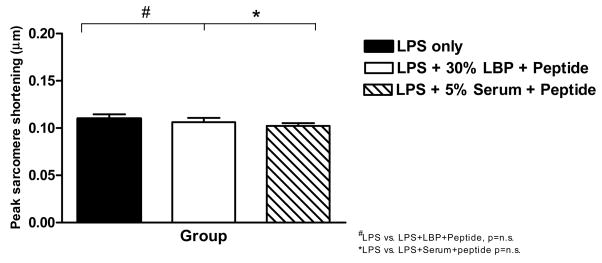
We assessed peak sarcomere shortening levels of cardiomyocytes isolated from sham-injured animals and exposed to LPS at a dose of 10 μg/mL (Figure 6, black bar), LPS 10 μg/mL + 30% rLBP + 20 μg/mL LBPK95A peptide (white bar) or LPS 10 μg/mL + 5% rat serum + 20 μg/mL LBPK95A peptide (Figure 6, striped bar). These experiments showed that the addition of the LBPK95A peptide at a dose of 20 μg/mL completely abolished the rLBP-induced normalization of peak sarcomere contraction seen previously in sham burn cardiomyocytes exposed to LPS (Figure 4A, sham injury). Interestingly, the addition of whole rat serum, with baseline levels of LBP, plus LBPK95A peptide led to a similar level of blockade. Thus, non-synthesized “natural” LBP present in rat serum can also be blocked by the LBPK95A peptide (Figure 6).
Figure 6. Addition of LBPK95A abolished the rLBP-induced improvement of peak sarcomere shortening in sham burn cardiomyocytes exposed to LPS.
Peak sarcomere shortening levels of cardiomyocytes isolated from sham-injured animals and exposed to 10 μg/mL LPS (black bar), LPS 10 μg/mL + 30% rLBP + 20 μg/mL LBPK95A peptide (white bar) or LPS 10 μg/mL + 5% whole rat serum + 20 μg/mL LBPK95A peptide (striped bar). (compare to Figure 3, left panel, sham injury, no compound group).
Discussion
In this study, we showed that high-dose rLBP normalizes cardiomyocyte contractility and abolishes the defects caused by endotoxin exposure. Burn injury causes apoptosis of cardiomyocytes and significant depression of sarcomere function which is exacerbated by exposure to LPS. The presence of rLBP can eliminate the suppressive effects of LPS on cardiomyocyte contractility and restore function to the level exhibited by sham injury cardiomyocytes. Exposure to lower doses of rLBP or a control protein (BVCat) had little effect towards preventing the reduction in cardiomyocyte contraction produced by exposure to LPS. However, this positive effect of rLBP on cardiac contractility in the presence of LPS can be abrogated by in vitro addition of a synthetic, LBP-blocking peptide.
The cellular interaction of the Gram-negative bacterial wall component lipopolysaccharide (LPS) or endotoxin has been a focus of extensive research efforts (18, 19). Currently, the cellular uptake mechanisms and the intracellular effects of LPS are still not completely known. Onset of a microbial burn wound infection in patients with a large TBSA burn can negatively impact survival and morbidity. Burn wound infections can be caused by Gram-negative enteric type pathogens such as Pseudomonas aeruginosa, E. coli, and Acinetobacter spp. Systemic spread of bacterial endotoxin (LPS) in the setting of burn wound infection leading to sepsis, critically suppresses the cardiac function of burn patients. This can palace these patients at risk for end-organ damage and death due to impaired circulation and shock (20).
Lipopolysaccharide-binding protein (LBP) is a protein which is upregulated and secreted in stress situations. Its exact role in modulating the cellular response to endotoxin remains unclear. As an acute phase response protein LBP is especially interesting because of findings indicating a potential dual role in the regulation of LPS action on target cells: Low baseline level concentrations of LBP have been shown to enhance LPS potency, whereas the high concentrations seen during the acute-phase can decrease the suppressive effect of LPS by shunting it into the HDL clearance pathway. It has been demonstrated in a mouse model that that the high serum concentrations of LBP are protective in the setting of sepsis and bacteremia (4). Zweigner et al., found that high concentrations of LBP resulted in significant inhibition of monocyte proinflammatory cytokine production (TNF-α), thus blunting the immune response normally mediated by LPS exposure in humans (5). Our results expand this knowledge into the arena of LBP and LPS effects on cardiac function using cultured cardiomyocytes isolated from burn or sham injured animals.
The first experiments in this investigation confirmed that burn injury results in cardiomyocyte apoptosis and a cardiodepressive proinflammatory response that produces myocardial suppression (6,7). Exposure of sham or burn injury cardiomyocytes to rLBP in the abacterial setting did not result in changes to cardiomyocyte contractility beyond those already created by the thermal injury (Figure 3). We then evaluated the modulating effect of LBP on cardiomyocytes when exposed to bacterial endotoxin (LPS). In cardiomyocytes derived from sham animals exposure to in vitro LPS (Figure 4A, sham injury) produced a suppression in sarcomere contractility that could be blocked by co-culture with high levels of rLBP. Similar to experiments using alveolar macrophages, we saw a dose-dependent protective effect of LBP. A dose of 30% rLBP led to normalization of peak contraction when compared to sham injury cardiomyocytes that were not exposed to LPS (Figure 3A and 4A) (21). Note that these experiments were conducted in vitro, and there still exists a question regarding the complete in vivo mechanism of the beneficial effects of high-levels of LBP. It is unclear if high-levels of LBP act only to bind and competitively inhibit LPS recognition, or if these high-levels result in recognition and shunting into bacterial/endotoxin clearance pathways. Hamann reported that A549 cells - a respiratory type II cell line - show increased LPS uptake with increasing LBP doses (21). This group also reported the LBP paradox of alveolar macrophages reacting to LPS at low levels of LBP, but that high levels of LBP resulted in inhibition of LPS induced activation of alveolar macrophages.
Experimental evidence has shown the existence of at least three cardiodepressive mechanisms following burn injury: 1) abacterial generation of systemic proinflammatory cytokines, 2) the complement system and cardiodepressive effect of the anaphylatoxin C5a, 3) the direct suppressive action of LPS on cardiomyocytes (7, 22). Tavener et al. have shown LPS medicated cardiac effects to be independent of toll-like receptor 4 (TLR4) presence on cardiomyocytes and instead dependent on leukocyte bound TLR4 (23). This data supports our findings and suggests that LPS acts directly on cardiomyocytes without involvement of TLR4-dependent pathways. The results of the LBPK95A blocking peptide experiment provides additional evidence of an LPS suppressive or clearance effect of LBP at high concentrations, potentially via cluster or micelle-formation of LPS-LBP complexes (Figure 6). In our experiments, the LBPK95A peptide was able to block both native rat serum LBP (Figure 6, hashed bar) and ex vivo generated recombinant rat LBP (Figure 6, white bar). Increased LBP levels mediate enhanced LPS binding in a Chinese hamster ovary cell culture model, without leading to increased NF-κB activation, indicating that LPS binding alone does not induce intracellular signaling (24). There is literature suggesting that “LPS-unresponsive cells” such as A549 cells may have an anti-inflammatory function by cellular uptake of LPS (21).
However, our current as well as previous results suggest that high, non-physiologic LPS concentrations are needed to elicit cardiomyocyte suppressive effects. This may indicate a dichotomous effect of LPS on cardiomyocytes: Lower LPS concentrations do not appear to affect cardiomyocyte function, and this endotoxin may be cleared by a yet unknown mechanism. In contrast, high LPS concentrations induce myocardial depression, indicative of a direct effect of LPS (TLR4-independent LPS action) occurring at a level where cellular LPS clearance functions are exceeded.
Acknowledgments
Grant Support: This work has been supported by the American College of Surgeons C. James Carrico Faculty Research Fellowship for the Study of Trauma and Critical Care (M.R.H.) and by the National Institutes of Health grants R01-GM54911 (S.C.W.) and K08-GM078610 (M.R.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fang CW, Yao YM, Zhai HX, Yu Y, Wu Y, Lu LR, Sheng ZY, Sheng CY. Tissue lipopolysaccharide-binding protein expression in rats after thermal injury: potential role of TNF-alpha. Burns. 2004;30:225–231. doi: 10.1016/j.burns.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Klein RD, Su GL, Aminlari A, Zhang H, Steinstraesser L, Alarcon WH, Wang SC. Skin lipopolysaccharide-binding protein and IL-1beta production after thermal injury. J Burn Care Rehabil. 2000;21:345–352. doi: 10.1067/mbc.2000.107542. [DOI] [PubMed] [Google Scholar]
- 3.Jack RS, Fan X, Bernheiden M, Rune G, Ehlers M, Weber A, Kirsch G, Mentel R, Furll B, Freudenberg M, Schmitz G, Stelter F, Schutt C. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature. 1997;389:742–745. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 4.Lamping N, Dettmer R, Schroder NW, Pfeil D, Hallatschek W, Burger R, Schumann RR. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J Clin Invest. 1998;101:2065–2071. doi: 10.1172/JCI2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98:3800–3808. doi: 10.1182/blood.v98.13.3800. [DOI] [PubMed] [Google Scholar]
- 6.Carlson DL, White DJ, Maass DL, Nguyen RC, Giroir B, Horton JW. I kappa B overexpression in cardiomyocytes prevents NF-kappa B translocation and provides cardioprotection in trauma. Am J Physiol Heart Circ Physiol. 2003;284:H804–814. doi: 10.1152/ajpheart.00394.2001. [DOI] [PubMed] [Google Scholar]
- 7.Niederbichler AD, Westfall MV, Su GL, Donnerberg J, Usman A, Vogt PM, Ipaktchi KR, Arbabi S, Wang SC, Hemmila MR. Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile. Shock. 2006;25:176–183. doi: 10.1097/01.shk.0000192123.91166.e1. [DOI] [PubMed] [Google Scholar]
- 8.Comstock KL, Krown KA, Page MT, Martin D, Ho P, Pedraza M, Castro EN, Nakajima N, Glembotski CC, Quintana PJ, Sabbadini RA. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–2775. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- 9.Cowan DB, Poutias DN, Del Nido PJ, McGowan FX., Jr CD14-independent activation of cardiomyocyte signal transduction by bacterial endotoxin. Am J Physiol Heart Circ Physiol. 2000;279:H619–629. doi: 10.1152/ajpheart.2000.279.2.H619. [DOI] [PubMed] [Google Scholar]
- 10.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, Carabello BA, Mann DL, Vallejo JG. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation. 2002;106:2608–2615. doi: 10.1161/01.cir.0000038110.69369.4c. [DOI] [PubMed] [Google Scholar]
- 11.Froon AH, Dentener MA, Greve JW, Ramsay G, Buurman WA. Lipopolysaccharide toxicity-regulating proteins in bacteremia. J Infect Dis. 1995;171:1250–1257. doi: 10.1093/infdis/171.5.1250. [DOI] [PubMed] [Google Scholar]
- 12.Fan MH, Klein RD, Steinstraesser L, Merry AC, Nemzek JA, Remick DG, Wang SC, Su GL. An essential role for lipopolysaccharide-binding protein in pulmonary innate immune responses. Shock. 2002;18:248–254. doi: 10.1097/00024382-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hemmila MR, Fan MH, Kim J, Sun JM, Steinstraesser L, Gong KQ, Arbabi S, Minter RM, Remick DG, Su GL, Wang SC. Improved survival in mice given systemic gene therapy in a gram negative pneumonia model. J Trauma. 2005;58:1110–1118. doi: 10.1097/01.ta.0000170855.37686.91. discussion 1118. [DOI] [PubMed] [Google Scholar]
- 14.Hemmila MR, Kim J, Sun JM, Cannon J, Arbabi S, Minter RM, Su GL, Remick DG, Wang SC. Gene therapy with lipopolysaccharide binding protein for gram-negative pneumonia: respiratory physiology. J Trauma. 2006;61:598–605. doi: 10.1097/01.ta.0000233763.18853.5b. discussion 605-596. [DOI] [PubMed] [Google Scholar]
- 15.Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932–936. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- 16.Arana Mde J, Vallespi MG, Chinea G, Vallespi GV, Rodriguez-Alonso I, Garay HE, Buurman WA, Reyes O. Inhibition of LPS-responses by synthetic peptides derived from LBP associates with the ability of the peptides to block LBP-LPS interaction. J Endotoxin Res. 2003;9:281–291. doi: 10.1179/096805103225002520. [DOI] [PubMed] [Google Scholar]
- 17.Su GL, Freeswick PD, Geller DA, Wang Q, Shapiro RA, Wan YH, Billiar TR, Tweardy DJ, Simmons RL, Wang SC. Molecular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol. 1994;153:743–752. [PubMed] [Google Scholar]
- 18.Wilson JD, Jr, Stewart RM, Fabian TC, Weinberg JA, Trenthem LL, Proctor KG. Plasma tumor necrosis factor and post-traumatic hyperdynamic sepsis evoked by endotoxin. Shock. 1994;1:176–183. doi: 10.1097/00024382-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Seatter SC, Li MH, Bubrick MP, West MA. Endotoxin pretreatment of human monocytes alters subsequent endotoxin-triggered release of inflammatory mediators. Shock. 1995;3:252–258. doi: 10.1097/00024382-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H, Traber LD, Traber DL. Altered systemic organ blood flow after combined injury with burn and smoke inhalation. Shock. 1998;9:369–374. doi: 10.1097/00024382-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Hamann L, Stamme C, Ulmer AJ, Schumann RR. Inhibition of LPS-induced activation of alveolar macrophages by high concentrations of LPS-binding protein. Biochem Biophys Res Commun. 2002;295:553–560. doi: 10.1016/s0006-291x(02)00710-6. [DOI] [PubMed] [Google Scholar]
- 22.Hoesel LM, Niederbichler AD, Schaefer J, Ipaktchi KR, Gao H, Rittirsch D, Pianko MJ, Vogt PM, Sarma JV, Su GL, Arbabi S, Westfall MV, Wang SC, Hemmila MR, Ward PA. C5a-blockade improves burn-induced cardiac dysfunction. J Immunol. 2007;178:7902–7910. doi: 10.4049/jimmunol.178.12.7902. [DOI] [PubMed] [Google Scholar]
- 23.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 24.Hamann L, Schumann RR, Flad HD, Brade L, Rietschel ET, Ulmer AJ. Binding of lipopolysaccharide (LPS) to CHO cells does not correlate with LPS-induced NF-kappaB activation. Eur J Immunol. 2000;30:211–216. doi: 10.1002/1521-4141(200001)30:1<211::AID-IMMU211>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]